Introduction
Immunoglobulin light-chain (AL) amyloidosis is a
rare and potentially fatal disease characterized by clonal plasma
cells in the bone marrow that produce abnormal κ or λ light chains
(1). The mechanisms of outbreak,
which arise from abnormal plasma cells in the bone marrow, are
known to be similar to that of multiple myeloma (MM). The
pathogenesis of MM is characterized primarily by the overproduction
of interleukin-6 (IL-6) (2), the
major growth and survival (anti-apoptotic) factor, which may also
be expressed in AL amyloidosis (3).
Currently, there is no standard treatment for AL
amyloidosis. However, lenalidomide (one of the more potent
immunomodulatory drugs) and low-dose dexamethasone therapy has been
approved for the treatment of AL amyloidosis (4) as this treatment has been demonstrated to
be effective against MM (5), a
condition with a similar pathology as AL amyloidosis. In addition,
a common cause of mortality of patients with AL amyloidosis is
cardiac dysfunction (6), including
atrial fibrillation, a condition where warfarin is used to lower
the risk of stroke.
Lenalidomide is excreted by the kidney, and the
drug-metabolizing enzyme cytochrome P450 (CYP) is not involved in
this metabolizing process (7,8). Lenalidomide is known to be a poor
substrate of P-glycoprotein (P-gp), but no clinically significant
drug interactions between lenalidomide and P-gp substrates and
inhibitors have been observed (9).
Warfarin interacts with numerous drugs demonstrating
pharmacokinetic or pharmacodynamic drug interactions (10–12). The
pharmacokinetic interaction of warfarin is mediated by the
inhibition of CYP2C9 or displacement of plasma protein binding. The
pharmacodynamic interactions are primarily attributed to the
additive or antagonistic effects on vitamin K-dependent cycle of
blood coagulation (13).
Dexamethasone is an inducer of CYP3A4, an enzyme that metabolizes
dexamethasone. The interaction between anticoagulant agents and
corticosteroids including dexamethasone has been reported (14–16). It
has been demonstrated that a high-dose of dexamethasone (40 mg/day
for 4 days) is able to increase the international normalized ratio
(INR) values in patients that take warfarin (14) and corticosteroids accelerated or
inhibited anticoagulant activity (15,16). In
the case of low-dose dexamethasone, increase in INR elevation was
not observed (17).
Recently, Weiss et al (18) demonstrated that co-administration of
lenalidomide with a single dose of warfarin did not alter the
plasma exposure or anticoagulant effect of warfarin or the plasma
exposure of lenalidomide in healthy volunteers, but this was not
the case for patients with overproduction of IL-6. However, it
remains unclear whether there are interactions between multiple
doses of warfarin and the combination of lenalidomide and low-dose
dexamethasone.
The present study focused on evaluating the
association of drug interactions between warfarin and the
combination of lenalidomide and low-dose dexamethasone in patients
with AL amyloidosis.
Materials and methods
Ethics approval
The present study was reviewed and approved by the
Institutional Review Boards of the Japan Community Health care
Organization Kyoto Kuramaguchi Medical Center (Kyoto, Japan;
approval no. 2015012602). Written patient consent was waived since
this was a retrospective and observational study.
Study population and design
A retrospective study was performed at the Japan
Community Health care Organization Kyoto Kuramaguchi Medical Center
(Kyoto, Japan). Initially, 4 patients with AL amyloidosis treated
with 1.5–4.0 mg doses of warfarin and treated with a combination of
lenalidomide and low-dose dexamethasone between March 2011 and
February 2015 were included. However, 1 patient from this group was
excluded from the assessment due to the increase of warfarin dose
during lenalidomide and low-dose dexamethasone combination
therapy.
The lenalidomide and low-dose dexamethasone regimen
consisted of 15 mg lenalidomide on days 1–21 and 12 or 40 mg
dexamethasone on days 1, 8, 15, 22 and 28 (28 days per cycle).
Data collection and evaluation
The anticoagulant activity of warfarin (INR values)
was measured prior to and during lenalidomide and low-dose
dexamethasone therapy. Throughout the combination chemotherapy,
maximum INR value was obtained. Among the 6 cycles of chemotherapy
for patients, the INR variation data for 4 cycles were analyzed.
