Introduction
Ovarian cancer (OC) begins in the ovary and can
result in abnormal cells invading numerous other parts of the body.
Symptoms are often vague and unapparent when the disorder begins.
There are numerous symptoms for OC, such as bloating (1), pelvic pain (2) and abdominal swelling (3). A number of areas of the body can be
affected by OC by the metastasis of abnormal cells, including the
abdomen (4), bowel (5), bladder (6), lymph nodes (7) and liver (8). In 2012, there were 239,000 females
affected by OC, which caused 152,000 mortalities worldwide
(9). OC is the sixth and eighth
leading cause of cancer mortality among women in developed and
developing countries, respectively (10).
Medical therapy is the most widely used type of
treatment for OC; examples include olaparib a biological therapy
agent and taxol a chemotherapy agent (10–13).
However, the majority of medicines used exhibit unwanted cell
toxicity (14–17). Chemotherapy can cause different side
effects, such as nausea and vomiting, distress, sexual dysfunction,
fatigue and memory loss (18). The
toxicity and side effects limit the usage and effectiveness of the
aforementioned treatments for OC. It is therefore necessary to find
an effective therapeutic method with fewer side effects for OC.
Non-pharmaceutical treatment has become a novel therapy for various
types of cancer. Blueberries (Vaccinium spp.), a type of
fruit, have been identified to exhibit therapeutic effects on
several types of cancer (19,20). For example, blueberries inhibit the
progression of triple negative breast cancer and triple negative
breast cancer-associated metastasis by the inhibition of
inflammation via specific cytokine-mediated signaling pathways
(21). Blueberries have been revealed
to suppress tumor growth and metastasis (21), and are becoming an important fruit due
to the associated chemopreventative and therapeutic potential
against the tumorigenesis of numerous types of cancer. A
blueberry-supplemented diet protects against 17β-estradiol-mediated
mammary tumorigenesis (22). However,
the molecular mechanisms of blueberries with respect to the
inhibition of various types of cancer are unclear, and the
therapeutic effect on OC remains unknown.
Cyclooxygenase (COX), termed
prostaglandin-endoperoxide synthase (23), is an enzyme responsible for the
formation of prostaglandins. A previous study reported the presence
of high levels of COX-1 mRNA in high-grade serous ovarian cancer
(24). COX-2 is also overexpressed in
human ovarian cancer cells, and targeting COX-2 may be a potential
approach for the treatment of OC (25). Blueberries may exhibit a therapeutic
effect on OC, and COX-1 and COX-2 may be involved in the process.
The present study aimed to explore the protective effect of a
blueberry diet against the development of OC, which is hypothesized
to act via the regulation of COX-mediated pathways.
Materials and methods
Materials
The blueberries were purchased from the Blueberry
Production Field (Guizhou, China). The blueberries were frozen upon
arrival at −20°C. A total of 100 g blueberries were homogenized in
a homogenizer (GYB60-65; Shanghai Donghua High Pressure Homogenizer
Factory, Shanghai, China). The blueberry juice was obtained by
centrifuge at 10,000 × g, 40°C for 10 min (1 ml blueberry juice was
produced from 2 g blueberry).
Cell culture
The human ovarian cancer SKOV3 cell line was
purchased from the Type Culture Collection of the Chinese Academy
of Sciences (Shanghai, China) and cultured with McCoy's 5A medium
without serum (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA). The medium was supplemented with 4 µg/ml transferrin
(Chemicon International Inc.; EMD Millipore, Billerica, MA, USA), 5
µg/ml insulin (Sigma-Aldrich, St. Louis, MO, USA). and 10 ng/ml
vascular endothelial growth factor (Chemicon International Inc.;
EMD Millipore). The cells were cultured in 5% CO2 at
37°C and 100% humidity, and transferred to fresh medium every 2
days.
Evaluation of cell viability by MTT
assay
Cell viability was determined using an MTT assay.
The SKOV3 cells were seeded into 96-well plates at a concentration
of 1×104 cells per well in 100 µl McCoy's 5A medium. The
cells were treated for 24, 48 or 72 h with 0, 1, 2, 4, 8 or 16
mg/ml blueberry juice. At the end of the culture, 5 µl MTT (10
mg/ml in PBS) was added to each well and incubated for an
additional 3 h at room temperature. Untreated cells were used as
control groups. The purple formazan was dissolved in dimethyl
sulfoxide. Absorbance was recorded at 570 nm using an ELISA reader
(Elisa Reader KD600; Shanghai Tongge Medical Devices Co., Ltd.,
Shanghai, China).
COX-1 and COX-2 expression
construction
COX-1 and COX-2 genes were amplified and cloned into
the Nhel-EcoRI sites of pcDNA3.1 plasmid (Invitrogen; Thermo Fisher
Scientific, Inc.), which were termed pcDNA3.1-COX-1 and
pcDNA3.1-COX-2. The details of the CPX primers are as follows:
COX-1 accession number, BC029840.1, sense,
5′-GTGAGCTAGCATGAGCCGGAGTCTCTTGC-3′ and antisense primers,
5′-CTGAGAATTCTCAGAGCTCTGTGGATGGTC-3′, generating a 1820-base pair
(bp) product; COX-2 accession number, AY462100.1, sense,
5′-GTGAGCTAGCATGCTCGCCCGCGCCCTGC-3′ and antisense primers,
5′-CTGAGAATTCCTACAGTTCAGTCGAACGTTC-3′, generating an 1835-bp
product. The constructed plasmids were amplified in Escherichia
coli (Takara Biotechnology Co., Ltd., Dalian, China), isolated
using a plasmid Miniprep Kit (Beijing TransGen Biotech Co., Ltd.,
Beijing, China) and were verified by sequencing (Takara
Biotechnology Co., Ltd.). The classical Sanger sequencing was used.
PCR reaction mixture was prepared using the following: 1 µl 100 ng
plasmid, 1 µl primer (5 pmol; 5′ Sequencing 1 Primer, CMV-F; 3′
Sequencing 1 Primer, BGH-rev), 2 µl BigDye, 4 µl 5X buffer, 0.5 µl
Taq DNA polymerase and ddH2O added to 20 µl. The PCR
reaction begins at 95°C 1 min, then 35 cycles of 96°C 10 sec, 52°C
15 sec and 72°C 1 min.
COX-1 and COX-2 RNAi
The lentivector pLKO.1 l was purchased from Academia
Sinica (Taipei, Taiwan). The siRNA sequence targeting COX-1,
5′-GTGAGCTATTACACTCGTATT-3′ and COX-2, 5′-GATTGACAGTCCACCAACTTA-3′
was synthesized by Takara Biotechnology Co., Ltd., and linked to
the lentivector pLKO.1, and therefore termed pLKO.1-COX-1-siRNA and
pLKO.1-COX-2-siRNA. The SKOV3 cells were cotransfected by the
reconstructed vectors by Lipofectamine® 2000 (Shanghai
Yijie Biotechnology Co., Ltd., Shanghai, China) and pPACK Packaging
Plasmid Mix (System Biosciences, Palo Alto, CA, USA).
Orthotopic implantation
All procedures were approved by the Animal Care and
Ethics Committee of Guangdong General Hospital (Guangzhou, China).
