Introduction
Breast-conserving therapy (BCT), which consists of
breast-conserving surgery (BCS) and radiation therapy, has become
the standard treatment for women with early-stage breast cancer
(1–4).
However, compared with those treated by mastectomy, patients
receiving BCS have increased rates of local in-breast recurrence
(3,5).
Previous studies revealed that local recurrence following BCT may
independently predict distant metastasis and poor disease-specific
survival (6,7). Identifying the potential risk factors of
local recurrence following BCS is helpful for the selection of
appropriate candidates for BCS and clinical decision-making.
Previous studies have revealed that the radiological
appearance of breast tumors may reflect pathological changes and
the aggressiveness of the cancer (8–12).
Calcification is an important radiological feature of breast
cancer, and a number of studies have suggested that calcification
on mammography is associated with an increased risk of local and
distant recurrence following mastectomy (13–16).
However, there remains little evidence on the association between
the radiological appearance of breast cancer and the risk of local
recurrence in patients who undergo BCS. It remains controversial
whether patients with calcification on mammography have increased
rates of local recurrence compared with those without calcification
following BCS. Calcification is also often encountered in breast
ultrasonography (BUS) examinations. It remains unclear whether
calcification on BUS has prognostic values in patients with breast
carcinoma.
In the present study, the association between
pre-surgery radiological appearance, (particularly the morphology
and distribution patterns of calcification) and post-surgery
pathological characteristics was firstly examined. Additionally,
the survival outcomes of patients receiving breast-conserving
surgery with or without calcification were compared, including
local in-breast recurrence, distant metastasis and overall survival
time (OS). The prognosis of patients with calcification on breast
ultrasonography examination was analyzed. It was then determined
whether the distribution and morphology affect local in-breast
recurrence, distant metastasis and OS of patients treated with BCS.
The associations between calcification features, including
morphology and distribution patterns, surgical margin status and
extensive intraductal component were also examined.
Patients and methods
Patients
The present study was approved by the Institutional
Review Board of the Tianjin Medical University Cancer Institute and
Hospital (Tianjin, China). In total, the records of 409 patients
with a diagnosis of breast carcinoma who were treated with BCS were
retrospectively reviewed between January 2005 and December 2008. To
be included in the present study, patients were required to have
received pre-surgery mammography and BUS at the Cancer Institute
and Hospital of Tianjin Medical University (Tianjin, China) and
have available results for review. The exclusion criteria were as
follows: Younger than 20 or older than 70 years old; previous
history of other malignant neoplasms including breast cancer;
distant metastasis at the time of diagnosis; and local relapse
within six months of the surgery.
Data
Demographic, diagnostic, clinical, pathological,
treatment and follow-up data were reviewed from the medical records
of the patients and the follow-up system at the center. Patients
were divided into three groups: No calcification; mammographic
calcification; and BUS calcification (calcification only in BUS).
The mammographic patterns were classified as mass, architectural
distortion, calcification or a combination of calcification with
mass or architectural distortion. The morphology of calcification
in mammograms of the patients was categorized as one of four types:
Micro-calcification; pleomorphic calcification; casting
calcification; and large/coarse/spherical calcification (benign
calcification). Patients with micro-calcification, pleomorphic
calcification and casting calcification were merged as one group in
the final analysis. The distribution patterns of calcification in
mammograms of the BCS patients were divided into clustered,
liner/segmental (ductal spreading), or scattered type.
Statistical analysis
The χ2 or Fisher's exact tests were used
for categorical parameters, while a t-test was used for the
analysis of continuous data. The association between calcification
types and lymph node status, with or without adjustment for tumor
size and histological grade, was analyzed using a binary logistic
regression model. The Kaplan-Meier method was applied as in the
survival analysis to calculate the local relapse free survival
(LRFS), disease free survival (DFS), and OS times. The log-rank
test was used to compare differences among survival curves. The Cox
proportional hazards model was used to estimate the effect of
mammographic calcification types on long-term prognosis, adjusting
for potential factors including margin status, tumor size,
histological grade, lymph node status, receptor status and
treatment modality.
Results
Patient characteristics
A total of 589 patients who had a histological
diagnosis of breast carcinoma and received BCS were identified.
Mammograms and BUS reports were available for review in 433
patients. A total of 24 patients were excluded by exclusion
criteria. Overall, 409 patients were included in the final
analysis. Among them, 238 patients did not have calcification on
either mammogram or BUS and were defined as the BCS group without
calcification. Of the remaining 171 patients who were defined as
the BCS group with calcification, 135 patients had calcification on
mammogram and 36 did not have calcification on mammogram, but had
calcification signs in BUS tests.
The majority (96.6%) of the patients included in the
present study were Han Chinese women. The demographic, surgical and
pathological characteristics of the patients are summarized in
Table I. The median age of the
patients was 50 years (24–70 years) and 51 years (20–70 years) in
the BCS groups with and without calcification, respectively. In the
calcification and non-calcification groups, 52.38 and 52.36% of the
women were premenopausal, respectively. A total of 6 (3.5%) and 2
(0.8%) of the patients received neoadjuvant chemotherapy in the BCS
groups with and without calcification, respectively. The majority
of patients had received quadrantectomy rather than lumpectomy.
Negative margin status was achieved in 93.6 and 92.9% of the
patients with and without calcification, respectively. A margin ≤5
mm was defined as close.
 | Table I.Characteristics of patients with or
without calcification. |
Table I.