The other 2 cycles were excluded as the INR value prior to the
combination chemotherapy was high as compared with that of the
standard value (second cycle in Patient B), or warfarin was not
administered prior to the combination chemotherapy (second cycle in
Patient C). The dose of warfarin was stable throughout the
combination chemotherapy.
Factors affecting the anticoagulant activity of
warfarin, including additional administration of CYP2C9 inhibitors,
hepatic function, serum albumin values, and chemotherapy-induced
nausea and appetite loss were also assessed. Changes in these
factors were compared prior and subsequent to the combination
chemotherapy. Parameters of hepatic function, aspartate
aminotransferase, glutamic-pyruvic transaminase and total bilirubin
were reviewed.
The association of drug interaction between
lenalidomide and warfarin were evaluated using Horn's Drug
Interaction Probability Scale (>8, highly probable; 5–8,
probable; 2–4, possible; <2, doubtful) (19).
Statistical analysis
Data are expressed as the mean ± standard deviation
or median ± range. A comparison of the INR values prior to the
start of combination chemotherapy and during the course of the
treatment was performed using the unpaired Student's t-test with
Microsoft Excel software (version 2013; Microsoft Corporation,
Redmond, WA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
The characteristics of patients enrolled in the
present study are shown in Table I.
All patients (n=3) were diagnosed with AL amyloidosis and were
administered warfarin due to cardiac dysfunction with atrial
fibrillation. No patient exhibited significant alterations in
hepatic function and serum albumin concentrations prior to and
following combination chemotherapy. In addition, the patients were
not treated with any other drugs possessing inhibitory activity of
CYP2C9, except for warfarin. Furthermore, chemotherapy-induced
nausea and appetite loss were not observed in any patient.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
| Patients |
|---|
|
|
|
|---|
| Characteristic | A | B | C |
|---|
| Age, years | 70 | 71 | 71 |
| Sex | Male | Female | Female |
| Diagnosis | AL amyloidosis | AL amyloidosis | AL amyloidosis |
| Type | BJP-λ | BJP-λ | IgG-λ |
| Cardiac