A total of 40 BALB/c 3-week-old nude mice (male:female=1:1; 15–20
g; height, 2–2.5 cm) purchased from the Animal Center of Guangdong
Academy of Medical Sciences (Guangzhou, China) were raised in a
high-efficiency particulate arrestance-filtered environment with a
12-h light/dark cycle. The SKOV3 cells were subcutaneously injected
into 32 nude mice. At ~1 cm3, the xenograft was excised
and minced, and implanted into other 4-week old BALB/c nude mice
according to the protocol of a previous study (26). A total of 4 days subsequent to the
implantation of the OC tumors, the effects of blueberries on the
mice was tested.
Experimental design
All the mice were assigned into either control or
model group, with the model group being administered different
concentrations of blueberry juice (100, 200 and 400 mg daily). The
control and model groups were fed a normal diet and water, and all
the model groups were administered a normal diet plus the
corresponding blueberry juice. Subsequent to 2 weeks, the mice were
sacrificed via cervical dislocation. Tumors were isolated under
sterile conditions on a nutrient culture medium. Tumor size was
determined using the ellipsoid formula v=4/3π abc, where v is
volume, a and c are equal to half tumor height, and b is half tumor
length. All the procedures for animal studies were consistent with
the Animal Care and Use Guidance of Guangdong Academy of Medical
Sciences (Guangzhou, China).
Quantitative reverse
transcriptase-polymerase chain reaction (RT-qPCR)
Total RNA was purified from ovarian tumors of animal
models or healthy mice using an RNA purification kit (cat. no.
74106; Qiagen, Inc., Valencia, CA, USA). Tumor tissues were cut
into slices <2 mm and homogenized in 500 µl TRIzol. Phase was
separated by the addition of 500 µl chloroform, and centrifuged at
12,000 × g for 10 min. The RNA was precipitated by adding 500 µl
isopropyl alcohol. RNA was purified using a purification column. A
total of 1 µg RNA from each sample was reverse-transcribed using
the High-Capacity cDNA Reverse Transcription kit (cat. no. 4368813;
Thermo Fisher Scientific, Inc., Carlsbad, CA, USA). A total of 1 µl
purified RNA, poly(A)+-selected RNA primed with oligo
(deoxy-thymidine) 100 nM, 1X annealing buffer and water were heated
to 65°C for 5 min and placed on ice for 1 min. This reaction mix
and 0.5 µl reverse transcriptase were added to the reaction for a
final volume of 40 µl, and the mixture was incubated at 50°C for 50
min. The mixture was then heated to 95°C for 5. The SYBR Green DNA
PCR kit was purchased from Applied Biosystems (Thermo Fisher
Scientific, Inc.) and used for RT-qPCR using Applied
Biosystems® 7500 Real-Time PCR Systems (Thermo Fisher
Scientific, Inc.). The PCR primers were as follows: COX-1 forward,
5′-CAGAGCCAGATGGCTGTGGG-3′ and reverse, 5′-AAGCTGCTCATCGCCCCAGG-3′;
COX-2 forward, 5′-AAGTGCGATTGTACCCGGAC-3′ and reverse,
5′-ACGTTCCAAAATCCCTTGAA-3′; GAP DH forward,
5′-AACTACATGGTTTACATGTT-3′ and reverse, 5′-CACTTGATTTTGGAGGGATC-3′.
After 1 min enzyme activation at 95°C, the reaction was cycled 45
times at 95°C for 10 s, 55°C for 10 s, and 72°C for 20 s. The
values of the target genes were calculated utilizing the
2−ΔΔCq method (27) for
the fold change compared with GAPDH. The mRNA levels of the target
genes were presented as relative increases compared with GAPDH. All
experiments were performed in triplicate.
ELISA analysis
The concentrations of COX-1 and COX-2 in the treated
and untreated samples were measured using Human COX-1 ELISA kit
(cat. no. MBS026381) and Human COX-2 ELISA kit (cat. no. MBS043833;
MyBioSource, San Diego, CA, USA), respectively.
Statistical analysis
Data were analyzed using one-way analysis of
variance in SPSS 20 (IBM SPSS, Armonk, NY, USA). Quantitative data
are shown as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of blueberry on the growth of
SKOV3 cells
To investigate the effects of blueberries on SKOV3
cells, the concentration-dependent effects of blueberry juice was
explored. It was found that blueberries inhibited cell growth in a
dose-dependent way. Subsequent to the exposure of SKOV3 cells to 16
mg/ml blueberry, the cell growth rate was reduced by up to 28%
compared with the control following the 3-day culture (P=0.018;
Fig. 1). Based on these results, the
present study used a higher blueberry concentration (16 mg/ml) to
treat the OC mouse models (400 mg daily for a 25 g mouse).
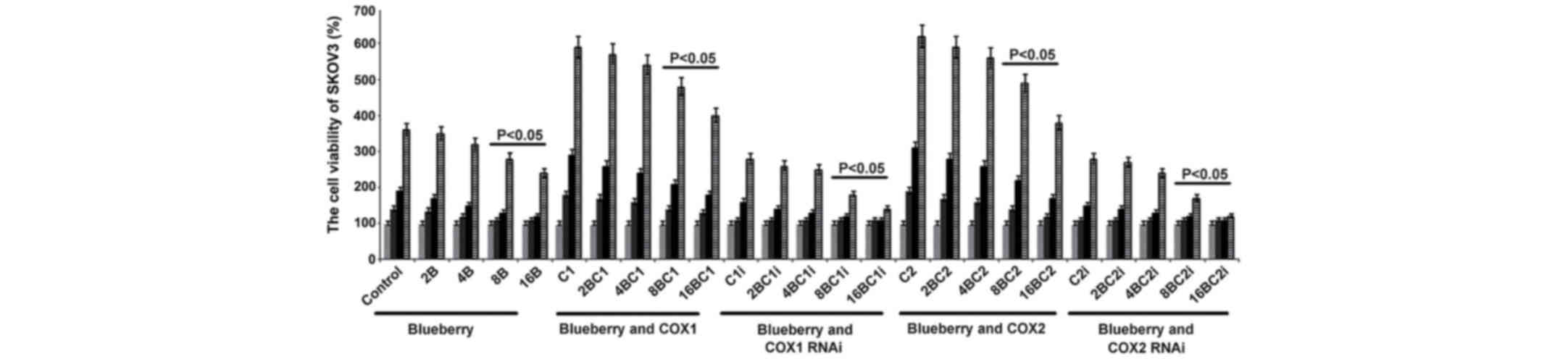 | Figure 1.Blueberries inhibit cell growth rate
of SKOV3 cells. SKOV3 cells were transfected with COX-1, COX-2,
COX-1 RNAi and COX-2 RNAi, and treated with 0, 2, 4, 8 and 16 mg/ml
blueberry juice. Cell concentrations were determined using MTT
assays at days 0, 1, 2 and 3. The data represent the results of 5
independent experiments and are presented as the mean ± standard
deviation. P<0.05 compared with 0 µg/ml blueberry subsequent to
a 1-, 2- or 3-day culture. COX, cyclooxygenase; RNAi, RNA
interference; Control, without transfection or blueberry; 2B, 2 mg
blueberry; 4B, 4 mg blueberry; 8B, 8 mg blueberry; 16B, 16 mg
blueberry; C1, COX-1; 2BC1, 2 mg blueberry and COX1 transfection;
4BC1, 4 mg blueberry and COX1 transfection; 8BC1, 8 mg blueberry
and COX1 transfection; 16BC1, 16 mg blueberry and COX1
transfection; C1i, COX-1 RNAi; 2BC1i, 2 mg blueberry and COX1 RNAi;
4BC1i, 4 mg blueberry and COX1 RNAi; 8BC1i, 8 mg blueberry and COX1
RNAi; 16BC1i, 16 mg blueberry and COX1 RNAi; C2, COX-2; 2BC2, 2 mg
blueberry and COX2 transfection; 4BC2, 4 mg blueberry and COX2
transfection; 8BC2, 8 mg blueberry and COX2 transfection; 16BC2, 16
mg blueberry and COX2 transfection; C2i, COX-2 RNAi; 2BC2i, 2 mg
blueberry and COX2 RNAi; 4BC2i, 4 mg blueberry and COX2 RNAi;
8BC2i, 8 mg blueberry and COX2 RNAi; 16BC2i, 16 mg blueberry and
COX2 RNAi. |
Effects of COX-1 and COX-2 on the
growth rate of SKOV3 cells
To investigate the effects of COX-1 and COX-2 on
SKOV3 cells, the genes were overexpressed by gene transformation or
inhibited by RNAi in the cells. Exposure of SKOV3 cells to 16 mg/l
blueberry resulted in a reduction of cell concentration detected by
MTT when compared with the control (P=0.019; Fig. 1). When COX-1 or COX-2 was inhibited by
RNAi in SKOV3 cells and treated with 16 mg/ml blueberry juice, the
growth rate of the cells was reduced by up to 50% compared with the
growth rate of the control group subsequent to 72 h culture
(Fig. 1). By contrast, if COX-1 or
COX-2 was overexpressed in SKOV3 cells, the growth rate increased
by up to 66% compared with the growth rate of the control group
subsequent to 72 h culture (Fig. 1).