Characteristics of patients with or
without calcification.
| Characteristic | BCS with
calcification, n | BCS without
calcification, n | P-value |
|---|
| Total | 171 | 238 |
|
| Age, median
(range) | 50 (24–70) | 51 (20–70) |
|
| Menopausal
status |
|
| 0.99 |
|
Premenopausal | 88 | 122 |
|
|
Postmenopausal | 80 | 111 |
|
| Surgery |
|
| 0.59 |
|
Quadrantectomy | 163 | 224 |
|
|
Lumpectomy |
8 | 14 |
|
| Margin status |
|
| 0.78 |
|
Negative | 160 | 221 |
|
|
Positive/closea | 11 | 17 |
|
| Neoadjuvant
chemotherapy |
|
| 0.07 |
| No | 165 | 236 |
|
|
Yes |
6 |
2 |
|
| Histological
type |
|
| 0.71 |
|
Invasive ductal carcinoma | 135 | 193 |
|
|
Invasive lobular
carcinoma |
4 |
8 |
|
| Ductal
carcinoma in situ |
4 |
3 |
|
|
Others | 28 | 34 |
|
| Histological
grade |
|
| 0.70 |
|
1 | 14 | 21 |
|
|
2 | 95 | 158 |
|
|
3 | 14 | 17 |
|
| Tumor size |
|
| 0.47 |
| Mean ±
standard deviation, cm | 1.84±0.86 | 1.79±0.79 |
|
| T1 | 132 | 195 |
|
| T2 | 35 | 38 |
|
| T3 |
2 |
2 |
|
| Axillary lymph node
status |
|
| 0.17 |
|
Negative | 128 | 190 |
|
|
Positive | 39 | 41 |
|
| ER/PR status |
|
| 0.33 |
|
Positive | 129 | 174 |
|
|
Negative | 37 | 63 |
|
| Her-2 status |
|
| 0.29 |
|
Positive | 22 | 23 |
|
|
Negative | 144 | 210 |
|
| Radiation
therapy |
|
| 0.74 |
|
Yes | 148 | 205 |
|
| No | 17 | 21 |
|
| Chemotherapy |
|
| 0.86 |
| Unknown
(n=7) |
3 |
4 |
|
|
Yes | 151 | 209 |
|
| No | 17 | 25 |
|
| Hormonal
therapy |
|
| 0.92 |
|
Yes | 119 | 161 |
|
| No | 10 | 13 |
|
Tumor attributes
The main histological type of the tumor was invasive
ductal carcinoma. The majority of tumors were grade 2 or 3. The
mean tumor sizes were 1.84 and 1.79 cm for patients with and
without calcification, respectively. Axillary lymph node status was
available for 398 patients; 23.4 and 17.7% of the patients had
involved lymph nodes in the groups with and without calcification,
respectively. Hormonal receptor (HR; estrogen receptors and/or
progesterone receptors) and human epidermal growth factor (Her-2)
status was available for 403 patients; 77.7 and 73.4% of the
patients were HR-positive, while 86.7 and 90.1% of the patients
were Her-2-negative in the groups with and without calcification,
respectively.
Post-surgery therapy
Of the 409 patients included in the present study,
391 received post-surgery radiation therapy (PSRT); the rate of
having PSRT was 89.7 and 90.7% in the groups with and without
calcification, respectively. Post-surgery adjuvant chemotherapy,
based mainly on anthracycline and taxane, was administered to 151
and 209 patients of the groups with and without calcification,
respectively. In HR positive patients, tamoxifen and aromatase
inhibitors were used for premenopausal and postmenopausal patients,
respectively. Anti-Her-2 therapy (Trastuzumab) was not routinely
used in our center prior to 2008.
Calcification and long-term outcome of
breast conserving surgery
To investigate whether calcification on pre-surgery
mammogram and/or BUS impacted the long-term outcome of BCS, the
rates of local/regional recurrence, distant metastasis and
mortality were studied in BCS patients with and without
calcification.
The median follow-up time of all the patients was 85
months. In total, 16 (9.4%) and 9 (3.78%) local/regional relapses
occurred in patients who received BCS with and without
calcification, respectively (P=0.02; Table II). Similarly, more distant
metastasis occurred in patients who had calcification [18 (10.53%)
vs. 10 (4.20%), respectively; P=0.01]. In patients with
calcification, 5 local/regional relapses occurred concurrently and
4 occurred at a different time with distant metastasis. In those
without calcification on mammography and who underwent BUS, 1
local/regional relapse occurred concurrently, and 3 occurred at a
different time with distant metastasis. All patients who had
local/regional recurrence without distant metastasis received
salvage mastectomies and additional lymph node dissection, if
necessary. In patients who had distant metastatic diseases, the
treatments were combinations of radiation therapy, chemotherapy,
hormonal therapy and supportive care.
 | Table II.Outcome of patients with or without
calcification. |
Table II.
Outcome of patients with or without
calcification.