dysfunction | Non-sustained
ventricular tachycardia | Complete
atrioventricular block | Sick sinus
syndrome |
| Prior therapy
received (no. received) | Yes (1) | Yes (1) | Yes (1) |
| Regimen |
|
|
|
| Dosage of
lenalidomide, mg | 15 | 15 | 15 |
| Treatment
schedule, day | 1–21 | 1–21 | 1–21 |
| Dosage of
dexamethasone, mg | 12 | 12 | 40 |
| Treatment
schedule, day | 1, 8, 15, 22, 28 | 1, 8, 15, 22, 28 | 1, 8, 15, 22, 28 |
| Enforced chemotherapy
(cycle) | 1 | 2 | 3 |
| Analyzed chemotherapy
(cycle) | 1 | 1 | 2 |
| Warfarin dose,
mg | 4 | 1.5 | 2 |
| Additional
administration of CYP2C9 inhibitors | No | No | No |
| Alteration of hepatic
function (AST, ALT and T-Bil) | No | No | No |
| Alteration of serum
albumin | No | No | No |
| Chemotherapy-induced
nausea and appetite loss | No | No | No |
Clinical time course of INR
values
Changes in INR values during the lenalidomide and
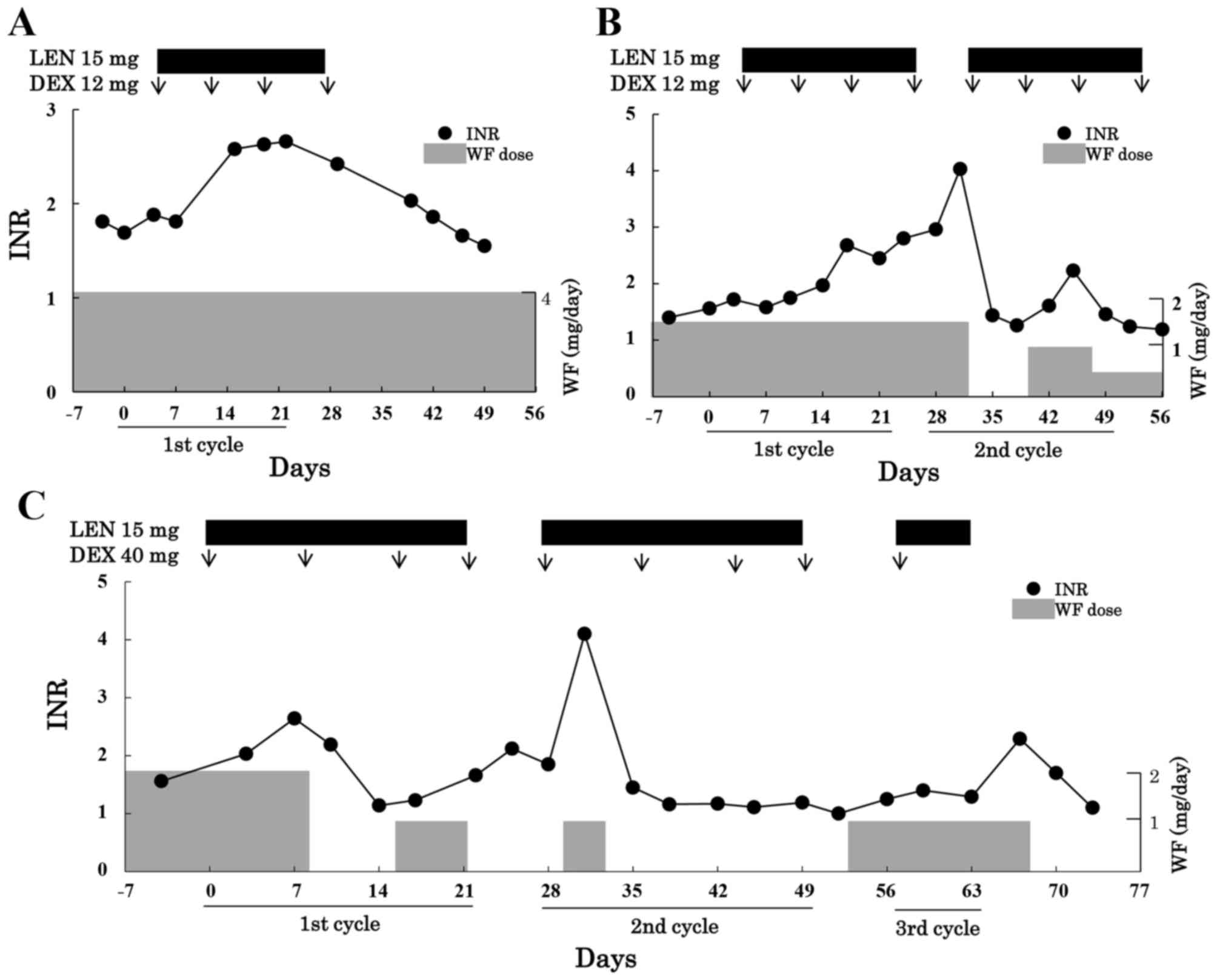
dexamethasone combination therapy are shown in Fig. 1. In all cases, INR values were stable
(mean, 1.52) prior to the chemotherapy. Although the dose of
warfarin was unchanged, the INR value prior to combination
chemotherapy was 1.69 in case 1 and increased to 2.66 on day 23
during chemotherapy. The INR value returned to 1.55 on day 50
during the withdrawal period.
Similar to the findings in case 1, the INR values in
case 2 increased from 1.56 to 2.80 on day 25. In addition, the INR
value markedly and quickly increased on day 4 of the second cycle
from 2.96 to 4.03 due to re-administration of lenalidomide. On day
18 of the second cycle, INR values quickly increased to 2.23, even
though the dose of warfarin was reduced from 1.5 to 1.0 mg/day and
was only re-administered if lenalidomide was administered
beforehand.
In case 3, INR values increased from 1.56 to 2.64 on
day 8 in the first cycle, resulting in discontinuation of warfarin.
The dose of warfarin was reduced to half and was subsequently
re-administered on day 2 of the second cycle. The marked and quick
increase in INR values from 1.85 to 4.10 was observed on day 4 of
the second cycle.
The Horn's Drug Interaction Probability Scale
(19) indicated that the total score
was 2, 4 and 4 in cases 1, 2 and 3, respectively. The scores
indicate a possibility of drug interaction in each case.