The increase was inhibited completely when 16 mg/ml blueberry juice
was added to the cells.
mRNA level of COX-1 and COX-2 in
SKOV3
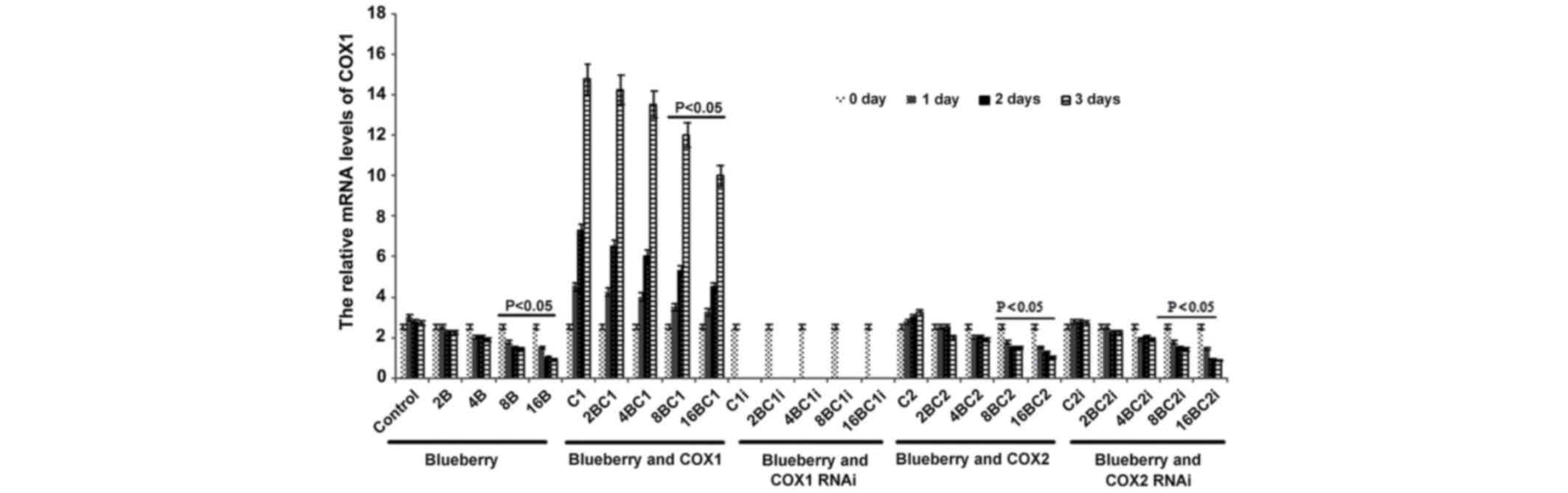
The mRNA level of COX-1 was significantly affected
by blueberry juice and was reduced by up to 50% when 16 mg/ml
blueberry juice was added compared with the control without the
blueberry treatment (P=0.002; Fig.
2). The mRNA level of COX-1 increased by up to 50% when SKOV3
cells were transfected with COX-1, compared with the control
without COX-1 transfection (P=0.002; Fig.
2). The increase was inhibited by the addition of blueberry
juice in a dose-dependent way. The mRNA level of COX-1 was reduced
to 0 when SKOV3 cells were transfected with COX-1 RNAi (Fig. 2). Neither the overexpression nor gene
silencing of COX-2 affected the mRNA level of COX-1 (P=0.235;
Fig. 2).