| Outcome | BCS with
calcification, n (%) | BCS without
calcification, n (%) | P-value |
|---|
| Local/regional
relapse |
|
| 0.02 |
|
Yes | 16
(9.4) |
9 (3.78) |
|
| No | 155 (90.6) | 229 (96.22) |
|
| Distant
metastasis |
|
| 0.01 |
|
Yes | 18
(10.53) | 10
(4.20) |
|
| No | 153 (89.47) | 228 (95.80) |
|
| Breast carcinoma
mortality |
|
| 0.01 |
|
Yes | 15
(8.8) |
7 (2.9) |
|
| No | 156 (91.2) | 231 (97.1) |
|
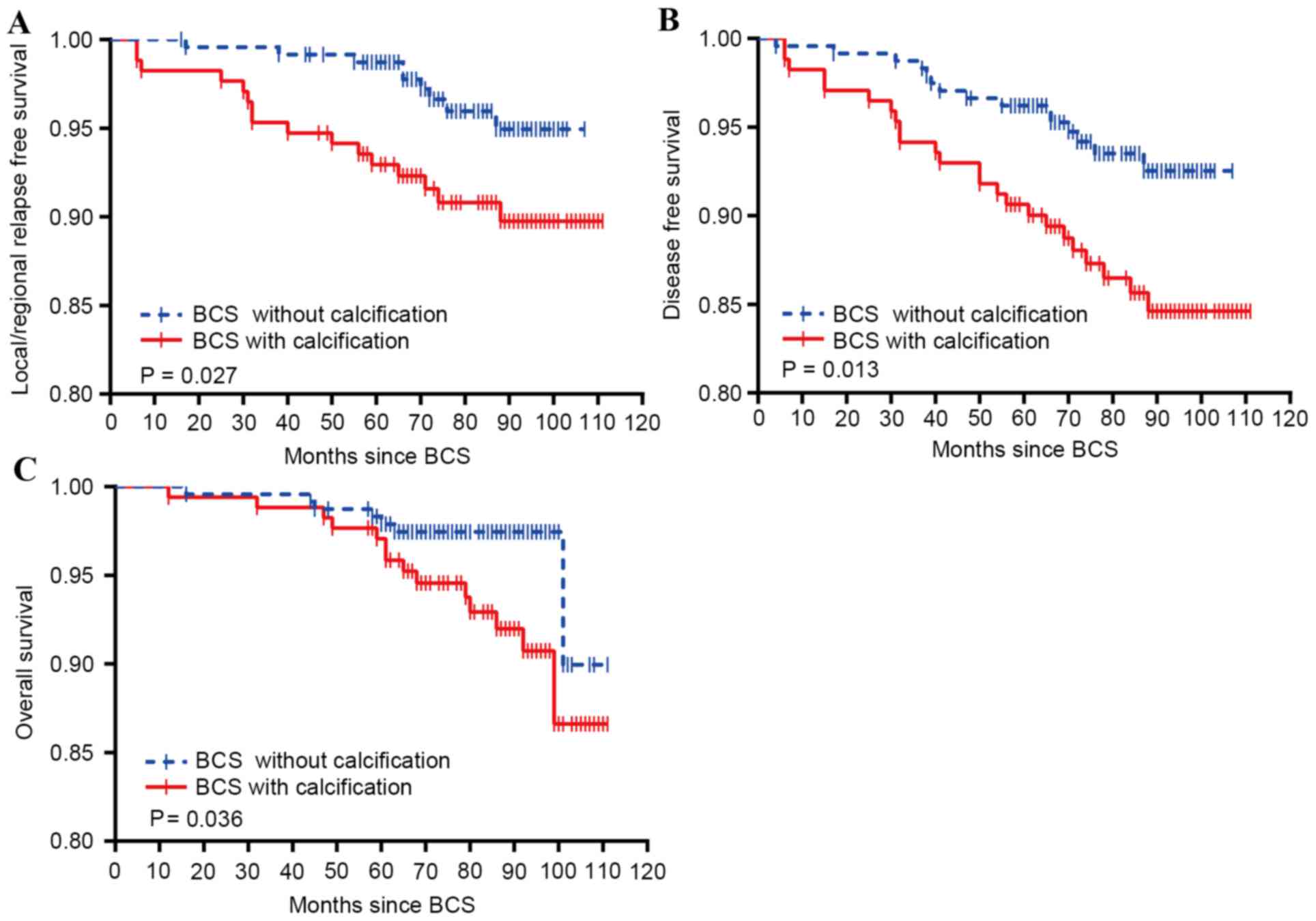
Survival analysis comparing the LRFS, DFS, and OS
was also performed in patients treated by BCS with or without
calcification. As shown in Fig. 1,
patients who had calcification on pre-surgery examination
(mammography and/or BUS) had poorer cumulative LRFS, DFS and OS,
compared with those who did not have calcification on either
mammography or BUS. Following the adjustment of potential
confounding factors, compared with patients without calcification,
those with calcification had a 2.46-fold [relative risk (RR), 2.46;
95% confidence interval (CI), 1.11–5.44], 2.24-fold (RR, 2.24; 95%
CI, 1.19–4.24), and 2.50-fold (RR, 2.50; 95% CI, 1.06–5.86)
increased risk of local/regional recurrence, distant metastasis and
mortality, respectively, subsequent to receiving BCS.
The data from Fig. 1
indicated that patients with calcification on mammograms or BUS may
have an increased risk of relapse, metastasis and mortality.
However, the causes for this phenomenon remain unclear. As
previously mentioned, calcification was divided into different
types according to the morphology and distribution; it was unclear
whether the morphology or the distribution pattern of calcification
in each group was responsible for the increased risk of BCS
failure. To clarify, subgroup analyses concerning the associations
between different calcification types or distribution patterns, and
the pathological and clinical outcomes of the cancer, were
performed.
Calcification and tumor
characteristics
The number of patients with different types and
distribution patterns of calcification are presented in Table III. Patients with calcification only
in BUS examination (n=36) were defined as BUS calcification. In the
following analyses, patients with benign calcification
(large/coarse/spherical calcification (n=16), were merged with
those with BUS calcification, while patients with casting
calcification (n=2), micro-calcification (n=23) and pleomorphic
calcification (n=94) were merged as one group, defined as
micro/pleomorphic calcification. All the calcifications with
diffuse/scattered distribution patterns (n=7) were benign
calcifications and were therefore merged with the BUS calcification
group. Patients who did not exhibit calcification on either
mammography or BUS (n=238) were used as the reference group. The
potential confounding factors, consisting of age, menopausal
status, tumor size, histological grade, HR status and Her-2 status,
were adjusted by inclusion of the factors in the logistic
regression analysis.
 | Table III.Number of patients with calcification
by morphological types and distribution patterns. |
Table III.
Number of patients with calcification
by morphological types and distribution patterns.
| Calcification | Number of patients,
n | Percentage, % |
|---|
| Morphology | 171 | 100.0 |
| BUS
calcification | 36 |
21.1 |
| Benign
calcification | 16 |
9.4 |
| Casting
calcification |
2 |
1.2 |
|
Micro-calcification | 23 |
13.5 |
|
Pleomorphic calcification | 94 |
55.0 |
| Distribution | 171 | 100.0 |
| BUS
calcification | 36 |
21.1 |
|
Diffuse/scattered |
7 |
4.1 |
|
Liner/segmental | 35 |
20.5 |
|
Clustered | 93 |
54.4 |
Positive/close margin status is an established risk
of local recurrence following BCS (1). Table IV
shows the post-surgery margin status of patients with calcification
of different types and distribution patterns. No evidence showed
that calcification increased the chance of positive/close margin
status on pathological examination. The histological grades of
tumors of patients with different types and distribution patterns
of calcification were also analyzed. No association was identified
between calcification and increased histological grades (grade 2
and 3; Table V). Patients with
calcification exhibited high rates of lymph node metastasis,
particularly in those with micro/pleomorphic calcification and
those with ductal spreading calcification (liner/segmental
distribution, calcification distributed along the ducts); however,
the differences were not statistically significant (P>0.05;
Table VI).
 | Table IV.Margin status of patients with
calcification by different morphological types and distribution
patterns. |
Table IV.