Anticoagulant activity of
warfarin
INR values prior to and following combination
chemotherapy and the times to reach maximum values are shown in
Table II. The mean value of INR was
1.52 prior to the treatment and increased 1.7-fold during
combination chemotherapy (P=0.0003). The median time to reach the
maximum value of INR was 17 days.
 | Table II.INR values prior to and following
combination chemotherapy and times to reach its maximum values. |
Table II.
INR values prior to and following
combination chemotherapy and times to reach its maximum values.
|
| Combination
chemotherapy |
|
|---|
|
|
|
|
|---|
| Parameter | Prior to | Subsequent to | P-value |
|---|
| INRa | 1.52±0.19 | 2.60±0.22 | 0.0003 |
| Times to reach
maximum value, daysb |
| 17 (8–25) |
|
Discussion
In the present analysis, the additional use of
warfarin with lenalidomide and low-dose dexamethasone combination
therapy significantly increased the INR values. Therefore, this
indicates a drug interaction between warfarin and the combination
of lenalidomide and low-dose dexamethasone, which is of clinically
importance.
The mechanism of the interaction between warfarin
and combination therapy with lenalidomide and low-dose
dexamethasone remains unclear, but a number of concepts were
considered. With regards to the combination of warfarin and
dexamethasone, it was reported that the high-dose of
corticosteroids potentiated the effects of warfarin, resulting in
increased INR values (14,15). By contrast, Yano et al
(17) reported that the increase in
INR values was not observed with concomitant use of low-dose (6.6
mg) dexamethasone, which is comparable to the results of the
present analysis. The extent of interaction between warfarin and
dexamethasone was poor, even though low-dose dexamethasone may
increase INR values.
Lenalidomide is not metabolized by cytochrome P450
enzymes in the liver (8), and the
ratio of binding to plasma protein was ~40% (7). By contrast, warfarin is metabolized by
CYP2C9 and has a high plasma protein binding ratio of ~99%
(10–12). Therefore, there is unlikely to be a
pharmacokinetic interaction between lenalidomide and warfarin. This
was supported by the observation that concomitant use of
multiple-dose lenalidomide with a single-dose warfarin had no
effect on the pharmacokinetics of total lenalidomide or R- and
S-warfarin in healthy volunteers (18). However, the alteration of INR values
was time-dependent and reproducible for lenalidomide in the present
analysis (Fig. 1). In addition, the
increase in INR values continued, even though treatment with
lenalidomide was discontinued (Fig.
1B), and increased markedly when lenalidomide was
re-administered in the next cycle. The Horn's Drug Interaction
Probability Scale (19) indicated
that the interaction between warfarin and lenalidomide was a
possibility. By contrast, patients exhibited no marked alterations
in hepatic function and serum albumin concentration prior and
subsequent to combination chemotherapy. Furthermore, and no
additional administration of CYP2C9 inhibitors or vitamin K
supplements was observed. In addition, chemotherapy-induced nausea
or appetite loss, which may alter vitamin K absorption and serum
albumin concentration, was not observed in any patients. These
findings suggest that the total clearance or protein binding of
warfarin remained unchanged. Accordingly, the combination of
warfarin and lenalidomide was considered to result in
pharmacodynamic interactions.