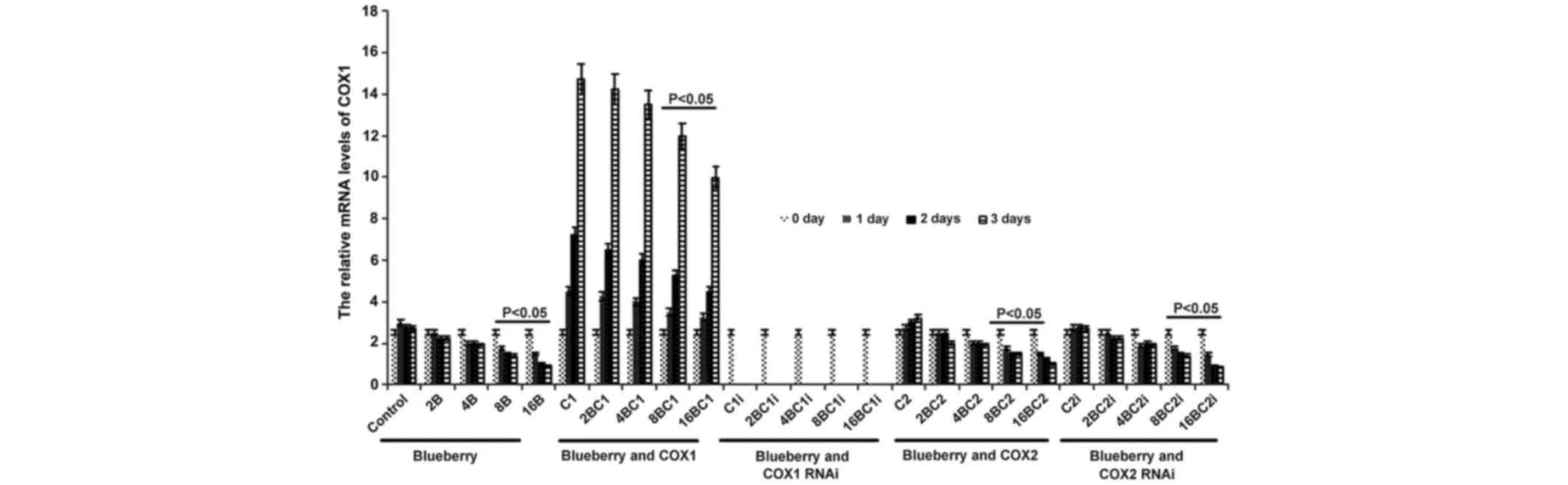 | Figure 2.Reverse transcription-qualitative
polymerase chain reaction analyses show that blueberries reduce
relative mRNA levels of COX-1 in SKOV3 cells. SKOV3 cells were
transfected with COX-1, COX-2, COX-1 RNAi or COX-2 RNAi, and
treated with 0, 2, 4, 8 or 16 mg/ml blueberry juice. The relative
mRNA levels of COX-1 were measured at days 0, 1, 2 and 3. GAPDH was
used as an internal control. The data represent the results of 5
independent experiments and are presented as the mean ± standard
deviation. P<0.05 compared with 0 µg/ml blueberry subsequent to
a 1-, 2- or 3-day culture. COX, cyclooxygenase; RNAi, RNA
interference; Control, without transfection or blueberry; 2B, 2 mg
blueberry; 4B, 4 mg blueberry; 8B, 8 mg blueberry; 16B, 16 mg
blueberry; C1, COX-1; 2BC1, 2 mg blueberry and COX1 transfection;
4BC1, 4 mg blueberry and COX1 transfection; 8BC1, 8 mg blueberry
and COX1 transfection; 16BC1, 16 mg blueberry and COX1
transfection; C1i, COX-1 RNAi; 2BC1i, 2 mg blueberry and COX1 RNAi;
4BC1i, 4 mg blueberry and COX1 RNAi; 8BC1i, 8 mg blueberry and COX1
RNAi; 16BC1i, 16 mg blueberry and COX1 RNAi; C2, COX-2; 2BC2, 2 mg
blueberry and COX2 transfection; 4BC2, 4 mg blueberry and COX2
transfection; 8BC2, 8 mg blueberry and COX2 transfection; 16BC2, 16
mg blueberry and COX2 transfection; C2i, COX-2 RNAi; 2BC2i, 2 mg
blueberry and COX2 RNAi; 4BC2i, 4 mg blueberry and COX2 RNAi;
8BC2i, 8 mg blueberry and COX2 RNAi; 16BC2i, 16 mg blueberry and
COX2 RNAi. |
The mRNA level of COX-2 was significantly affected
by blueberry juice, decreasing by up to 50% when 16 mg/ml blueberry
was added compared with the control without the blueberry treatment
(P=0.008; Fig. 3). The mRNA level of
COX-2 also increased by up to 150% when the SKOV3 cells were
transfected with COX-2 compared with the control without COX-2
transfection (P=0.002; Fig. 3). The
increase was inhibited by the addition of blueberry juice in a
dose-dependent way. The mRNA level of COX-2 decreased to 0 when the
SKOV3 cells were transfected with COX-2 RNAi (Fig. 3). Neither the overexpression nor gene
silencing of COX-1 affected the mRNA level of COX-2 (P=0.321;
Fig. 3).
 | Figure 3.Reverse transcription
qualitative-polymerase chain reaction analyses show that
blueberries reduce relative mRNA levels of COX-2 in SKOV3 cells.
SKOV3 cells were transfected with COX-1, COX-2, COX-1 RNAi or COX-2
RNAi, and treated with 0, 2, 4, 8 or 16 mg/ml blueberry juice. The
relative mRNA levels of COX-2 were measured at days 0, 1, 2 and 3.
GAPDH was used as an internal control. The data represent the
results of 5 independent experiments and are presented as the mean
± standard deviation. P<0.05 compared with 0 µg/ml blueberry
subsequent to a 1-, 2- or 3-day culture. COX, cyclooxygenase; RNAi,
RNA interference; Control, without transfection or blueberry; 2B, 2
mg blueberry; 4B, 4 mg blueberry; 8B, 8 mg blueberry; 16B, 16 mg
blueberry; C1, COX-1; 2BC1, 2 mg blueberry and COX1 transfection;
4BC1, 4 mg blueberry and COX1 transfection; 8BC1, 8 mg blueberry
and COX1 transfection; 16BC1, 16 mg blueberry and COX1
transfection; C1i, COX-1 RNAi; 2BC1i, 2 mg blueberry and COX1 RNAi;
4BC1i, 4 mg blueberry and COX1 RNAi; 8BC1i, 8 mg blueberry and COX1
RNAi; 16BC1i, 16 mg blueberry and COX1 RNAi; C2, COX-2; 2BC2, 2 mg
blueberry and COX2 transfection; 4BC2, 4 mg blueberry and COX2
transfection; 8BC2, 8 mg blueberry and COX2 transfection; 16BC2, 16
mg blueberry and COX2 transfection; C2i, COX-2 RNAi; 2BC2i, 2 mg
blueberry and COX2 RNAi; 4BC2i, 4 mg blueberry and COX2 RNAi;
8BC2i, 8 mg blueberry and COX2 RNAi; 16BC2i, 16 mg blueberry and
COX2 RNAi. |
Levels of COX-1 and COX-2 in
SKOV3
The results of the levels of COX-1 and COX-2 in
SKOV3 were similar compared with the results from the mRNA levels
investigation. The concentration of COX-1 was significantly
affected by the blueberry juice, being reduced by up to 50% when 16
mg/ml blueberry juice was added, compared with the control without
the blueberry treatment (P=0.003; Fig.
4). The concentration of COX-1 increased by up to 50% when the
SKOV3 cells were transfected with COX-1, compared with the control
without COX-1 transfection (P=0.002; Fig.
4). The increase was inhibited by the addition of blueberry
juice in a dose-dependent way. The concentration of COX-1 decreased
to 0 when the SKOV3 cells were transfected with COX-1 RNAi
(Fig. 4). Neither the overexpression
nor gene silencing of COX-2 affected the concentration of COX-1
(P=0.287; Fig. 4).
