Introduction
Kikuchi-Fujimoto disease (KFD), also known as
histiocytic necrotizing lymphadenitis, was first described in the
Japanese literature in 1972 (1,2). Up until
2013, 733 patients with KFD have been reported (3). Most patients with KFD present with
localized unilateral cervical lymphadenopathy (axillary and
inguinal are infrequently) with or without pain and fever (4). KFD occurs worldwide (5) with a relatively high prevalence,
particularly among young women and girls in Japan, as well as in
other Asian countries (6). KFD is a
rare but well known self-limiting disorder that typically affects
the cervical lymph nodes (LNs), with a possibility of recurrence
(6,7).
However, the etiology of KFD remains unknown, and its clinical
features of lymphadenopathy and necrotic lesions are frequently
misdiagnosed as other LN diseases, including malignancy. Metastatic
LNs of cancer also generally demonstrate necrosis and recurrence;
thus, clinicians may suspect LN metastasis in patients with
ipsilateral cervical lymphadenopathy and head and neck cancer.
Metastatic LNs may exhibit central necrosis, particularly in cases
of advanced metastasis, and preoperative therapy for cancer may
also cause LN necrosis. Regional recurrence of neck metastases is
common in patients with head and neck cancer.
The present study reports the case of a patient with
tongue cancer and ipsilateral cervical lymphadenopathy.
Pathological examination revealed two types of lesions in three
nodes, including one metastatic lesion and two KFD lesions, all
with evidence of necrosis. Ipsilateral cervical lymphadenopathy
recurred 6 years after the initial surgery, and clinical
examination was unable to differentiate between the second primary
tumor and KFD.
Case report
A 48-year-old man visited the Department of Oral and
Maxillofacial Surgery, University Hospital of the Ryukyus (Okinawa,
Japan) in April 2008 for treatment of a tongue mass. The patient
had a 2-month-long history of pain associated with the right
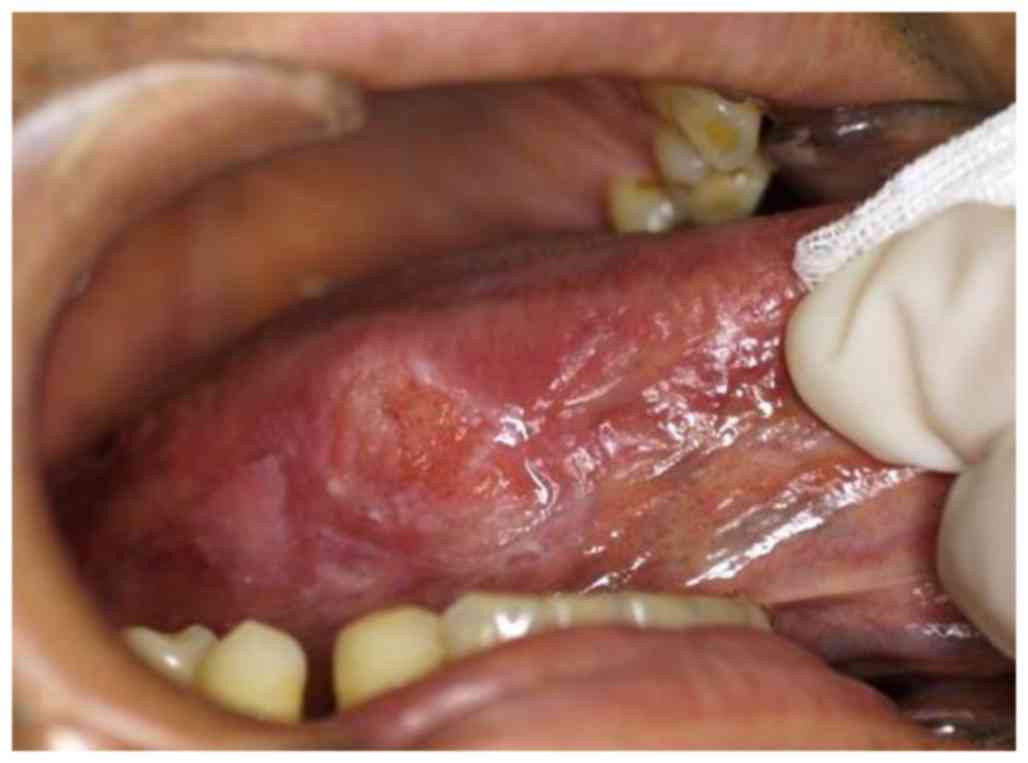
lateral tongue edge. Physical examination revealed a hard elastic
2.0×1.5 cm mass on the right side of the tongue (Fig. 1). Initial examination revealed no
palpable lymphadenopathy in the head and neck area. The patient's
medical history was unremarkable, except for Cushing's syndrome and
glaucoma. Initial contrast-enhanced computed tomography (CT) with
iopamidol (Oypalomin 370; Fuji Pharma, Tokyo, Japan), ultrasound
(US) and magnetic resonance imaging (MRI) identified no cervical LN
lesions. Incisional biopsy of the tongue mass (May 2008) led to a
histrogical diagnosis of squamous cell carcinoma (SCC) (cT2N0M0)
(8). Considering the patient's young
age, intravenous neoadjuvant bleomycin was administered (15 mg/day
for 6 days; total dose 90 mg) plus oral uracil/tegafur (450 mg/day)
as previously described (9,10). The tongue mass partially responded to
preoperative chemotherapy, based on revised RECIST guidelines
(version 1.1) (11). However,
ipsilateral cervical lymphadenopathy developed several days prior
to the scheduled surgery. One LN at level I was mildly enlarged,
firm, round, movable and painless on palpation, suggesting
metastasis. The other LNs exhibited no signs of metastasis.