Margin status of patients with
calcification by different morphological types and distribution
patterns.
|
| Margin status, n
(%) | OR (95% CI) |
|---|
|
|
|
|
|---|
| Characteristic | Negative | Positive/close | Crude |
Adjusteda |
| Morphology |
|
|
|
|
| No
calcification | 217 (92.7) | 17 (7.3) | 1.00 | 1.00 |
| BUS
calcification+benign calcification | 49
(94.2) |
3 (5.8) | 0.80
(0.22–2.82) | 0.74
(0.20–2.69) |
|
Micro/pleomorphic-calcification | 111 (93.3) |
8 (6.7) | 0.94
(0.39–2.24) | 0.99
(0.40–2.43) |
| Distribution |
| No
calcification | 217 (92.7) | 17
(7.3) |
1.00 |
1.00 |
| BUS
calcification+benign calcification | 49
(94.2) |
3 (5.8) | 0.78
(0.22–2.78) | 0.73
(0.20–2.66) |
|
Clustered | 78
(92.9) |
6 (7.1) | 0.98
(0.37–2.59) | 1.02
(0.38–2.76) |
| Ductal
spreading | 33
(94.3) |
2 (5.7) | 0.77
(0.17–3.50) | 0.86
(0.18–4.06) |
 | Table V.Histological grade of tumors of
patients with calcification by different morphological types and
distribution patterns. |
Table V.
Histological grade of tumors of
patients with calcification by different morphological types and
distribution patterns.
|
| Histological grade,
n (%) | OR (95% CI) |
|---|
|
|
|
|
|---|
| Characteristic | 1 | 2+3 | Crude |
Adjusteda |
|---|
| Morphology |
|
|
|
|
| No
calcification | 19
(10.4) | 163 (89.6) |
1 |
1 |
| BUS
calcification+benign calcification |
4 (9.3) | 39
(90.7) | 1.14
(0.37–3.53) | 1.30
(0.41–4.15) |
|
Micro/pleomorphic-calcification | 10
(12.5) | 70
(87.5) | 0.82
(0.36–1.84) | 0.72
(0.31–1.68) |
| Distribution |
|
|
|
|
| No
calcification | 19
(10.4) | 163 (89.6) |
1 |
1 |
| BUS
calcification+benign calcification |
4 (9.3) | 39
(90.7) | 1.14
(0.37–3.53) | 1.30
(0.41–4.14) |
|
Clustered |
9 (15.3) | 50
(84.7) | 0.65
(0.28–1.52) | 0.57
(0.23–1.39) |
| Ductal
spreading |
1 (4.8) | 20
(95.2) | 2.33
(0.30–18.36) | 2.09
(0.17–3.50) |
 | Table VI.Regional lymph node status of
patients with calcification by different morphological types and
distribution patterns. |
Table VI.
Regional lymph node status of
patients with calcification by different morphological types and
distribution patterns.
|
| Lymph node status,
n (%) | OR (95% CI) |
|---|
|
|
|
|
|---|
| Characteristic | Negative | Positive | Crude |
Adjusteda |
|---|
| Morphology |
| No
calcification | 174 (81.7) | 39 (18.3) | 1 | 1 |
| BUS
calcification+benign calcification | 40 (76.9) | 12 (23.1) | 1.34
(0.64–2.79) | 1.35
(0.59–3.11) |
|
Micro/pleomorphic-calcification | 88 (76.5) | 27 (23.5) | 1.37
(0.79–2.38) | 1.55
(0.80–3.01) |
| Distribution |
| No
calcification | 174 (81.7) | 39 (18.3) | 1 | 1 |
| BUS
calcification+benign calcification | 40 (76.9) | 12 (23.1) | 1.34
(0.64–2.79) | 1.35
(0.59–3.11) |
|
Clustered | 63 (76.8) | 19 (23.2) | 1.35
(0.72–2.50) | 1.62
(0.78–3.36) |
| Ductal
spreading | 25 (75.8) | 8 (24.2) | 1.43
(0.60–3.40) | 1.36
(0.43–4.31) |
Outcome of patients with calcification
of different types and distribution patterns
These data indicated that mammographic calcification
may be an independent risk factor of local/regional relapse. The
cumulative rates of local/regional relapse, distant metastasis and
mortality in patients with calcification of different types and
distribution patterns were then analyzed.