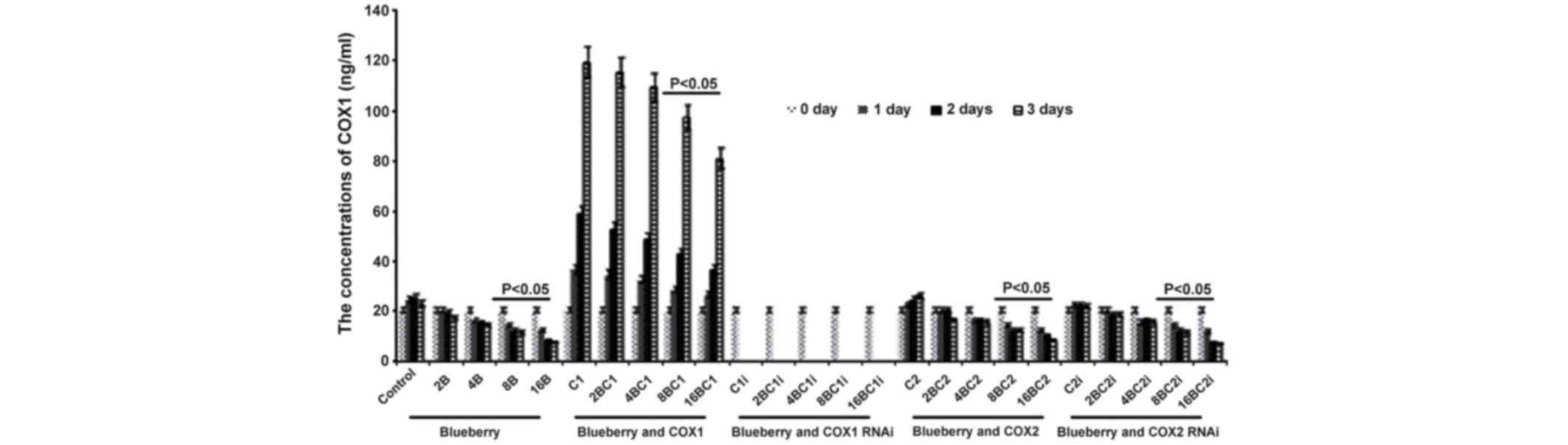 | Figure 4.ELISA analyses show that blueberries
reduce the protein levels of COX-1 in SKOV3 cells. SKOV3 cells were
transfected with COX-1, COX-2, COX-1 RNAi or COX-2 RNAi, and
treated with 0, 2, 4, 8 or 16 mg/ml blueberry juice. The
concentrations of COX-1 were measured at days 0, 1, 2 and 3. The
data represent the results of 5 independent experiments and are
presented as the mean ± standard deviation. P<0.05 compared with
0 µg/ml blueberry subsequent to a 1-, 2- or 3-day culture. COX,
cyclooxygenase; RNAi, RNA interference; Control, without
transfection or blueberry; 2B, 2 mg blueberry; 4B, 4 mg blueberry;
8B, 8 mg blueberry; 16B, 16 mg blueberry; C1, COX-1; 2BC1, 2 mg
blueberry and COX1 transfection; 4BC1, 4 mg blueberry and COX1
transfection; 8BC1, 8 mg blueberry and COX1 transfection; 16BC1, 16
mg blueberry and COX1 transfection; C1i, COX-1 RNAi; 2BC1i, 2 mg
blueberry and COX1 RNAi; 4BC1i, 4 mg blueberry and COX1 RNAi;
8BC1i, 8 mg blueberry and COX1 RNAi; 16BC1i, 16 mg blueberry and
COX1 RNAi; C2, COX-2; 2BC2, 2 mg blueberry and COX2 transfection;
4BC2, 4 mg blueberry and COX2 transfection; 8BC2, 8 mg blueberry
and COX2 transfection; 16BC2, 16 mg blueberry and COX2
transfection; C2i, COX-2 RNAi; 2BC2i, 2 mg blueberry and COX2 RNAi;
4BC2i, 4 mg blueberry and COX2 RNAi; 8BC2i, 8 mg blueberry and COX2
RNAi; 16BC2i, 16 mg blueberry and COX2 RNAi. |
Similarly, the concentration of COX-2 was
significantly affected by blueberry juice, being reduced by up to
50% when 16 mg/ml blueberry juice was added, compared with the
control without the blueberry treatment (P=0.002; Fig. 5). The mRNA level of COX-2 increased by
up to 150% when SKOV3 cells were transfected with COX-2, compared
with the control without COX-2 transfection (P=0.003; Fig. 5). The increase was inhibited by the
addition of blueberry juice in a dose-dependent way. The
concentration of COX-2 decreased to 0 when the SKOV3 cells were
transfected with COX-2 RNAi (Fig. 5).
Neither the overexpression nor gene silencing of COX-1 affected the
concentration of COX-2 (P=0.329; Fig.
3).
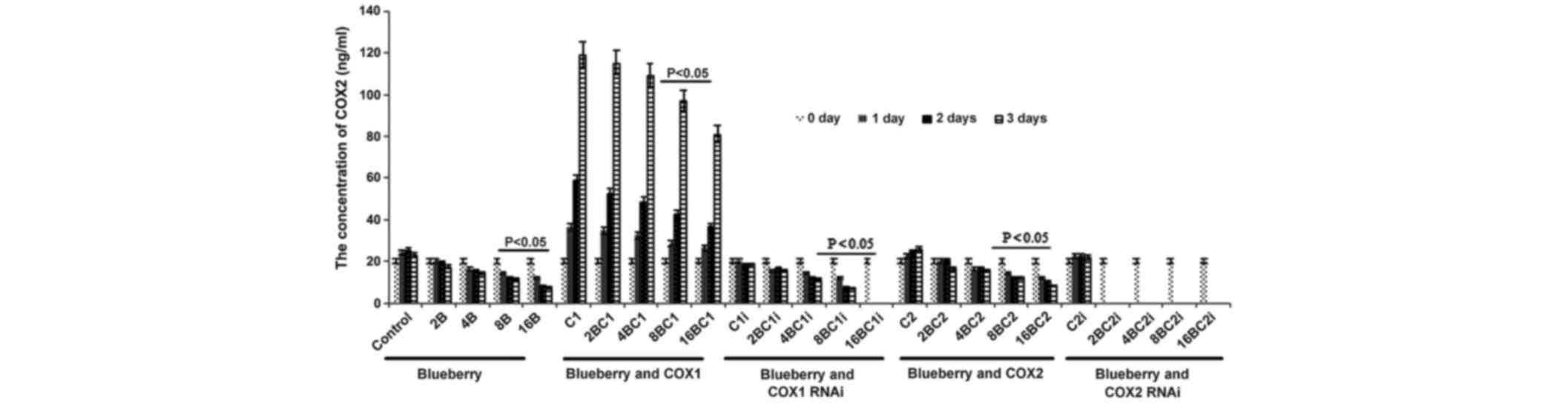 | Figure 5.ELISA analyses show that blueberries
reduce the protein levels of COX-2 in SKOV3 cells. SKOV3 cells were
transfected with COX-1, COX-2, COX-1 RNAi or COX-2 RNAi, and
treated with 0, 2, 4, 8 or 16 mg/ml blueberry juice. The
concentrations of COX-2 were measured at days 0, 1, 2 and 3. The
data represents the results of 5 independent experiments and are
presented as the mean ± standard deviation. P<0.05 compared with
0 µg/ml blueberry subsequent to a 1-, 2- or 3-day culture. COX,
cyclooxygenase; RNAi, RNA interference; Control, without
transfection or blueberry; 2B, 2 mg blueberry; 4B, 4 mg blueberry;
8B, 8 mg blueberry; 16B, 16 mg blueberry; C1, COX-1; 2BC1, 2 mg
blueberry and COX1 transfection; 4BC1, 4 mg blueberry and COX1
transfection; 8BC1, 8 mg blueberry and COX1 transfection; 16BC1, 16
mg blueberry and COX1 transfection; C1i, COX-1 RNAi; 2BC1i, 2 mg
blueberry and COX1 RNAi; 4BC1i, 4 mg blueberry and COX1 RNAi;
8BC1i, 8 mg blueberry and COX1 RNAi; 16BC1i, 16 mg blueberry and
COX1 RNAi; C2, COX-2; 2BC2, 2 mg blueberry and COX2 transfection;
4BC2, 4 mg blueberry and COX2 transfection; 8BC2, 8 mg blueberry
and COX2 transfection; 16BC2, 16 mg blueberry and COX2
transfection; C2i, COX-2 RNAi; 2BC2i, 2 mg blueberry and COX2 RNAi;
4BC2i, 4 mg blueberry and COX2 RNAi; 8BC2i, 8 mg blueberry and COX2
RNAi; 16BC2i, 16 mg blueberry and COX2 RNAi. |
The aforementioned results suggest that blueberries
reduce the concentration of COX-1 and COX-2 in a dose-dependent
way. Blueberries inhibit the growth rate of SKOV3 by decreasing the
levels of COX-1 and COX-2, while COX-1 and COX-2 promote the growth
of SKOV3.