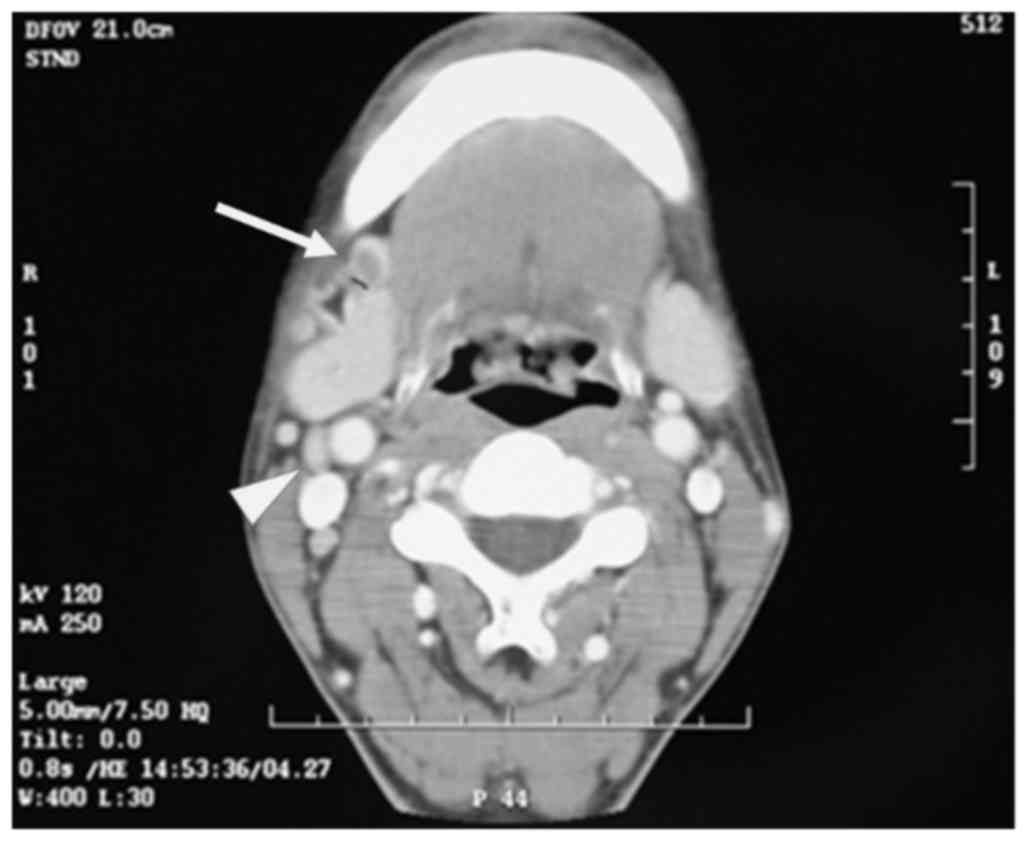
Subsequent CT was performed to reevaluate the clinical stage. The
level I LN appeared as a 1.2×0.9 cm round mass with central
necrosis and rim enhancement, suggesting LN metastasis. However, CT
also revealed two enlarged oval-shaped LNs of ≥1.0 cm at levels II
and III, with homogeneous contrast enhancement (Fig. 2), which could not be conclusively
diagnosed as cancerous. A clinical diagnosis of tongue SCC
(cT2N1M0) was made using UICC TNM classification 7th edition
(8).
Radical neck dissection was performed (May 2008)
with dissection and pathological diagnosis of LNs at all levels.
The patient simultaneously underwent local excision of the tongue
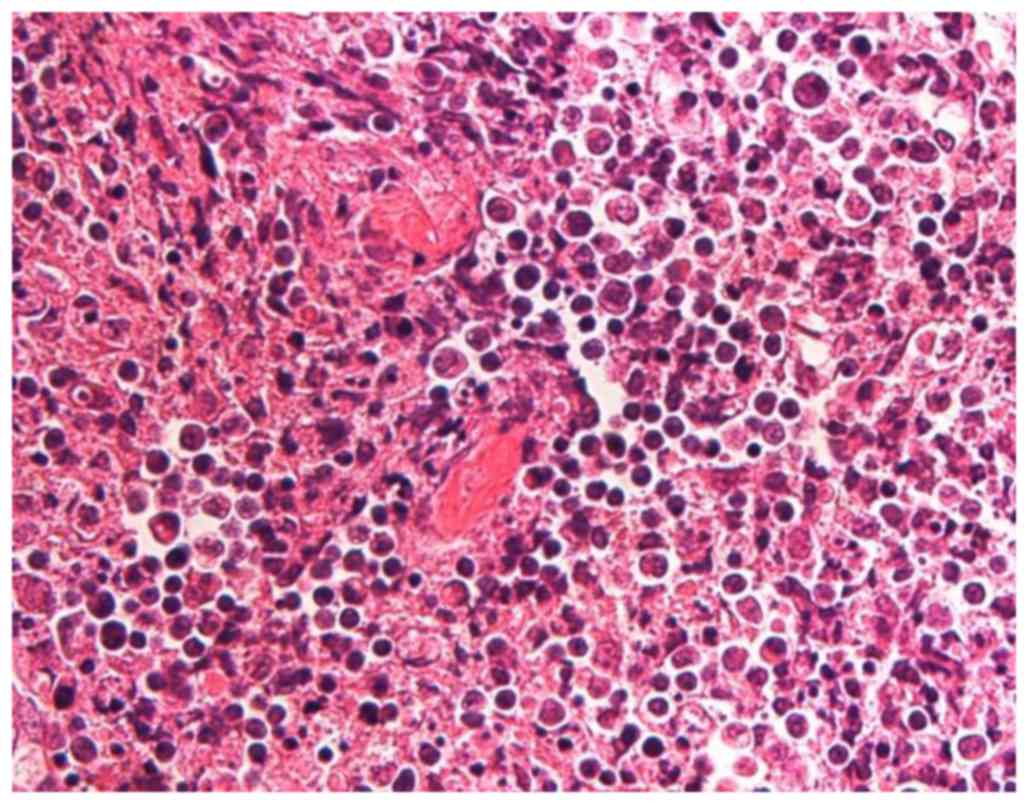
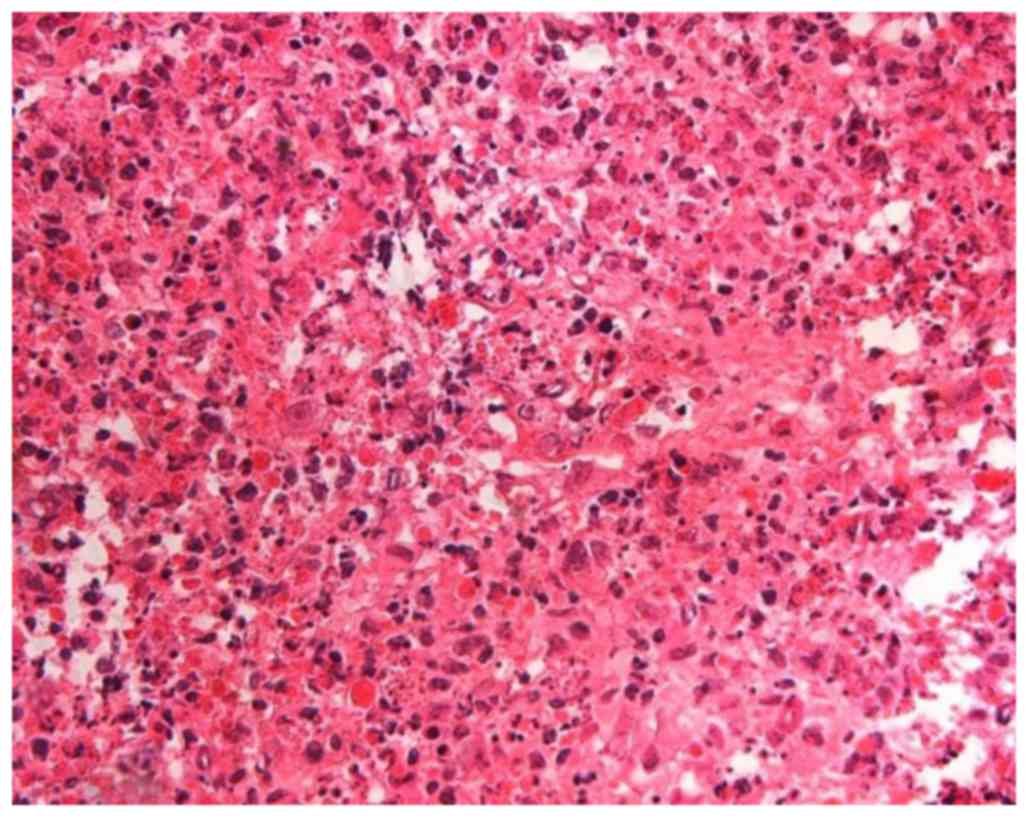
cancer. Histopathological examination revealed tongue SCC with one
metastatic node at level I with necrosis (Fig. 3; a 3-µm section was cut and stained