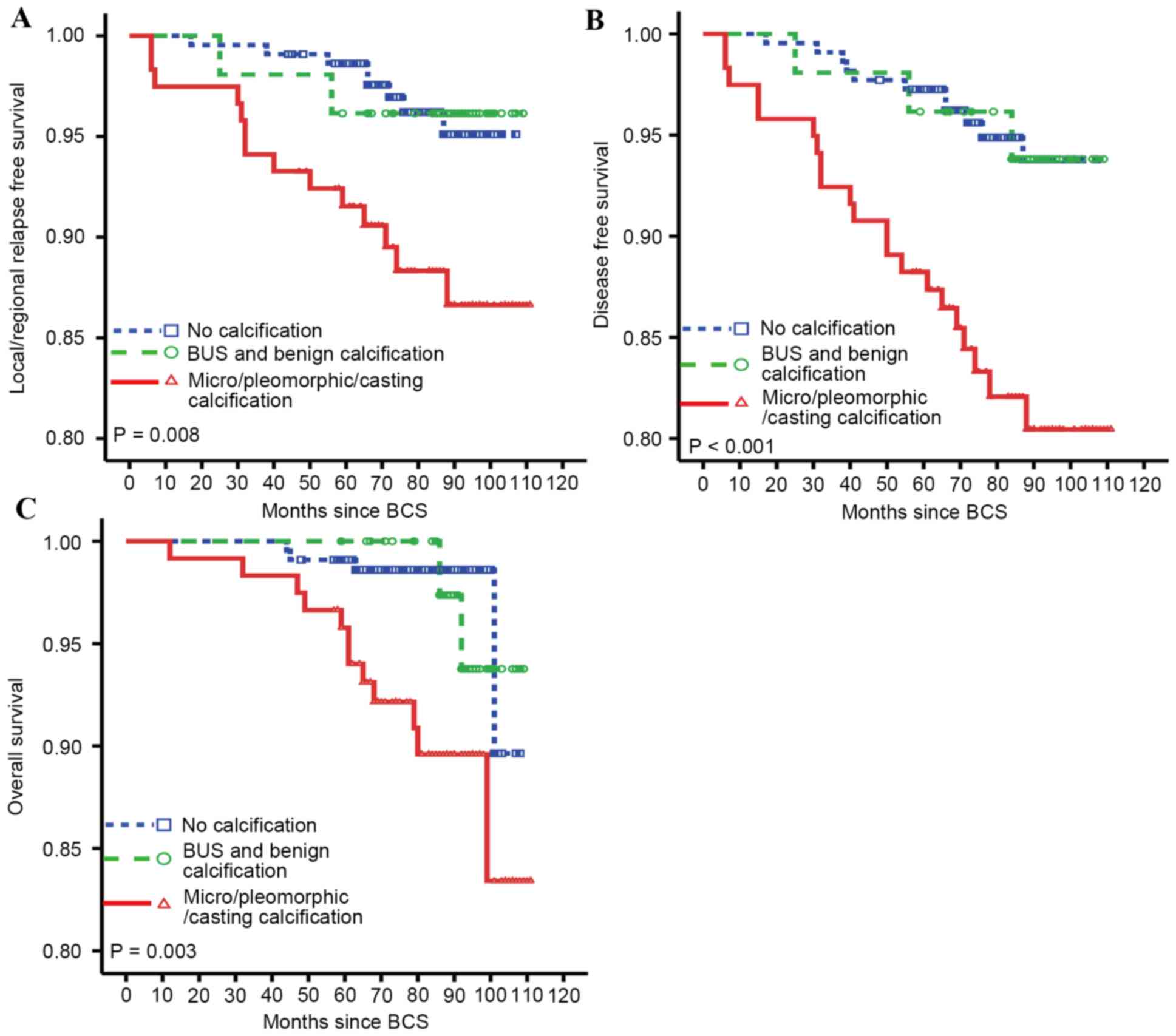
Firstly, associations between calcification types
and the cumulative rates of local/regional relapse, distant
metastasis and mortality were investigated. Fig. 2 shows the outcomes of the LRFS, DFS
and OS of patients with BCS, based on the calcification types.
Patients with micro-calcification, pleomorphic calcification and
casting calcification had significantly increased rates of local
(P=0.008) and distant relapse (P<0.001) as well as decreased OS
(P=0.003). To study whether calcification or the distribution
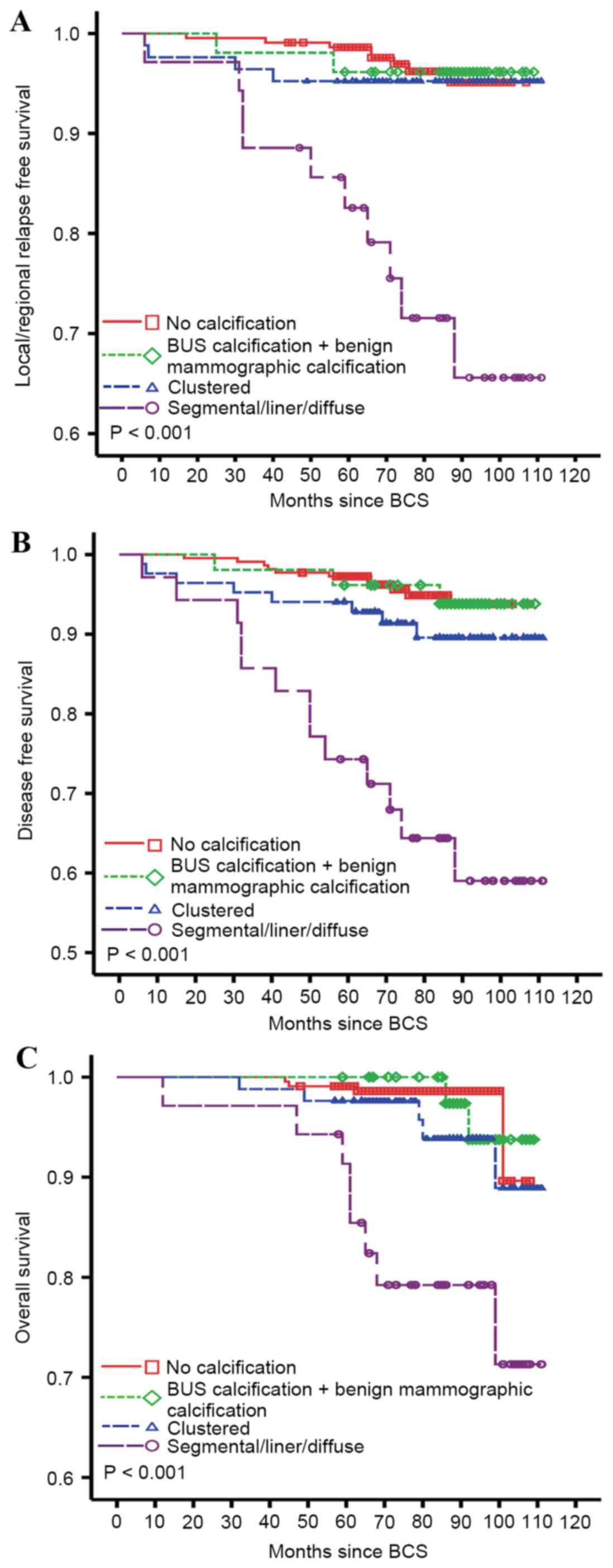
patterns impacted the long-term prognosis of patients with BCS,
survival analyses by distribution patterns, which were adjusted for
age, menopausal status, histological grade, tumor size, lymph node
status, HR status and Her-2 status, were performed with Cox
regression analysis. As shown in Fig.