Effects of blueberry on tumor
volume
Prior to the blueberry treatment, the volumes of the
OC tumors were similar between groups. Subsequent to the blueberry
treatment, the volumes of OC in the 500 mg group significantly
decreased by 40% compared with the group that received no blueberry
treatment (P=0.0008). The high-efficiency inhibitory concentrations
of blueberry were between 8 and 16 mg. The weights of the OC tumors
were 4.82±0.41, 4.25±0.35, 3.16±0.23 and 2.47±0.26 g in blank, 0,
100, 200 and 400 mg blueberry groups, respectively, yielding growth
inhibitions of 12.5, 35.4, and 49.4%. From the aforementioned
results, the 400 mg blueberry group showed significant inhibitory
results for OC (P=0.014).
Blueberry juice affects the mRNA
levels of COX-1 and COX-2 of OC in the mouse model
The present study investigated the effect of
blueberries on the mRNA levels of COX-1 and COX-2 of OC in a mouse
model, which are biomarkers of OC (24,28). The
mRNA levels of COX-1 and COX-2 of OC in the mice were the highest
in the control group compared with those in the models treated with
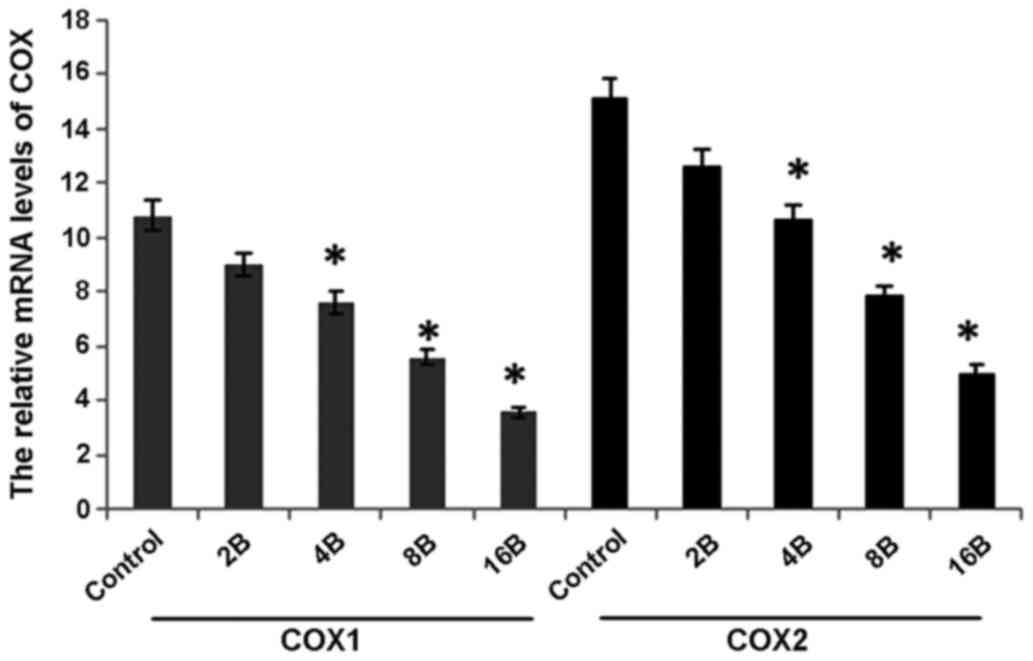
blueberry juice (P=0.006; Fig. 6).
Comparatively, the levels of COX-1 and COX-2 significantly
decreased by up to 63.6 and 68.7%, respectively, when the mice were
treated with 400 mg blueberry juice daily compared with those from
the control without the addition of blueberry juice (P=0.002;
Fig. 6). The results suggested that
blueberry juice significantly affects the mRNA levels of COX-1 and
COX-2 of OC in the mouse model studied.
Blueberry juice affects the levels of
COX-1 and COX-2 of OC in the mouse model
In concordance with the mRNA results, ELISA analysis
showed higher levels of COX-1 and COX-2 of OC in mice in the
control group when compared with the groups treated with blueberry
juice (P=0.012; Fig. 7).
Comparatively, the levels of COX-1 and COX-2 significantly
decreased by up to 57.1 and 54.5%, respectively, when the mice were
treated with 400 mg blueberry juice daily compared with the protein
levels of the control without the addition of blueberry juice
(P=0.006; Fig. 7). The aforementioned
results suggested that blueberry juice significantly affects the
levels of COX-1 and COX-2 of OC in the mouse model studied.
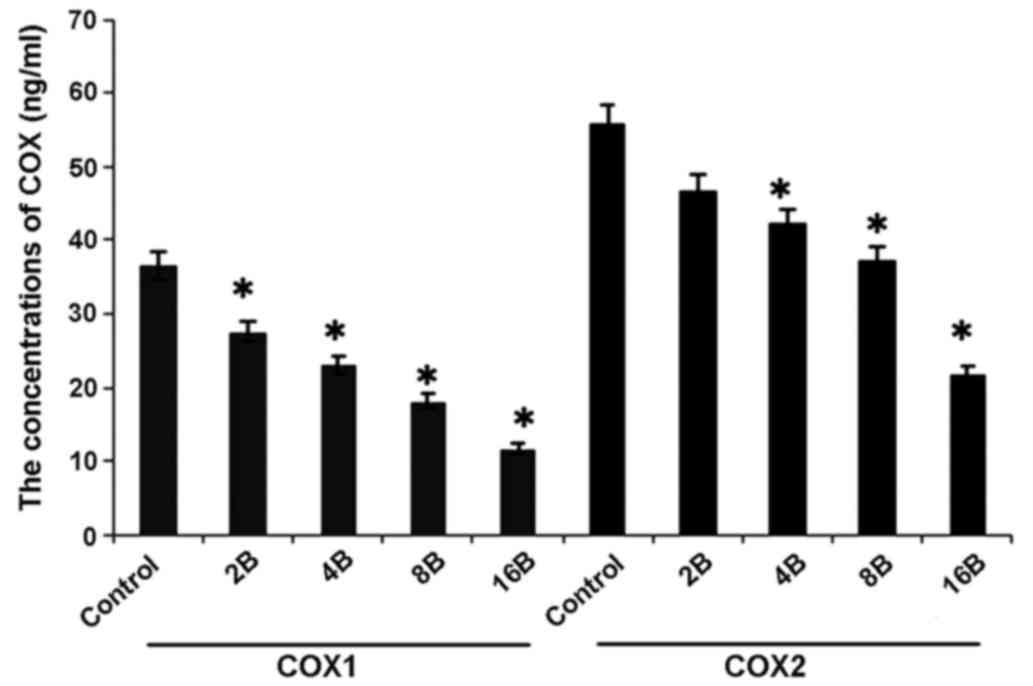 | Figure 7.ELISA analyses show that blueberries
reduce protein levels of COX-1 and COX-2 of ovarian cancer in a
mouse model. The mice were treated with 0, 2, 4, 8 or 16 mg/ml
blueberry juice. The protein levels of COX-1 and COX-2 were
measured subsequent to a 14-day culture. The data represent the
results of 5 independent experiments and are presented as the mean
± standard deviation. *P<0.05 compared with 0 µg/ml blueberry.