with hematoxylin and eosin and evaluated with brightfield
microscopy), with no extracapsular nodal spread or positive
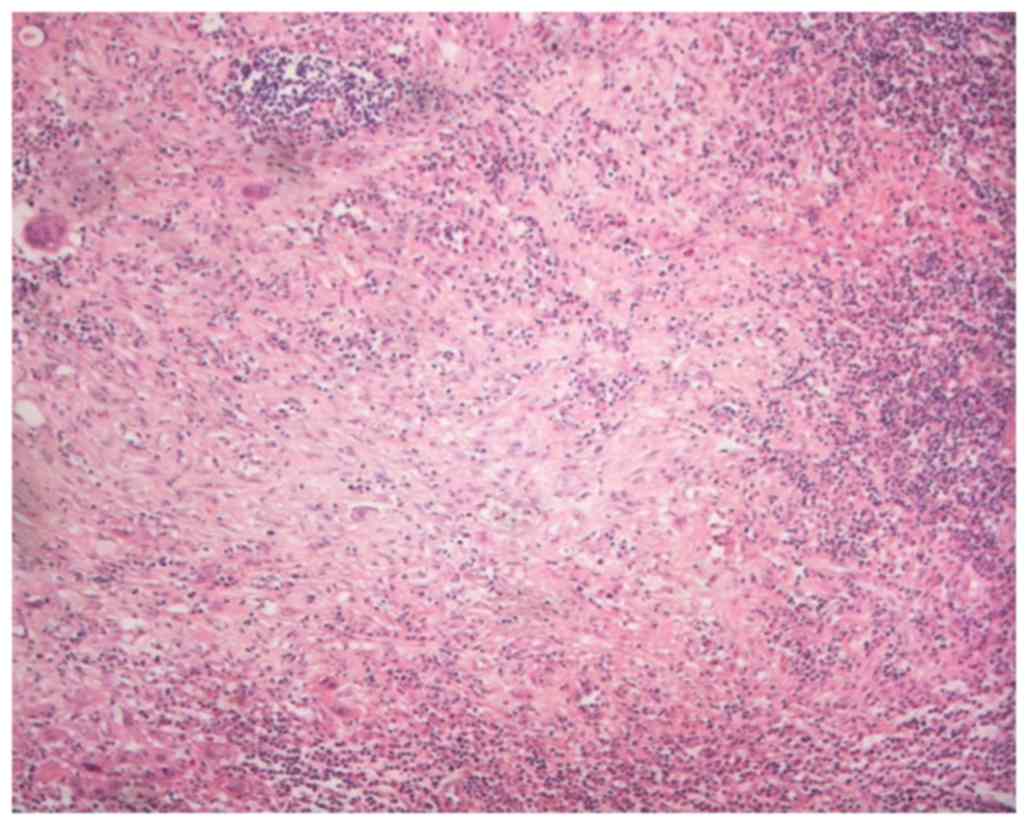
margins. The level II and III LNs revealed necrotizing
granulomatous lesions, and the presence of coagulative necrosis
surrounded by numerous epithelioid cells accompanying nuclear
debris, clinical featured of KFD (Fig.
4; a 3-µm section was cut and stained with hematoxylin and
eosin and evaluated with brightfield microscopy).
Immunohistochemical analysis was performed with the following
antibodies: AE1/AE3 (dilution, 1:800; catalog no. M3515; Dako;
100°C/60 min of incubation); cluster of differentiation (CD) 3
(dilution, 1:50; catalog no. M7254; Dako; 100°C/60 min of
incubation); CD20 (dilution, 1:200; catalog no. M0755; Dako;
100°C/60 min of incubation); CD68 (dilution, 1:1,600; catalog no.
M0814; Dako; room temperature/8 min of incubation).
