Introduction
Intestinal obstruction caused by malignant tumors is
a common complication of late cancer, and is especially common in
patients with gastrointestinal malignant cancer and gynecologic
malignant cancer (1,2). The small intestine has a relatively
concentrated distribution range, making it easy to adhere after
abdominal surgery. It has abundant blood supply and is thus
vulnerable to tumor metastasis in the peritoneal cavity and
development of intestinal canal stenosis and compression which
causes small intestinal obstruction (2). These patients are typically at a late
stage of cancer, and mostly are unable to undergo surgery. The
traditional treatment method is feeding via the nasogastric tube.
This method cannot thoroughly drain to the small intestine proximal
end, thus its effect on small intestine distal end drainage is not
that effective.
In the present study, we used nasotracheal small
intestine decompression tubes to perform small intestine
decompression drainage on this population of patients, which can
relieve symptoms of intestinal obstruction in the short-term.
Subsequently, we used decompression tubes for selective
opacification of the distal end of the small intestine to assist
the diagnosis, and carried out enteral nutrition treatment and
achieved positive treatment effects.
Materials and methods
Patients
In total, 22 cases of middle and late stage
malignant tumor causing-secondary small intestine obstruction by
nasogastric small intestine decompression were treated. Of the 22
cases, there were 12 males and 10 females, aged 28–81 years, median
age 49 years. A total of 16 cases had a history of surgery related
to malignant tumors (9 cases of stomach cancer, 1 case of porta
hepatis bile duct cancer, 1 case of hepatocarcinoma, 4 cases of
ovarian cancer, and 1 case of cervical cancer), 6 cases had primary
lesions and secondary peritoneal metastasis (3 cases of stomach
cancer, 1 case of liver cancer, 1 case of pancreatic cancer and 1
case of cervical cancer), and 4 cases had a history of
radiotherapy. All the patients were diagnosed with small intestine
obstruction by clinical diagnosis, as well as plain film and CT of
the abdomen. A total of 16 cases were clinically considered
intestinal obstruction by abdominal adhesion and 6 cases were of
intestinal obstruction caused by tumor compression and invasion.
The patients showed symptoms including stomach and abdominal
distension, emesis, and inability to defecate and pass flatus. This
study was approved by the Ethics Committee of Xuzhou Central
Hospital. Signed written informed consents were obtained from all
participants before the study.
Tube array method and small intestine
decompression
A nasogastric decompression tube (with 2 cavities, 2
air sacs and multiple lateral drainage apertures on the anterior of
the tube) was used. The tube was placed through the nostril,
stomach and duodenum to the upper jejunum (distal of Treitz
ligament) with X-ray monitoring and assistance by a metal
guidewire. For patients with significant structural changes to the
upper digestive tract or difficulty in inserting the tube after
surgery, we used a gastroscope to assist the tube in reaching the
jejunum, and inserted the tube into the distal end of the small
intestine as far as possible with the combination of the tube and a
guidewire. When tube insertion was hindered, we injected 100 ml
lohexol by the decompression tube and evaluated the extent of small
intestine expansion. We injected 5–15 ml sterilized distilled water
to the anterior air sacs, and the water-filled sacculus stimulated
the alimentary canal to spur the tube to gradually move to the
distal end of the small intestine. The suction socket of the tube
was connected to negative pressure drainage. The tube was fixed to
the nose by adhesive tape to prevent the decompression tube from
sliding out of the body. Each day, patients were supervised and
tube position adjusted, and observed for improvements in the
condition of gas-liquid levels within the enteric cavity, whether
there was free gas under the diaphragm, and the position of
previously injected contrast medium in the intestine. We inserted
the guidewire again to adjust the tube when necessary. Each day the
amount of tubal drainage was recorded, and we carried out regular
microorganism tests for the first drainage, and supervised the
changes in body weight and biochemistry of blood each week.