3, patients with calcification distributing along the ducts, or
calcification with liner and segmental distribution were at a
significantly increased risk of local recurrence, distant
metastasis and mortality. The relative risk was 6.20 (95% CI,
2.26–16.98), 6.81 (95% CI, 2.86–16.20) and 9.14 (95% CI,
2.53–33.00) for LRFS, DFS and OS, respectively. Although patients
with calcification of clustered distribution also demonstrated
increased risk of disease relapse and mortality, the trends were
not statistically significant. Furthermore, the outcome of patients
with BUS calcification was as good as those without
calcification.
Calcification distribution patterns
and extensive intraductal component
As demonstrated in Fig.
2, the incidence of recurrence, metastasis and mortality were
higher in patients with micro/pleomorphic calcification compared
with those without calcification. The following subgroup analysis
of the distribution of calcification revealed that patients with
micro/pleomorphic calcification with a clustered distribution did
not possess a significantly increased risk of relapse, metastasis
and mortality. However, patients with micro/pleomorphic
calcification with a liner/ segmental distribution were at a
significantly increased risk of local recurrence, distant
metastasis and mortality compared with patients with no
calcification. These data suggest that the distribution patterns
rather than the morphological types account for the increased risk
of recurrence following BCS. An extensive intraductal component
(EIC) is defined as >25% of the mass of an invasive tumor in
intraductal carcinoma. Intraductal carcinoma was observed within
and outside of the tumors with an EIC. Previous studies revealed
that mammographic calcification of liner, segmental or diffuse
distribution pattern were correlated with EIC (17). Therefore, the presence of EIC in
tumors of patients with different calcification distribution
patterns was analyzed. As shown in Table VII, >20% of patients with
calcification of ductal spreading pattern had tumors with EIC; the
rate was significantly increased (P<0.001). No association was
observed between EIC and positive/close margin status. Among
patients with calcification of ductal spreading pattern, those with
EIC had a significantly increased incidence of local/regional
recurrence (P=0.03), while the rates of distant metastasis and
mortality were not statistically different (Table VIII).
 | Table VII.Incidence of extensive intraductal
components in tumors of patients with different calcification
distribution patterns. |
Table VII.
Incidence of extensive intraductal
components in tumors of patients with different calcification
distribution patterns.
|
| Extensive
intraductal component, n (%) |
|---|
|
|
|
|
|---|
| Distribution of
calcification | No | Yes | P-value |
|---|
| No
calcification | 237 (99.6) |
1 (0.4) |
<0.001 |
| BUS
calcification | 50
(96.2) |
2 (3.8) |
| +benign
calcification |
| Clustered | 79
(94.0) |
5 (6.0) |
| Ductal
spreading | 27
(77.1) |
8 (22.9) |
 | Table VIII.Outcomes of patients with
calcification of ductal spreading distribution pattern by extensive
intraductal component. |
Table VIII.
Outcomes of patients with
calcification of ductal spreading distribution pattern by extensive
intraductal component.
|
| Extensive
intraductal component, n (%) |
|---|
|
|
|
|
|---|
| Outcome | No | Yes | P-value |
|---|
| Local/regional
relapse |
|
| 0.03 |
|
Yes |
5 (18.5) |
5 (62.5) |
| No | 22
(81.5) |
3 (37.5) |
| Distant
metastasis |
|
| 0.12 |
|
Yes |
8 (29.6) |
5 (62.5) |
| No | 19
(70.4) |
3 (37.5) |
| Breast cancer
mortality |
|
| 0.35 |
|
Yes |
5 (18.5) |
3 (37.5) |
| No | 22
(81.5) |
5 (62.5) |
Discussion
BCS, followed by radiation therapy, is now the
standard treatment for early breast cancer; it may achieve an OS
equivalent to mastectomy (1–4). Patients who can tolerate radiation
therapy and have lesions that can be removed with adequate margins
and acceptable cosmetic results are appropriate candidates for BCS
(1,18). Calcification is an important
radiological feature of breast cancer (19). Calcification is not an absolute
contraindication of BCS; however, patients with diffuse
malignant-appearing micro-calcifications are not recommended to
undergo BCS (10,20). Previous studies have evaluated the
role of mammographic features of breast carcinoma as prognostic
factors for women with breast carcinoma (8–11,13–16);
however, to the best of our knowledge, there is little previous
study regarding the predictive value of calcifications found on
pre-surgery radiological tests, particularly mammography, for
breast cancer patients who received BCS. In addition, calcification
can also be identified in BUS examinations, and it remains unclear
whether calcification on BUS has prognostic values in patients with
breast carcinoma.
Several studies have investigated the predictive
value of mammographic tumor features on women with small invasive
breast cancer (14–16). Thurfjell et al (16) revealed that mammographic appearance
presenting as casting or pleomorphic calcifications alone had a
significantly worse prognosis than other types of mammographic
appearance in small invasive breast cancer, which was confirmed by
the later series studies (14,15).
Conversely, there are certain studies doubting the ability of
mammographic calcification to predict the survival outcomes of
patients (21–23). In the present study, the outcome of
409 breast cancer patients with or without calcification, who had
received BCS and post-surgery adjuvant treatment, were
retrospectively studied. Initial analyses demonstrated that
patients with calcification had an increased risk of local and
distant relapse following BCS compared with those who had BCS
without calcification, which was consistent with the majority of
current studies (13–16). In addition, subgroup analysis of the
present study suggested that the distribution patterns rather than
morphological types of calcification correlated with the increased
risk of local and distant failure in patients with calcification
following BCS. Patients with calcification distributed along the
ducts, or calcification with liner and segmental distribution, had
a significantly increased risk of local recurrence, distant
metastasis and mortality, which suggested that BCS was not suitable
for those patients with calcification spreading along the ducts.