COX, cyclooxygenase, Control, without transfection or blueberry;
2B, 2 mg blueberry; 4B, 4 mg blueberry; 8B, 8 mg blueberry; 16B, 16
mg blueberry. |
Discussion
OC is a common cause of female mortality worldwide
and blueberry therapy has been identified to be effective in the
treatment of various types of carcinoma (21,22,29). The
theoretical benefit of blueberry juice as a salvage therapy is
associated with the anti-inflammation capacity (30,31) and
ability to prevent the progression of various types of cancer
(32,33). However, the molecular mechanism of the
inhibition of OC by blueberries remains unclear. Therefore, the
present study aimed to address the issue, and revealed a
significant result with respect to the use of blueberry juice for
the treatment of OC in the BALB/c nude mouse model. Based on the
aforementioned information, the present study firstly reported the
molecular mechanisms for the inhibition of OC by blueberry,
revealing that a suitable dosage of blueberry juice decreases the
expression of COX-1 and COX-2, which are 2 biomarkers for the
development of OC. The aforementioned findings suggest that
blueberry juice decreases the levels of COX-1 and COX-2, and
inhibits the progression of OC. Furthermore, detecting the levels
of COX-1 and COX-2 aid in the prediction of patients at risk for
OC, as reported in previous studies (24,34).
Blueberry juice therapy may therefore provide a non-pharmaceutical
treatment for patients with OC.
Regarding the promotion of the growth of SKOV3 by
COX-1 and COX-2, the issue that growth rate may be affected by
other molecules must be considered. Therefore, the present study
investigated the overexpression and gene silencing of COX-1 and
COX-2, revealing that when COX-1 and COX-2 were overexpressed
without blueberry treatment, the growth rate of SKOV3 reached the
highest level of the present study. By contrast, when COX-1 and
COX-2 were silenced by RNAi and treated with blueberry juice at a
high concentration, the growth rate of SKOV3 reached the lowest
level of the present study (Fig. 1).
Furthermore, blueberry juice reduces the levels of COX-1 and COX-2
in a dose-dependent way (Figs.
2–5). The aforementioned results
suggest that COX-1 and COX-2 promote the growth of OC while
blueberry juice inhibits the development of OC by downregulating
the levels of COX-1 and COX-2.
There were certain limitations of the present study.
For example, blueberry juice possesses a number of different
components, which were not separated or purified to identify the
more effective agents for the treatment of OC. The functions of
blueberries are not unique and more functions require exploration;
a limitation of the present study is that the precise mechanism of
blueberries with respect to the downregulation of the levels of
COX-1 and COX-2 remains unknown. The components of blueberry juice
should be analyzed in detail, which may offer information to
understand the exact molecular mechanisms for the role of blueberry
juice in the therapy of OC.
The present study revealed that the efficacy of
using blueberries to treat OC is significant. To make full use of
blueberries, all associated molecular mechanisms should be
investigated to maximize the potential benefit of blueberries and
minimize the risk of side effects. The present study demonstrated
that the concentration at which blueberries exhibit significant
inhibition is 16 mg/ml for OC cells. At this concentration,
blueberries effectively treated the OC models. The results of the
present study provide information for subsequent clinical trials
and may be beneficial to utilize blueberries, effectively and
safely, as a non-pharmaceutical OC therapy. Considering the
effectiveness and safety of blueberries, a larger sample size and
long-term follow-up test in a larger sample of mice is
recommended.
In conclusion, blueberries have been demonstrated to
be effective and safe in controlling the size of OC in a
dose-dependent way. Blueberries inhibit the proliferation of OC
cells by downregulating the levels of COX-1 and COX-2. The present
study established animal models of OC by the injection of SKOV3
cells into nude mice. Blueberry (400 mg daily) consumption
decreased tumor size significantly in the mice with OC compared
with the controls without blueberry treatment (P<0.05). The
results of the present study suggest that blueberries should be
developed as a potential non-pharmaceutical therapy for OC.
References
|
1
|
Donovan KA, Donovan HS, Cella D, Gaines
ME, Penson RT, Plaxe SC, von Gruenigen VE, Bruner DW, Reeve BB and
Wenzel L: Recommended patient-reported core set of symptoms and
quality-of-life domains to measure in ovarian cancer treatment
trials. J Natl Cancer Inst. 106:pii: dju1282014. View Article : Google Scholar
|
|
2
|
Chen CH, Chiu LH, Chan C and Liu WM:
Management of ovarian cancer in 14th gestational week of pregnancy
by robotic approach with preservation of the fetus. Gynecol Obstet
Invest. 80:139–144. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang XJ, Zheng FY, Xu YS and Ou RY:
Ovarian cancer initially presenting with isolated ipsilateral
superficial inguinal lymph node metastasis: A case study and review
of the literature. J Ovarian Res. 7:202014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shim SH, Kim DY, Seo MJ, Lee SW, Park JY,
Lee JJ, Kim JH, Kim YM, Kim YT and Nam JH: Preoperative fluorine 18
fluorodeoxyglucose tumoral uptake ratio between upper and lower
abdomen in primary advanced-stage ovarian cancer. Int J Gynecol
Cancer. 23:1383–1392. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peng X, Wang P, Li S, Zhang G and Hu S:
Randomized clinical trial comparing octreotide and scopolamine
butylbromide in symptom control of patients with inoperable bowel
obstruction due to advanced ovarian cancer. World J Surg Oncol.
13:502015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mir MC, Stephenson AJ, Grubb RL III, Black
A, Kibel AS and Izmirlian G: Predicting risk of bladder cancer
using clinical and demographic information from prostate, lung,
colorectal, and ovarian cancer screening trial participants. Cancer
Epidemiol Biomarkers Prev. 22:2241–2249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagano H, Muraoka M and Takagi K:
Recurrent ovarian cancer with multiple lymph nodes metastases
successfully treated with lymphadenectomy as secondary
cytoreductive surgery: A case report. Int J Surg Case Rep.