Selective small intestine
opacification diagnosis
After 24 h, if the tube did not naturally continue
to move deeper, or if it still could not move forward by
adjustments, then we immediately injected contrast medium through
the decompression tube mouth to carry out selective small intestine
opacification. When there was opacification, the anterior sacculus
was placed under pressure and we used 20–30 ml gas to fill the
posterior sacculus to let it tightly bind to the intestinal wall,
and to prevent the reflux of contrast medium. When opacification
occurred, we mainly observed the extent of intestinal tract
expansion, unobstruction, passage and stenosis. When the
opacification was finished, the posterior sacculus was placed under
pressure and we filled the anterior sacculus with 5 ml sterilized
distillated water. We then continued the decompression drainage
(3).
Enteral nutrition treatment
At 48 h after the disappearance of abdominal
distension, felt by the patient him/herself, and under the
circumstance of clear clinical diagnosis that the patients did not
have complications such as perforation of the intestine and
intestinal necrosis, we immediately began enteral nutrition
treatment. For patients with selective small intestinal
opacification showing unobstructed intestine, we injected 200–500
ml saline to the small intestine through the decompression tube
(100 ml/h), and turned off the drainage pathway and observed for
3–4 h. If there was no obvious discomfort, then we intermittently
turned off the drainage pathway and changed to oral administration
of short peptides and glutamine. We gradually increased the amount.
When the total amount reached >800 ml per day, we gradually
increased nutrition with small amounts of chyme until normal diet
was achieved, and the patients could remove the tube. During this
period, close observation of abdominal symptoms and signs were
still needed, and when abdominal distension or abdominal pain
appeared when taking food, we immediately turned on the drainage
tube to carry out decompression drainage. For patients with
intestinal obstruction and with obvious stenosis, distortion or
obstruction detected by selective small intestine opacification,
and for where the calculated length of small intestinal canal upper
obstruction was >1 m, we gave oral saline (100 ml), and if
abdominal distension did not significantly increase, then we began
oral administration of short peptides without glutamine. We then
gradually increase the amount, meanwhile continuously carrying out
negative pressure drainage by the decompression tube. Approximately
1 week later, we re-evaluated small intestine opacification to
observe the extent of intestinal canal expansion and improvement
from stenosis. The lacking part of dietary caloric intake was
accompanied by parenteral nutrition.
Results
Tube array and treatment
A total of 21 cases of patients were successful in
terms of insertion of the nasogastric decompression tube to the
jejunum position on the first attempt, with the operation time
lasting 12–86 min, averaging 29 min. For 1 case, insertion of the
decompression tube was successful 1 day after stomach intestinal
decompression. After 10 h to 1 week from tube decompression
(average ~2.2 days), all patients had lessened symptoms of
abdominal distension, abdominal pain and vomiting, and tube
drainage liquid volume was 350–3,650 ml (average 1,280 ml) per day
within 1 week. After 1 week of decompression, plain film of the
abdomen (in decubitus position) showed that 17 patients had lost
liquid-gas levels in the intestinal cavity, and did not have
obvious intestine expansion, with insertion depth 1.6–2.9 m
(average ~2.2 m). Among the 17 patients, 12 patients had the
ability to exhaust and a small amount of excrement to exit the
body. The 17 patients were prescribed special enteral nutrition
through the mouth with the tube, and 2 patients underwent surgical
small intestine stoma treatment and we removed the tubes after 5
weeks when their symptoms related to intestinal obstruction abated.
As for the other patients, 5 had lessened symptoms related to
intestinal obstruction but still had air-fluid level at the distal
intestinal tube, 4 patients were not suitable for enteral nutrition
since the obstruction position was ~1 m from the jejunum, 1 patient
carried out the special-prescription enteral nutrition through tube
feeding but insufficient nutrition was supported by parenteral
nutrition. None of the patients had complications related to the
operation such as alimentary tract hemorrhage or piercing.
Selective small intestine
opacification
For all the 22 patients, the anterior part of the
tube stopped moving forward to the deep part of small intestine
after several adjustments, and after 24 h, contrast medium was
injected through the tube to carry out intestinal opacification.