Immunohistochemical examination for the pancytokeratin marker
AE1/AE3 revealed no epithelial component, indicating that the
lesion was not a tumor metastasis (data not shown). No abnormal
distributions of CD3+/CD20+ lymphocytes were
identified (data not shown). Detection of the histiocyte marker
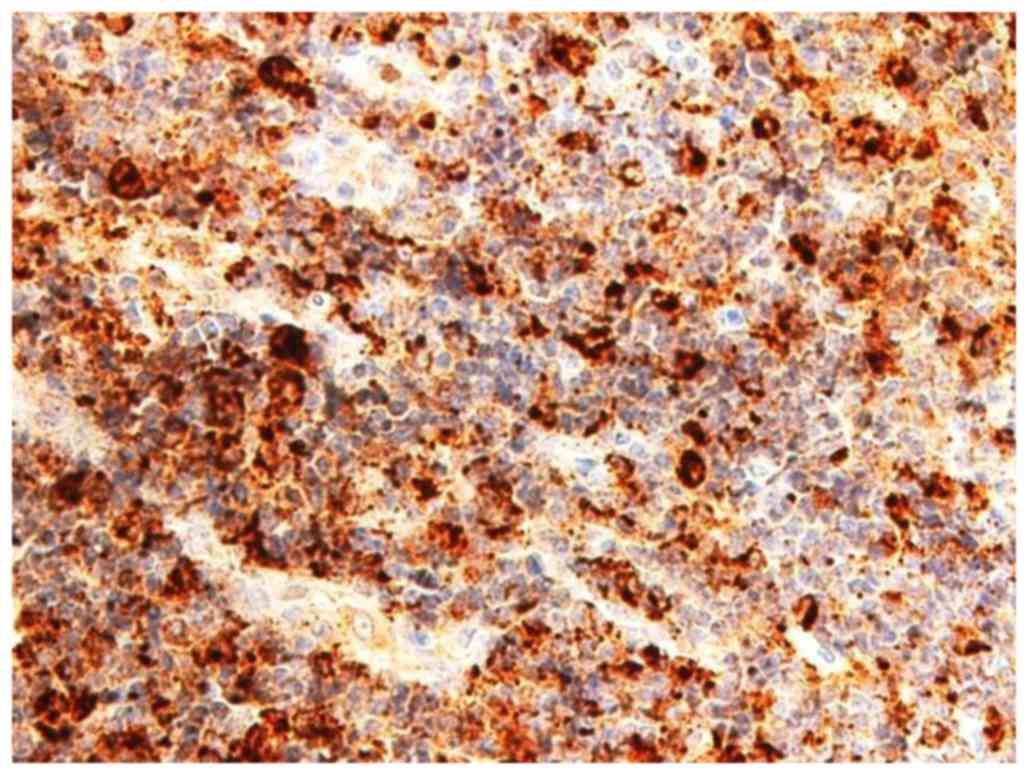
CD68 confirmed the epithelioid cells as histiocytes (Fig. 5). Based on histological and
immunohistochemical findings it was therefore concluded that the
level II and III LN lesions were KFD (lymphohistiocytic type).
The patient remained disease-free for 6 years after
the initial surgery, following which the right side of the
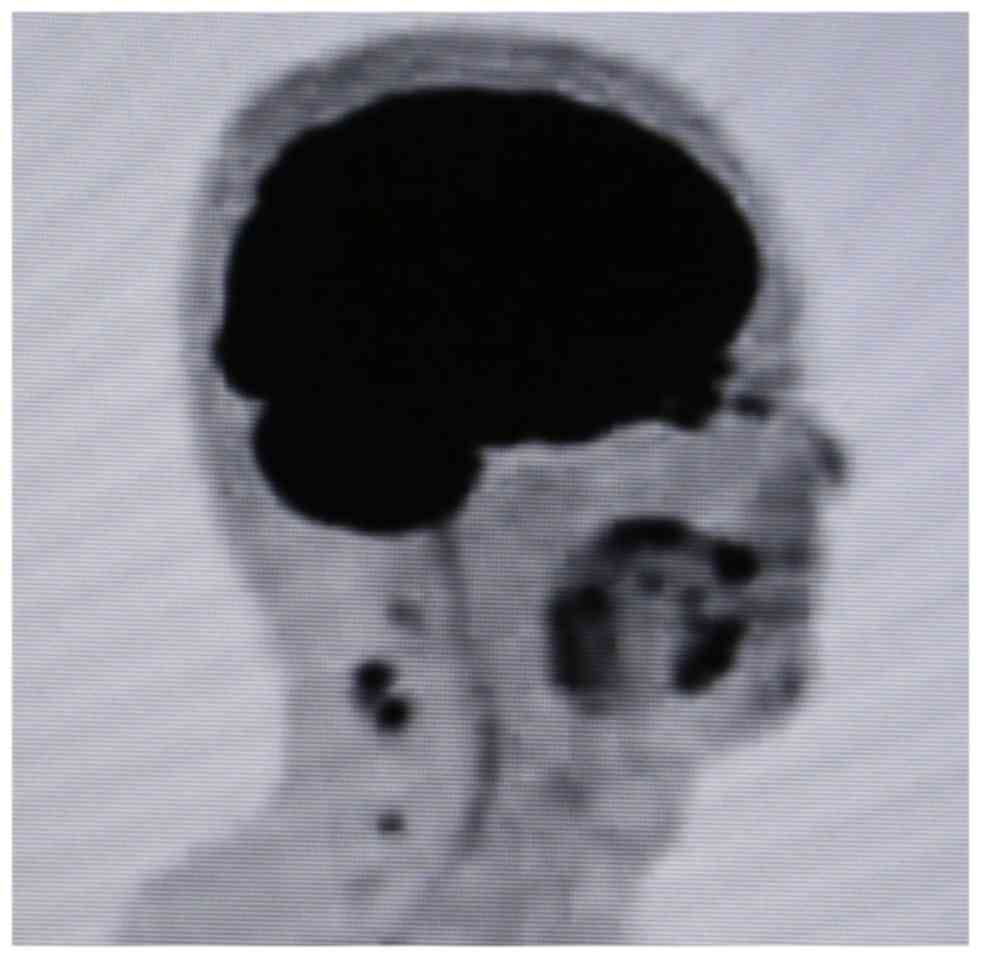
patient's posterior neck became swollen, and CT revealed multiple
swollen posterior LNs, including spinal accessory nodes outside the
scope of the previous neck dissection. High
2-[18F]-fluoro-2-deoxy-D-glucose (FDG) uptake by these LNs was
demonstrated by FDG positron emission tomography (FDG-PET)/CT
(Fig. 6). No axillary or inguinal
lymphadenopathy was found, and a second primary tumor of the neck
or recurrent KFD was suspected. To diagnose the lesion clearly, an
excision biopsy (July 2014) and subsequent histological examination
of six posterior cervical LNs was performed, with the results
suggesting KFD (Fig. 7; a 3-µm
section was cut and stained with hematoxylin and eosin and
evaluated with brightfield microscopy). No malignant lesions,
including regional recurrent SCC or lymphoma, were detected
pathologically. No preoperative or postoperative therapy was
performed before or after the second LN excision (July 2014). The
residual lymphadenopathy gradually resolved on palpation and
postoperative CT (5 months after surgery) confirmed the
disappearance of the lymphadenopathy without treatment [Lee et
al (12) described the resemble
symptom]. Therefore, based on clinical and pathological findings,
the lesion was diagnosed as recurrent KFD. The patient remained
well, with no clinical or radiological signs of recurrence or
metastasis, for 17 months after the second LN excision (July
2014).