5:412–415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bacalbasa N and Popescu I: Ovarian cancer
liver metastases-should we apply the principle of optimal
cytoreduction to the liver? A review. Hepatogastroenterology.
62:355–357. 2015.PubMed/NCBI
|
|
9
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Frampton JE: Olaparib: A review of its use
as maintenance therapy in patients with ovarian cancer. BioDrugs.
29:143–150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ledermann J, Harter P and Gourley C:
Correction to Lancet Oncol 2014; 15: 856. Olaparib maintenance
therapy in patients with platinum-sensitive relapsed serous ovarian
cancer: A preplanned retrospective analysis of outcomes by BRCA
status in a randomised phase 2 trial. Lancet Oncol.
16:e1582015.PubMed/NCBI
|
|
12
|
Fu X, Zhang Y, Wang X, Chen M, Wang Y, Nie
J, Meng Y and Han W: Low dose decitabine combined with taxol and
platinum chemotherapy to treat refractory/recurrent ovarian cancer:
An open-label, single-arm, Phase I/II study. Curr Protein Pept Sci.
16:329–336. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McGrail DJ, Khambhati NN, Qi MX, Patel KS,
Ravikumar N, Brandenburg CP and Dawson MR: Alterations in ovarian
cancer cell adhesion drive taxol resistance by increasing
microtubule dynamics in a FAK-dependent manner. Sci Rep.
5:95292015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pettitt SJ, Rehman FL, Bajrami I, Brough
R, Wallberg F, Kozarewa I, Fenwick K, Assiotis I, Chen L, Campbell
J, et al: A genetic screen using the PiggyBac transposon in haploid
cells identifies Parp1 as a mediator of olaparib toxicity. PLoS
One. 8:e615202013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McNeil EM, Ritchie AM and Melton DW: The
toxicity of nitrofuran compounds on melanoma and neuroblastoma
cells is enhanced by Olaparib and ameliorated by melanin pigment.
DNA Repair (Amst). 12:1000–1006. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bouquet W, Ceelen W, Adriaens E, Almeida
A, Quinten T, de Vos F, Pattyn P, Peeters M, Remon JP and Vervaet
C: In vivo toxicity and bioavailability of Taxol and a
paclitaxel/beta-cyclodextrin formulation in a rat model during
HIPEC. Ann Surg Oncol. 17:2510–2517. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Arany I, Clark JS, Reed D, Szabo I, Ember
I and Juncos LA: The role of p66shc in taxol- and dichloroacetic
acid-dependent renal toxicity. Anticancer Res. 33:3119–3122.
2013.PubMed/NCBI
|
|
18
|
Sun CC, Bodurka DC, Weaver CB, Rasu R,
Wolf JK, Bevers MW, Smith JA, Wharton JT and Rubenstein EB:
Rankings and symptom assessments of side effects from chemotherapy:
Insights from experienced patients with ovarian cancer. Support
Care Cancer. 13:219–227. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Faria A, Pestana D, Teixeira D, de Freitas
V, Mateus N and Calhau C: Blueberry anthocyanins and pyruvic acid
adducts: Anticancer properties in breast cancer cell lines.
Phytother Res. 24:1862–1869. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zu XY, Zhang ZY, Zhang XW, Yoshioka M,
Yang YN and Li J: Anthocyanins extracted from Chinese blueberry
(Vaccinium uliginosum L.) and its anticancer effects on DLD-1 and
COLO205 cells. Chin Med J (Engl). 123:2714–2719. 2010.PubMed/NCBI
|
|
21
|
Kanaya N, Adams L, Takasaki A and Chen S:
Whole blueberry powder inhibits metastasis of triple negative
breast cancer in a xenograft mouse model through modulation of
inflammatory cytokines. Nutr Cancer. 66:242–248. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jeyabalan J, Aqil F, Munagala R, Annamalai
L, Vadhanam MV and Gupta RC: Chemopreventive and therapeutic
activity of dietary blueberry against estrogen-mediated breast
cancer. J Agric Food Chem. 62:3963–3971. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thuresson ED, Lakkides KM and Smith WL:
PGG2, 11R-HPETE and 15R/S-HPETE are formed from different
conformers of arachidonic acid in the prostaglandin endoperoxide H
synthase-1 cyclooxygenase site. Adv Exp Med Biol. 507:67–72. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wilson AJ, Fadare O, Beeghly-Fadiel A, Son
DS, Liu Q, Zhao S, Saskowski J, Uddin MJ, Daniel C, Crews B, et al:
Aberrant over-expression of COX-1 intersects multiple
pro-tumorigenic pathways in high-grade serous ovarian cancer.
Oncotarget. 6:21353–21368. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin Y, Cui M, Xu T, Yu W and Zhang L:
Silencing of cyclooxygenase-2 inhibits the growth, invasion and
migration of ovarian cancer cells. Mol Med Rep. 9:2499–2504.
2014.PubMed/NCBI
|
|
26
|
Hui X, Chen H, Zhang S, Ma X, Wang X and
Huang B: Antitumor activities of recombinant human interferon
(IFN)-λ1 in vitro and in xenograft models in vivo for colon cancer.
Cancer Lett. 311:141–151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang WL, Roland IH, Godwin AK and Xu XX:
Loss of TNF-alpha-regulated COX-2 expression in ovarian cancer
cells. Oncogene. 24:7991–8002. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Montales MT, Rahal OM, Kang J, Rogers TJ,
Prior RL, Wu X and Simmen RC: Repression of mammosphere formation
of human breast cancer cells by soy isoflavone genistein and
blueberry polyphenolic acids suggests diet-mediated targeting of
cancer stem-like/progenitor cells. Carcinogenesis. 33:652–660.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Esposito D, Chen A, Grace MH, Komarnytsky
S and Lila MA: Inhibitory effects of wild blueberry anthocyanins
and other flavonoids on biomarkers of acute and chronic
inflammation in vitro. J Agric Food Chem. 62:7022–7028. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Paulis G, Cavallini G, Giorgio GD,
Quattrocchi S, Brancato T and Alvaro R: Long-term multimodal
therapy (verapamil associated with propolis, blueberry, vitamin E
and local diclofenac) on patients with Peyronie's disease (chronic
inflammation of the tunica albuginea). Results of a controlled
study. Inflamm Allergy Drug Targets. 12:403–409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ge I, Rudolph A, Shivappa N, Flesch-Janys
D, Hébert JR and Chang-Claude J: Dietary inflammation potential and
postmenopausal breast cancer risk in a German case-control study.
Breast. 24:491–496. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Khachatryan N and Kempen JH:
Immunosuppressive therapy and cancer risk in ocular inflammation
patients: Fresh evidence and more questions. Ophthalmology.
122:219–221. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Magnowska M, Zaborowski M, Surowiak P,
Nowak-Markwitz E, Zabel M and Spaczyński M: COX-2 expression
pattern is related to ovarian cancer differentiation and prognosis,
but is not consistent with new model of pathogenesis. Ginekol Pol.
85:335–341. 2014. View
Article : Google Scholar : PubMed/NCBI
|