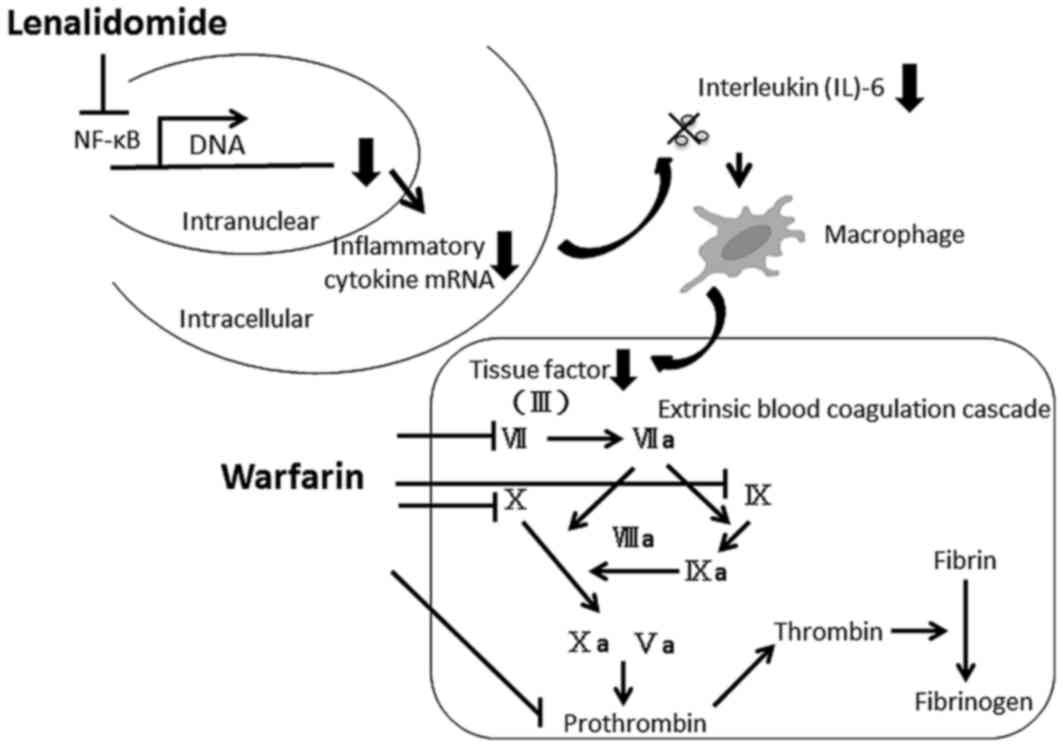
The mechanism of pharmacodynamic interaction between
warfarin and lenalidomide was considered. Lenalidomide is known to
have immunomodulatory effects, which alters the production of
Th2-type cytokines, including IL-6 and tumor necrosis factor α and
is primarily modulated by a decrease in IL-6 levels (20,21). IL-6
has been demonstrated to be associated with extrinsic blood
coagulation cascades, which produces a tissue factor in macrophages
(22). Lenalidomide may decrease the
synthesis of a tissue factor by inhibiting the production of IL-6
and thus resulting in an increase of anticoagulant activity of
warfarin, as shown in Fig. 2. The
mechanism proposed by the authors of the present study was
supported by a study into the drug interaction of capecitabine and
warfarin, which demonstrated that capecitabine effects the factor
VII activity and contributes to the increase in INR values
(23). Collectively, the interaction
between lenalidomide and warfarin may have occurred due to
pharmacodynamic interaction by inhibiting the production of IL-6
but not pharmacokinetic interaction. Analysis of levels of IL-6 and
tissue factors may provide further insights into the precise
mechanism of this interaction.
Clinically important interactions between warfarin
and combination therapy with lenalidomide and low-dose
dexamethasone were observed in AL amyloidosis, where there was a
significant increase in INR values. Therefore, patients taking
warfarin should be monitored closely and regularly for alterations
in INR values during lenalidomide and low-dose dexamethasone
combination therapy, and the dose of warfarin should be reduced if
necessary.
References
|
1
|
Gertz MA: Immunoglobulin light chain
amyloidosis: 2013 pdate on diagnosis, prognosis, and treatment. Am
J Hematol. 88:416–425. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lauta VM: A review of the cytokine network
in multiple myeloma: Diagnostic, prognostic, and therapeutic
implications. Cancer. 97:2440–2452. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nagasawa T, Yanagisawa H, Hasegawa Y,
Kanma H and Abe T: Polycythemia associated with primary systemic
amyloidosis: Elevated levels of hemopoietic factors and cytokines.