Discussion
To the best of our knowledge, this is the first
reported case of a patient with simultaneous tongue cancer,
regional LN metastasis and KFD. This highlights the need for
clinicians to consider KFD when they encounter LNs with necrotic
lesions and without cancerous cells, and indicates that a
combination of clinical and pathological assessments may aid in the
identification of KFD, in addition to ruling out metastasis in
initial and recurrent lymphadenopathies. This is also the first
report, to the best of our knowledge, of recurrent KFD on the same
side as that of the original lesion (metastasis and KFD), and
presents the oldest reported patient with recurrent KFD, occurring
at 54 years old (initial presentation, 48 years old). Prior to our
case, the oldest patient of recurrent KFD was 50 years old
(13).
The literature from 1972–2016 was searched using
PubMed and Google Scholar with the following key words or phrases:
‘Kikuchi's disease AND cancer’, ‘Kikuchi's disease AND malignancy’,
‘Kikuchi-Fujimoto disease AND cancer’, ‘Kikuchi-Fujimoto disease
AND malignancy’, ‘histiocytic necrotizing lymphadenitis AND
cancer’, ‘or’ ‘histiocytic necrotizing lymphadenitis AND
malignancy.’ This led to the identification of 13 cases of patients
with KFD and cancer (14–24) (Table I).
However, none of the previously reported cases had recurrent KFD.
Three reports in the English literature-described metastatic LNs
together with KFD lesions (18,23,24), where
KFD was detected incidentally during cancer treatment. Cancer
clinicians often assume that regional LN swelling is due to
metastasis, and fail to consider the possibility of KFD.
 | Table I.Reports of patients with KFD
associated with cancer. |
Table I.
Reports of patients with KFD
associated with cancer.
| Case no. | Age | Sex | Type of cancer | Metastatic LN
presence | LN site of KFD | Recurrence of
KFD | (Refs.) no. |
|---|
| 1 | 57 | Female | Recurrent malignant
fibrous histiocytoma of thigh | (−) | Inguinal
(regional) | (−) | (18) |
| 2 | 37 | Male | Stomach
adenocarcinoma | (−) | Cervical | (−) | (19) |
| 3 | 66 | Female | Invasive lobular
carcinoma of breast | (−) | Axillary
(regional) | (−) | (20) |
| 4 | 27 | Female | Remission period of
diffuse large B-cell lymphoma | (−) | Cervical | (−) | (21) |
| 5 | 30 | Female | Remission period of
diffuse large B-cell lymphoma | (−) | Axillary | (−) | (21) |
| 6 | NA | Male | Buccal mucosa
cancer | (+) | Cervical
(regional) | (−) | (22) |
| 7 | 41 | Female | Post-treatment of
thyroid cancer and cervical metastasis | (−) | Cervical
(regional) | NA | (23) |
| 8 | 58 | Male | Prostate cancer
(postoperative) | (−) | Cervical | (−) | (24) |
| 9 | NA | NA | NA | NA | NA | NA | (25) |
| 10 | NA | NA | NA | NA | NA | NA | (25) |
| 11 | NA | NA | Resolved
lymphoma | (−) | NA | (−) | (26) |
| 12 | 37 | Female | Melanoma of
thigh | (+) | Inguinal
(regional) | (−) | (27) |
| 13 | 38 | Male | Regional recurrence
of papillary thyroid carcinoma | (+) | Cervical
(regional) | (−) | (28) |
| 14 | 48 | Male | Tongue SCC | (+) | Cervical
(regional) | (+) | Current study |
The etiology of KFD remains unknown (25). Although various factors, including
Yersinia enterocolitica, Toxoplasma gondii,
Epstein-Barr virus, human herpes virus types 6, 7 and 8, systemic
lupus erythematosus, Hashimoto's thyroiditis, silicone breast
implants and pacemaker implantation have been implicated in its
development (26–30), it remains unclear if these are
causative factors or coincidental phenomena. Cancer may also serve
a role in the development of KFD, and is the most important factor
for clinicians to be aware of. Kikuchi (1) and Fujimoto et al (2) pointed out the potential for the
misdiagnosis of KFD as cancer metastasis in 1972, but few reports
have described metastasis as a differential diagnosis of KFD
(18,21), and only three reports have described
metastatic LNs together with KFD lesions (18,23,24). To
the best of our knowledge, the current report is the first to
describe methods for differentiating between these two diseases.
The results of the present report lead to the hypothesis that
previous cases of KFD may have been misdiagnosed as metastasis in
patients with cancer, thus leading to the underestimation of its
incidence. National Comprehensive Cancer Network guidelines
(31) suggest that risk factors,
including multiple metastasis positive nodes, N2 or N3 nodal
disease, and nodal disease in levels IV or V, should be considered
as indications for postoperative therapy in patients with head and
neck cancer, indicating that the misdiagnosis of KFD may lead to
unnecessary adjuvant therapy.
The detection of LNs with necrotic lesions with no
clinical or pathological indication of cancerous cells should alert
clinicians to the possibility of KFD. The case reported in the
present study exhibited apparent SCC in level I, but necrotic
lesions without cancerous cells in levels II and III. Although the
level II and III lesions were ultimately diagnosed as KFD by
immunohistochemical examination, it was essential to rule out the
possibility of metastases, given that necrotic lesions lacking
cancerous cells, but within the regional LN area of the cancer, may
also represent a pathologic complete response, defined as no cancer
lesion remaining after therapy (11).
Necrosis can be determined clinically, as well as pathologically,
and KFD LNs with necrosis may be misdiagnosed as metastases in
patients with head and neck cancer.