Similar trends were identified in those with calcification of
clustered distribution, but the trends were not statistically
significant. Namely, clustered micro-calcification and pleomorphic
calcification are not absolute contraindications of BCS, but
patients should be informed that they have a potentially increased
risk of local failure following BCS and should be followed up
closely. For patients with BUS calcification, the outcome was as
good as the outcome for patients without calcification, and thus
should be treated similarly.
Previous studies revealed that certain types of
calcification, particularly casting calcification, were associated
with more aggressive characteristics of invasive ductal carcinoma,
as well as ductal carcinoma in situ (DCIS), such as
increased histological grades, negative HR status, positive Her-2
status, positive lymph node status and comedo necrosis in DCIS
(8–12). Casting calcification is considered a
relative contraindication of BCS in the Tianjin Medical University
Cancer Institute and Hospital, and there were only two cases of
casting calcification in the present study. No association was
identified between calcification, either by morphological types or
by distribution patterns, and more invasive tumor characteristics,
including larger tumor size, increased histological grade and a
triple-negative phenotype. However, it was identified that patients
with calcification spreading along the ducts on mammograms were
more likely to have tumors with EIC. It was reported that EIC
associated with an increased risk of residual disease and patients
with EIC had an increased risk of in-breast recurrence (17,24–28).
Numerous other studies showed that negative margins were adequately
safe for patients with EIC-positive tumors (29,30).
However, as reported by Holland et al (31), the majority of tumors with EIC
involved up to a whole quadrant. Furthermore, since these lesions
are frequently non-palpable, it is challenging to obtain adequate
negative margins during the surgery. In the present study, it was
identified that patients with calcification spreading along the
ducts on mammograms possessed an increased rate of EIC, and
patients with EIC had an increased risk of local/regional relapse.
However, no association was identified between EIC and
positive/close margin status. The present results indicated that
EIC may reflect a diffuse and multifocality growth pattern of the
tumor, which may increase the false negative rate of surgical
margins, and thus lead to increased local recurrence.
Previous studies demonstrated that although patients
treated with BCS had increased rates of local recurrence compared
with those treated with mastectomy, the overall survival rates were
similar (1–5). However, in the present study, patients
with calcification were identified to have increased rates of
distant metastasis and mortality, mainly caused by calcification
spreading along the ducts. Previous studies identified that
patients with calcification, particularly with casting type
calcification, had a poorer outcome, regardless of the local
treatments (13–16). It remains unclear whether mastectomy
could improve the outcome of patients with calcification spreading
along the ducts. Additional studies are required to compare the
outcome of patients with calcification spreading along the ducts
treated by BCS and mastectomy.
In conclusion, the present study demonstrated that
patients with calcification have an increased risk of developing
local/regional and distant relapse subsequent to BCS, compared with
patients without calcification. The distribution pattern, rather
than the morphological type of calcification, was correlated with a
poor outcome in patients with calcification. In addition, the
tumors with calcification spreading along the ducts on mammograms
were more likely to have an EIC, and the existence of an EIC was a
predictive factor of local failure in patients with calcification
treated with BCS. Therefore, from a clinical point of view, it was
hypothesized that patients with calcification spreading along the
ducts should not be recommended to undergo BCS; at least not prior
to additional prospective studies showing that patients with this
type of calcification have a similar outcome whether they receive
BCS or mastectomy. Alternative surgery approaches, including those
involving oncoplastic technologies, are preferable choices.
Clustered micro-calcification and pleomorphic calcification are not
absolute contraindications of BCS, but patients should be informed
that they have a potentially increased risk of local failure
subsequent to BCS and should be followed up closely. Patients who
do not have calcification on mammograms, but do have calcification
on BUS, have a similar outcome subsequent to BCS as those without
calcification, and therefore should be treated similarly.
Acknowledgements
The present study is supported by the National
Natural Science Foundation of China (grant no. 81502300).
References
|
1
|
Fisher B, Anderson S, Bryant J, Margolese
RG, Deutsch M, Fisher ER, Jeong JH and Wolmark N: Twenty-year
follow-up of a randomized trial comparing total mastectomy,
lumpectomy, and lumpectomy plus irradiation for the treatment of
invasive breast cancer. N Engl J Med. 347:1233–1241. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Litière S, Werutsky G, Fentiman IS,
Rutgers E, Christiaens MR, van Limbergen E, Baaijens M, Bogaerts J
and Bartelink H: Breast conserving therapy versus mastectomy for
stage I–II breast cancer: 20 year follow-up of the EORTC 10801
phase 3 randomised trial. Lancet Oncol. 13:412–419. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Veronesi U, Cascinelli N, Mariani L, Greco
M, Saccozzi R, Luini A, Aguilar M and Marubini E: Twenty-year
follow-up of a randomized study comparing breast-conserving surgery
with radical mastectomy for early breast cancer. N Engl J Med.
347:1227–1232. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ye JC, Yan W, Christos PJ, Nori D and Ravi
A: Equivalent survival with mastectomy or breast-conserving surgery
plus radiation in young women aged <40 years with early-stage
breast cancer: A national registry-based stage-by-stage comparison.