Am J Hematol. 43:57–60. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sanchorawala V, Wright DG, Rosenzweig M,
Finn KT, Fennessey S, Zeldis JB, Skinner M and Seldin DC:
Lenalidomide and dexamethasone in the treatment of AL amyloidosis:
Results of a phase 2 trial. Blood. 109:492–496. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dimopoulos MA, Chen C, Spencer A,
Niesvizky R, Attal M, Stadtmauer EA, Petrucci MT, Yu Z, Olesnyckyj
M, Zeldis JB, et al: Long-term follow-up on overall survival from
the MM-009 and MM-010 phase III trials of lenalidomide plus
dexamethasone in patients with relapsed or refractory multiple
myeloma. Leukemia. 23:2147–2152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Palladini G and Comenzo RL: The challenge
of systemic immunoglobulin light-chain amyloidosis (Al). Subcell
Biochem. 65:609–642. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen N, Zhou S and Palmisano M: Clinical
pharmacokinetics and pharmacodynamics of Lenalidomide. Clin
Pharmacokinet. 56:139–152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kumar G, Lau H and Laskin O: Lenalidomide:
In vitro evaluation of the metabolism and assessment of cytochrome
P450 inhibition and induction. Cancer Chemother Pharmacol.
63:1171–1175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen N, Weiss D, Reyes J, Liu L, Kasserra
C, Wang X, Zhou S, Kumar G, Weiss L and Palmisano M: No clinically
significant drug interactions between lenalidomide and
P-glycoprotein substrates and inhibitors: Results from controlled
phase I studies in healthy volunteers. Cancer Chemother Pharmacol.
73:1031–1039. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Holbrook AM, Pereira JA, Labiris R,
McDonald H, Douketis JD, Crowther M and Wells PS: Systematic
overview of warfarin and its drug and food interactions. Arch
Intern Med. 165:1095–1106. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jones CB and Fugate SE: Levofloxacin and
warfarin interaction. Ann Pharmacother. 36:1554–1557. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Greenblatt DJ and von Moltke LL:
Interaction of warfarin with drugs, natural substances, and foods.
J Clin Pharmacol. 45:127–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Strandell J and Wahlin S: Pharmacodynamic
and pharmacokinetic drug interactions reported to VigiBase, the WHO
global individual case safety report database. Eur J Clin
Pharmacol. 67:633–641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sellam J, Costedoat-Chalumeau N, Amoura Z,
Aymard G, Choquet S, Trad S, Vignes BL, Hulot JS, Berenbaum F,
Lechat P, et al: Potentiation of fluindione or warfarin by
dexamethasone in multiple myeloma and AL amyloidosis. Joint Bone
Spine. 74:446–452. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaufman M: Treatment of multiple sclerosis
with high-dose corticosteroids may prolong the prothrombin time to
dangerous levels in patients taking warfarin. Mult Scler.
3:248–249. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chatterjea JB and Salomon L: Antagonistic
effect of A.C.T.H. and cortisone on the anticoagulant activity of
ethyl biscoumacetate. Br Med J. 2:790–792. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yano R, Kurokawa T, Tsuyoshi H, Shinagawa
A, Sawamura Y, Matsunaga A, Nakamura T, Yoshida Y, Yoneda M,
Kotsuji F and Masada M: Transient elevation of international
normalized ratio during cisplatin-based chemotherapy in patients
who are taking warfarin. Ann Pharmacother. 45:e552011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weiss D, Knight R, Zhou S, Palmisano M and
Chen N: Evaluation of pharmacokinetic and pharmacodynamic
interactions when lenalidomide is co-administered with warfarin in
a randomized clinical trial setting. Clin Drug Investig.
35:455–461. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Horn JR, Hansten PD and Chan LN: Proposal
for a new tool to evaluate drug interaction cases. Ann
Pharmacother. 41:674–680. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Quach H, Ritchie D, Stewart AK, Neeson P,
Harrison S, Smyth MJ and Prince HM: Mechanism of action of
immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia.
24:22–32. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rushworth GF, Leslie SJ, Forsyth P and
Vincent C: Evidence-based case report: Multiple thrombotic episodes
associated with lenalidomide and dexamethasone therapy for multiple
myeloma. Ther Adv Drug Saf. 3:115–122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ikeda U, Ito T and Shimada K:
Interleukin-6 and acute coronary syndrome. Clin Cardiol.
24:701–704. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Camidge R, Reigner B, Cassidy J, Grange S,
Abt M, Weidekamm E and Jodrell D: Significant effect of
capecitabine on the pharmacokinetics and pharmacodynamics of
warfarin in patients with cancer. J Clin Oncol. 23:4719–4725. 2005.
View Article : Google Scholar : PubMed/NCBI
|