Clinically, initial preoperative CT in the case
discussed in the present report revealed central necrosis in the
level I metastatic LN and homogeneous contrast enhancement in the
level II and III KFD LNs. However, Kwon et al (32) reported that KFD LNs frequently exhibit
central necrosis and homogeneous contrast enhancement CT patterns,
suggesting that the CT appearance of KFD can be variable and mimic
metastasis (33); therefore, KFD
cannot be diagnosed clinically on the basis of CT alone. All three
LN lesions in the case discussed in the present report were
subsequently found to be necrotic pathologically; however, ~50% of
metastatic nodes also have necrotic lesions (34). The capability of MRI and US to
differentiate between KFD necrotic lesions and metastases are also
controversial (33,35–37).
The results of previous studies suggest that it is
difficult to distinguish KFD from metastasis clinically on the
basis of physical findings, imaging or time to recurrence. With
regard to physical findings, our patient's initial KFD LNs were
≥1.0 cm in diameter. However, a firm/hard consistency, the absence
of tenderness and a diameter ≥1.0 cm on palpation are symptoms of
suspected metastasis (11,38–40). While
KFD lymphadenopathy may be firm and sometimes painful (41), several studies have described KFD
lesions as firm or non-tender (41–43). Kwon
et al (32) reported that the
mean maximum diameter of the affected cervical LNs was 1.62 cm in
1,196 KFD LNs from 96 patients (32).
Furthermore, KFD LNs may be >2.5 cm (4,43). KFD LNs
may thus resemble metastatic lesions, based on palpation, though
clinical palpation of the neck has been reported to be unreliable
(44). KFD also has extranodal
manifestations that mimic malignant LN disease, including
extracapsular nodal spread of cancer metastasis (9,45). KFD can
occur in all levels of the cervical LNs (32). Cervical LNs affected by KFD are
commonly located in the posterior cervical triangle (18,32), but
the submandibular nodes (levels I and II; oral cavity, oropharynx,
hypopharynx, larynx) may also be affected, in addition to being the
regional LNs involved in head and neck cancer (32,46).
No definitive imaging modality for distinguishing
between KFD and malignancy has yet been identified (47). US findings in KFD tend to be
suspicious of malignancy, with findings including a round shape,
gross necrosis and a loss of central fatty hila (36,37). Youk
et al (48) also indicated
that KFD tended to be misdiagnosed as malignancy on the basis of US
findings. Furthermore, FDG uptake in FDG-PET/CT is not specific for
cancer (4,47). Regarding time to recurrence, KFD
typically recurs within 1 year of its initial resolution (5,49), while
LN metastasis also occurs within 1 year (50). Time to recurrence is therefore not
diagnostic, as KFD recurrence and LN metastasis may occur at any
time (51,52).
Unexpected findings are encountered during
pathologic examination in >3% of neck dissections (53); however, the presence of metastatic LNs
remains the most important finding for clinicians. Effective
communication between the clinician and pathologist is needed to
coordinate the clinical and pathologic characteristics needed to
make an accurate diagnosis of KFD (18). The combination of clinical and
pathological features may facilitate the diagnosis of KFD and rule
out metastasis in initial and recurrent lymphadenopathies. In the
case discussed in the present report, pathological findings
supported the sparse clinical findings to lead to the diagnosis of
KFD. However, for the recurrent lesions, the pathological findings
were inconclusive, while the clinical findings of resolution of the
lymphadenopathy on palpation and CT confirmed a diagnosis of KFD.
The initial KFD was diagnosed pathologically by immunohistochemical
examination of AE1/AE3, CD3, CD20 and CD68. In the case of the
recurrent lesions, although pathological diagnosis suggested
possible recurrent KFD based on the presence of histiocytes with
nuclear debris, as observed in the initial KFD lesion, the lesion
was too small to make a definitive diagnosis. However, following
surgery, lymphadenopathy of the residual posterior LNs that
resolved upon palpation and postoperative CT (5 months after
surgery) confirmed a diagnosis KFD. Although the cytological and
histological examinations were not conclusive in a study by Lee
et al (12), the occurrence of
enlarged LNs as recurrent KFD was diagnosed based on clinical
findings, including complete resolution of lymphadenopathy on CT
performed 1 month after LNs enlarged. In addition, KFD is difficult
to diagnose with fine-needle aspiration (13,54–57).
Although recurrent lymphadenopathy suggests a diagnosis of KFD on
clinical grounds, this may not be confirmed by pathological
examination following LN excision (13,51), and
some studies have reported a diagnosis of recurrent KFD based on
clinical findings alone, without rebiopsy (3,13,42,51,58). The
diagnostic criteria for recurrent KFD are therefore obscure. In the
current case, pathological examination was performed to rule out a
second primary tumor. However, when the pathological results are
inconclusive for KFD, as in the current case, postoperative
follow-up and a CT scan may be required to confirm a diagnosis.
Notably, KFD can recur 18–19 years after its initial presentation,
indicating the need for long-term follow-up (51,52).
To the best of our knowledge, this is the first
report of a patient with simultaneous tongue cancer, regional LN
metastasis and KFD. The results of the present study and previous
studies indicate that clinicians should consider KFD when they
encounter LNs with necrotic lesions without cancerous cells. A
combination of clinical and pathological techniques may aid in the
diagnosis of KFD, in addition to ruling out metastasis or a second
primary cancer in initial and recurrent lymphadenopathies. In
particular, careful attention should be paid to a lack of cancerous
cells in necrotic LNs in patients with head and neck cancer, given
that a misdiagnosis of KFD as metastasis may lead to unnecessary
adjuvant therapy.
Acknowledgements
The authors would like to thank Dr Takeaki Tomoyose
(Division of Endocrinology, Diabetes and Metabolism, Hematology and
Rheumatology, Second Department of Medicine, Graduate School of
Medicine, University of the Ryukyus, Nishihara, Japan) who
contributed to the patient diagnosis by excluding malignant
lymphoma. The authors would also like to thank the staff of Edanz
Group Japan for providing editing assistance for this
manuscript.
Glossary
Abbreviations
Abbreviations:
|
KFD
|
Kikuchi-Fujimoto disease
|
|
LN
|
lymph node
|
|
CT
|
computed tomography
|
|
US
|
ultrasound
|
|
MRI
|
magnetic resonance imaging
|
|
SCC
|
squamous cell carcinoma
|
|
CD
|
cluster of differentiation
|
|
FDG-PET
|
2-[18F]-fluoro-2-deoxy-D-glucose
positron emission tomography
|
References
|
1
|
Kikuchi M: Lymphadenitis showing focal
reticulum cell hyperplasia with nuclear debris and phagocytes: A
clinicopathological study. Acta Hematol Jpn. 35:379–380. 1972.(In
Japanese).
|
|
2
|
Fujimoto Y, Kozima Y and Yamaguchi K:
Cervical subacute necrotizing lymphadenitis: a new
clinicopathologic entity. Naika. 20:920–927. 1972.(In
Japanese).
|
|
3
|
Kim TY, Ha KS, Kim Y and Lee J, Lee K and
Lee J: Characteristics of Kikuchi-Fujimoto disease in children
compared with adults. Eur J Pediatr. 173:111–116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ranabhat S, Tiwari M, Kshetri J, Maharjan
S and Osti BP: An uncommon presentation of Kikuchi Fujimoto
disease: A case report with literature review. BMC Res Notes.