Clin Breast Cancer. 15:390–397. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kroman N, Holtveg H, Wohlfahrt J, Jensen
MB, Mouridsen HT, Blichert-Toft M and Melbye M: Effect of
breast-conserving therapy versus radical mastectomy on prognosis
for young women with breast carcinoma. Cancer. 100:688–693. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clarke M, Collins R, Darby S, Davies C,
Elphinstone P, Evans V, Godwin J, Gray R, Hicks C, James S, et al:
Effects of radiotherapy and of differences in the extent of surgery
for early breast cancer on local recurrence and 15-year survival:
An overview of the randomised trials. Lancet. 366:2087–2106. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meric F, Mirza NQ, Vlastos G, Buchholz TA,
Kuerer HM, Babiera GV, Singletary SE, Ross MI, Ames FC, Feig BW, et
al: Positive surgical margins and ipsilateral breast tumor
recurrence predict disease-specific survival after
breast-conserving therapy. Cancer. 97:926–933. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gajdos C, Tartter PI, Bleiweiss IJ,
Hermann G, de Csepel J, Estabrook A and Rademaker AW: Mammographic
appearance of nonpalpable breast cancer reflects pathologic
characteristics. Ann Surg. 235:246–251. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Killelea BK, Chagpar AB, Bishop J,
Horowitz NR, Christy C, Tsangaris T, Raghu M and Lannin DR: Is
there a correlation between breast cancer molecular subtype using
receptors as surrogates and mammographic appearance? Ann Surg
Oncol. 20:3247–3253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Malik HZ, Wilkinson L, George WD and
Purushotham AD: Preoperative mammographic features predict
clinicopathological risk factors for the development of local
recurrence in breast cancer. Breast. 9:329–333. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tamaki K, Ishida T, Miyashita M, Amari M,
Ohuchi N, Uehara K, Kamada Y, Tamaki N and Sasano H: Retrospective
analysis of mammographic findings for Japanese women: A potential
predictor for breast malignancies. Cancer Sci. 103:472–476. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zunzunegui RG, Chung MA, Oruwari J,
Golding D, Marchant DJ and Cady B: Casting-type calcifications with
invasion and high-grade ductal carcinoma in situ: A more aggressive
disease? Arch Surg. 138:537–540. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Holmberg L, Wong YN, Tabár L, Ringberg A,
Karlsson P, Arnesson LG, Sandelin K, Anderson H, Garmo H and Emdin
S: Mammography casting-type calcification and risk of local
recurrence in DCIS: Analyses from a randomised study. Br J Cancer.
108:812–819. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tabár L, Chen HH, Duffy SW, Yen MF, Chiang
CF, Dean PB and Smith RA: A novel method for prediction of
long-term outcome of women with T1a, T1b, and 10–14 mm invasive
breast cancers: A prospective study. Lancet. 355:429–433. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tabar L, Chen HH Tony, Amy Yen MF, Tot T,
Tung TH, Chen LS, Chiu YH, Duffy SW and Smith RA: Mammographic
tumor features can predict long-term outcomes reliably in women
with 1–14-mm invasive breast carcinoma. Cancer. 101:1745–1759.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thurfjell E, Thurfjell MG and Lindgren A:
Mammographic finding as predictor of survival in 1–9 mm invasive
breast cancers. Worse prognosis for cases presenting as
calcifications alone. Breast Cancer Res Treat. 67:177–180. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alrahbi S, Chan PM, Ho BC, Seah MD, Chen
JJ and Tan EY: Extent of margin involvement, lymphovascular
invasion, and extensive intraductal component predict for residual
disease after wide local excision for breast cancer. Clin Breast
Cancer. 15:219–226. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Osteen RT: Selection of patients for
breast conserving surgery. Cancer. 74 1 Suppl:S366–S371. 1994.
View Article : Google Scholar
|
|
19
|
Tse GM, Tan PH, Cheung HS, Chu WC and Lam
WW: Intermediate to highly suspicious calcification in breast
lesions: A radio-pathologic correlation. Breast Cancer Res Treat.
110:1–7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
MacMillan RD, Purushotham AD, Cordiner C,
Dobson H, Mallon E and George WD: Predicting local recurrence by
correlating pre-operative mammographic findings with pathological
risk factors in patients with breast cancer. Br J Radiol.
68:445–449. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Evans AJ, Pinder SE, James JJ, Ellis IO
and Cornford E: Is mammographic spiculation an independent, good
prognostic factor in screening-detected invasive breast cancer? AJR
Am J Roentgenol. 187:1377–1380. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
James JJ, Evans AJ, Pinder SE, Macmillan
RD, Wilson AR and Ellis IO: Is the presence of mammographic comedo
calcification really a prognostic factor for small screen-detected
invasive breast cancers? Clin Radiol. 58:54–62. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Månsson E, Bergkvist L, Christenson G,
Persson C and Wärnberg F: Mammographic casting-type calcifications
is not a prognostic factor in unifocal small invasive breast
cancer: A population-based retrospective cohort study. J Surg
Oncol. 100:670–674. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dzierzanowski M, Melville KA, Barnes PJ,
MacIntosh RF, Caines JS and Porter GA: Ductal carcinoma in situ in
core biopsies containing invasive breast cancer: Correlation with
extensive intraductal component and lumpectomy margins. J Surg
Oncol. 90:71–76. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lally BE, Haffty BG, Moran MS, Colasanto
JM and Higgins SA: Management of suspicious or indeterminate
calcifications and impact on local control. Cancer. 103:2236–2240.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fatouros M, Roukos DH, Arampatzis I,
Sotiriadis A, Paraskevaidis E and Kappas AM: Factors increasing
local recurrence in breast-conserving surgery. Expert Rev
Anticancer Ther. 5:737–745. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kurtz JM, Jacquemier J, Amalric R,
Brandone H, Ayme Y, Hans D, Bressac C, Roth J and Spitalier JM:
Risk factors for breast recurrence in premenopausal and
postmenopausal patients with ductal cancers treated by conservation
therapy. Cancer. 65:1867–1878. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Macmillan RD, Purushotham AD and George
WD: Local recurrence after breast-conserving surgery for breast
cancer. Br J Surg. 83:149–155. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fisher ER, Sass R, Fisher B, Gregorio R,
Brown R and Wickerham L: Pathologic findings from the national
surgical adjuvant breast project (protocol 6). II. Relation of
local breast recurrence to multicentricity. Cancer. 57:1717–1724.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schnitt SJ, Abner A, Gelman R, Connolly
JL, Recht A, Duda RB, Eberlein TJ, Mayzel K, Silver B and Harris
JR: The relationship between microscopic margins of resection and
the risk of local recurrence in patients with breast cancer treated
with breast-conserving surgery and radiation therapy. Cancer.
74:1746–1751. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Holland R, Hendriks JH, Vebeek AL,
Mravunac M and Stekhoven JH Schuurmans: Extent, distribution and
mammographic/histological correlations of breast ductal carcinoma
in situ. Lancet. 335:519–522. 1990. View Article : Google Scholar : PubMed/NCBI
|