8:4782015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dumas G, Prendki V, Haroche J, Amoura Z,
Cacoub P, Galicier L, Meyer O, Rapp C, Deligny C, Godeau B, et al:
Kikuchi-Fujimoto disease: Retrospective study of 91 cases and
review of the literature. Medicine (Baltimore). 93:372–382. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim JE, Lee EK, Lee JM, Bae SH, Choi KH,
Lee YH, Hah JO, Choi JH, Kong EJ and Cho IH: Kikuchi-Fujimoto
disease mimicking malignant lymphoma with
2-[(18)F]fluoro-2-deoxy-D-glucose PET/CT in children. Korean J
Pediatr. 57:226–231. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dalton J, Shaw R and Democratis J:
Kikuchi-Fujimoto disease. Lancet. 383:10982014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sobin LH and Wittekind C: Head and neck
tumoursTNM Classification of Malignant Tumours. 6th. John Wiley
& Sons; Hoboken, NJ: pp. 19–56. 2002
|
|
9
|
Sturgis EM, Moore BA, Glisson BS, Kies MS,
Shin DM and Byers RM: Neoadjuvant chemotherapy for squamous cell
carcinoma of the oral tongue in young adults: A case series. Head
Neck. 27:748–756. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Licitra L, Grandi C, Guzzo M, Mariani L,
Lo Vullo S, Valvo F, Quattrone P, Valagussa P, Bonadonna G,
Molinari R and Cantù G: Primary chemotherapy in resectable oral
cavity squamous cell cancer: A randomized controlled trial. J Clin
Oncol. 21:327–333. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee SK, Bahn YE and Kim DE: Features of
sequential CT and US-guided biopsy in recurrent Kikuchi disease of
the neck: a Case report. Ear Nose Throat. 92:442–448. 2013.
|
|
13
|
Bogusz AM and Bhargava P: Recurrent
histiocytic necrotizing lymphadenitis with a long latency in a
patient with autoimmunity: A case report and review of literature.
Int J Surg Pathol. 21:287–296. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chan JK and Ng CS: Kikuchi's histiocytic
necrotizing lymphadenitis in the regional lymph nodes of malignant
fibrous histiocytoma: Causal or coincidental? Histopathology.
12:448–451. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Radhi JM, Skinnider L and McFadden A:
Kikuchi's lymphadenitis and carcinoma of the stomach. J Clin
Pathol. 50:530–531. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aqel NM and Peters EE: Kikuchi's disease
in axillary lymph nodes draining breast carcinoma. Histopathology.
36:280–281. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoshino T, Mannami T, Ichimura K, Takenaka
K, Nose S, Yamadori I and Akagi T: Two cases of histiocytic
necrotizing lymphadenitis (Kikuchi-Fujimoto's disease) following
diffuse large B-cell lymphoma. Hum Pathol. 31:1328–1331. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin HC, Su CY, Huang CC, Hwang CF and
Chien CY: Kikuchi's disease: A review and analysis of 61 cases.
Otolaryngol Head Neck Surg. 128:650–653. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dequanter D: Lothaire, Haller, Saint
Aubain Somerhausen and Andry: Kikuchi-Fujimoto disease mimicking
thyroid metastasis. Rev Stomatol Chir Maxillofac. 106:302–303.
2005.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Waters A, Williams C and Pal A: Kikuchi
Disease: A unique case of fever of unknown origin. Kansas J Med.
6:60–64. 2013.
|
|
21
|
Yu SC, Chen CN, Huang HI, Chen TC, Wang
CP, Lou PJ, Ko JY, Hsiao TY and Yang TL: Diagnosis of
Kikuchi-Fujimoto disease: A comparison between open biopsy and
minimally invasive ultrasound-guided core biopsy. PLoS One.
9:e958862014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoo IH, Na H, Bae EY, Han SB, Lee SY,
Jeong DC and Kang JH: Recurrent lymphadenopathy in children with
Kikuchi-Fujimoto disease. Eur J Pediatr. 173:1193–1199. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Urbanellis P, Chin-Lenn L, Teman CJ and
McKinnon JG: Kikuchi-Fujimoto lymphadenitis imitating metastatic
melanoma on positron emission tomography: A case report. BMC Surg.
15:502015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park JJ, Seo YB, Choi HC, Kim JW, Shin MK,
Lee DJ and Lee J: Kikuchi-Fujimoto disease coexistent with
papillary thyroid carcinoma in a single lymph node. Soonchunhyang
Med Sci. 21:10–14. 2015. View Article : Google Scholar
|
|
25
|
Sharma V and Rankin R: Fatal Kikuchi-like
lymphadenitis associated with connective tissue disease: A report
of two cases and review of the literature. Springerplus. 4:1672015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deaver D, Horna P, Cualing H and Sokol L:
Pathogenesis, diagnosis, and management of Kikuchi-Fujimoto
disease. Cancer Control. 21:313–321. 2014.PubMed/NCBI
|
|
27
|
Lee DH, Lee JH, Shim EJ, Cho DJ, Min KS,
Yoo KY and Min K: Disseminated Kikuchi-Fujimoto disease mimicking
malignant lymphoma on positron emission tomography in a child. J
Pediatr Hematol Oncol. 31:687–689. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rubio SI, Plewinsky TS, Sabatini M and
Poretsky L: Kikuchi's disease associated with Hashimoto's
thyroiditis. J Endocrinol Invest. 19:136–137. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sever CE, Leith CP, Appenzeller J and
Foucar K: Kikuchi's histiocytic necrotizing lymphadenitis
associated with ruptured silicone breast implant. Arch Pathol Lab
Med. 120:380–385. 1996.PubMed/NCBI
|
|
30
|
Charalabopoulos K, Charalabopoulos A,
Binolis J, Papalimneou V and Ioachim E: Is implant pacemaker a
physicochemical cause triggering Kikuchi-Fujimoto disease? In Vivo.
16:73–76. 2002.PubMed/NCBI
|
|
31
|
National Comprehensive Cancer Network:
Head and Neck Cancers Version 1. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdfJune
8–2015
|
|
32
|
Kwon SY, Kim TK, Kim YS, Lee KY, Lee NJ
and Seol HY: CT findings in Kikuchi disease: Analysis of 96 cases.
AJNR Am J Neuroradiol. 25:1099–1102. 2004.PubMed/NCBI
|
|
33
|
Na DG, Chung TS, Byun HS, Kim HD, Ko YH
and Yoon JH: Kikuchi disease: CT and MR findings. AJNR Am J
Neuroradiol. 18:1729–1732. 1997.PubMed/NCBI
|
|
34
|
King AD, Tse GM, Ahuja AT, Yuen EH,
Vlantis AC, To EW and van Hasselt AC: Necrosis in metastatic neck
nodes: Diagnostic accuracy of CT, MR imaging, and US. Radiology.
230:720–726. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sumi M and Nakamura T: Diagnostic
importance of focal defects in the apparent diffusion
coefficient-based differentiation between lymphoma and squamous
cell carcinoma nodes in the neck. Eur Radiol. 19:975–981. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yoo JL, Suh SI, Lee YH, Seo HS, Kim KM,
Shin BK, Song JY and Seol HY: Gray scale and power Doppler study of
biopsy-proven Kikuchi disease. J Ultrasound Med. 30:957–963. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee KH and Ryu J: Real-time elastography
of cervical lymph nodes in Kikuchi disease. J Ultrasound Med.
33:2201–2205. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hay ID, Lee RA, Davidge-Pitts C, Reading
CC and Charboneau JW: Long-term outcome of ultrasound-guided
percutaneous ethanol ablation of selected ‘recurrent’ neck nodal
metastases in 25 patients with TNM stages III or IVA papillary
thyroid carcinoma previously treated by surgery and 131I therapy.
Surgery. 154:1448–1455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lang S and Kansy B: Cervical lymph node
diseases in children. GMS Curr Top Otorhinolaryngol Head Neck Surg.
13:Doc082014.PubMed/NCBI
|
|
40
|
Cheng CY, Sheng WH, Lo YC, Chung CS, Chen
YC and Chang SC: Clinical presentations, laboratory results and
outcomes of patients with Kikuchi's disease: Emphasis on the
association between recurrent Kikuchi's disease and autoimmune
diseases. J Microbiol Immunol Infect. 43:366–371. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tariq H, Gaduputi V, Rafiq A and Shenoy R:
The enigmatic kikuchi-fujimoto disease: A case report and review.
Case Rep Hematol. 2014:6481362014.PubMed/NCBI
|
|
42
|
Phupong V and Poomtavorn Y: Kikuchi
disease during pregnancy. Arch Gynecol Obstet. 274:393–396. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nieman RB: Diagnosis of Kikuchi's disease.
Lancet. 335:2951990. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Langhans L, Bilde A, Charabi B,
Therkildsen MH and von Buchwald C: Evaluation of sentinel lymph
node size and shape as a predictor of occult metastasis in patients
with squamous cell carcinoma of the oral cavity. Eur Arch
Otorhinolaryngol. 270:249–254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Thakral B, Zhou J and Medeiros LJ:
Extranodal hematopoietic neoplasms and mimics in the head and neck:
An update. Hum Pathol. 46:1079–1100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nakamura T and Sumi M: Nodal imaging in
the neck: Recent advances in US CT and MR imaging of metastatic
nodes. Eur Radiol. 17:1235–1241. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ito K, Morooka M and Kubota K: Kikuchi
disease: 18F-FDG positron emission tomography/computed tomography
of lymph node uptake. Jpn J Radiol. 28:15–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Youk JH, Kim EK, Ko KH and Kim MJ:
Sonographic features of axillary lymphadenopathy caused by Kikuchi
disease. J Ultrasound Med. 27:847–853. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Song JY, Lee J, Park DW, Sohn JW, Suh SI,
Kim IS, Kim WJ, Kim MJ and Cheong HJ: Clinical outcome and
predictive factors of recurrence among patients with Kikuchi's
disease. Int J Infect Dis. 13:322–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kim JW, Roh JL, Kim JS, Lee JH, Cho KJ,
Choi SH, Nam SY and Kim SY: (18)F-FDG PET/CT surveillance at 3–6
and 12 months for detection of recurrence and second primary cancer
in patients with head and neck squamous cell carcinoma. Br J
Cancer. 109:2973–2979. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Smith KG, Becker GJ and Busmanis I:
Recurrent Kikuchi's disease. Lancet. 340:1241992. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kosch M, Hausberg M, Barenbrock M, Rahn KH
and Kisters K: Histiocytic, necrotizing lymphadenitis as rare cause
of cervical lymphadenopathy and fever of unknown origin-a case of
biopsy proven recurrence over 19 years. Eur J Haematol. 63:282–283.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sheahan P, Hafidh M, Toner M and Timon C:
Unexpected findings in neck dissection for squamous cell carcinoma:
Incidence and implications. Head Neck. 27:28–35. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Park HS, Sung MJ, Park SE and Lim YT:
Kikuchi-Fujimoto disease of 16 children in a single center of
Korea. Pediatr Allergy Immunol. 18:174–178. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Phelan E, Lang E, Gormley P and Lang J:
Kikuchi-Fujimoto disease: A report of 3 cases. Ear Nose Throat J.
86:412–413. 2007.PubMed/NCBI
|
|
56
|
Tong TR, Chan OW and Lee KC: Diagnosing
Kikuchi disease on fine needle aspiration biopsy: A retrospective
study of 44 cases diagnosed by cytology and 8 by histopathology.
Acta Cytol. 45:953–957. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zar R and McClintock C: Kikuchi-Fujimoto
disease: A case report and review of literature. Conn Med.
70:491–494. 2006.PubMed/NCBI
|
|
58
|
Alijotas-Reig J, Casellas-Caro M,
Ferrer-Oliveras R, Cabero-Roura L and Vilardell-Tarres M: Recurrent
Kikuchi-Fujimoto disease during pregnancy: Report of case evolving
into systemic lupus erythematosus and review of published work. J
Obstet Gynaecol Res. 34:595–598. 2008. View Article : Google Scholar : PubMed/NCBI
|