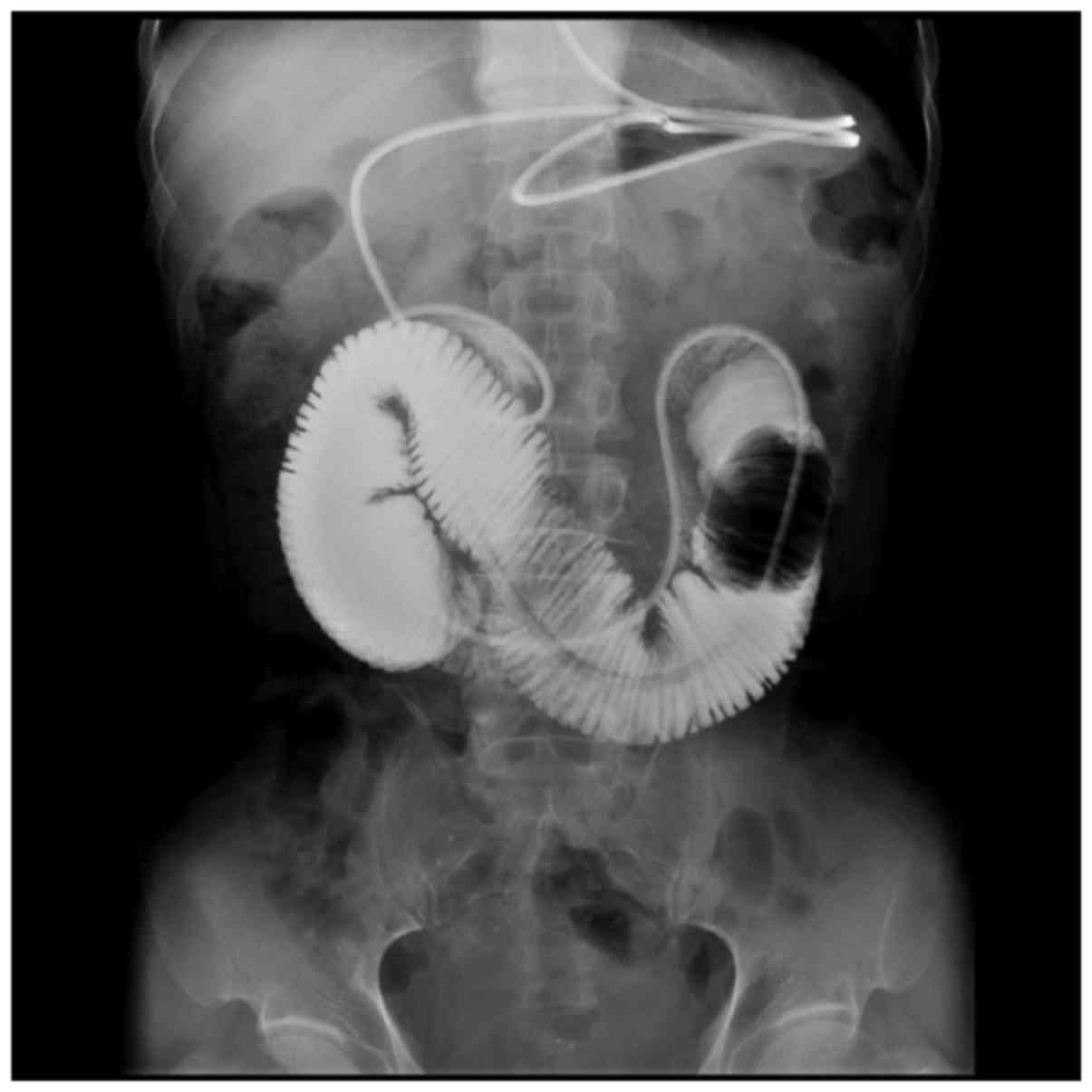
Opacification of 11 patients showed that the small intestine canal
was obstructed at the anterior of the decompression end, as
contrast medium cannot pass the obstructed segment of the
intestinal canal, and the proximal intestinal canal expands
(Fig. 1). Opacification of the 8
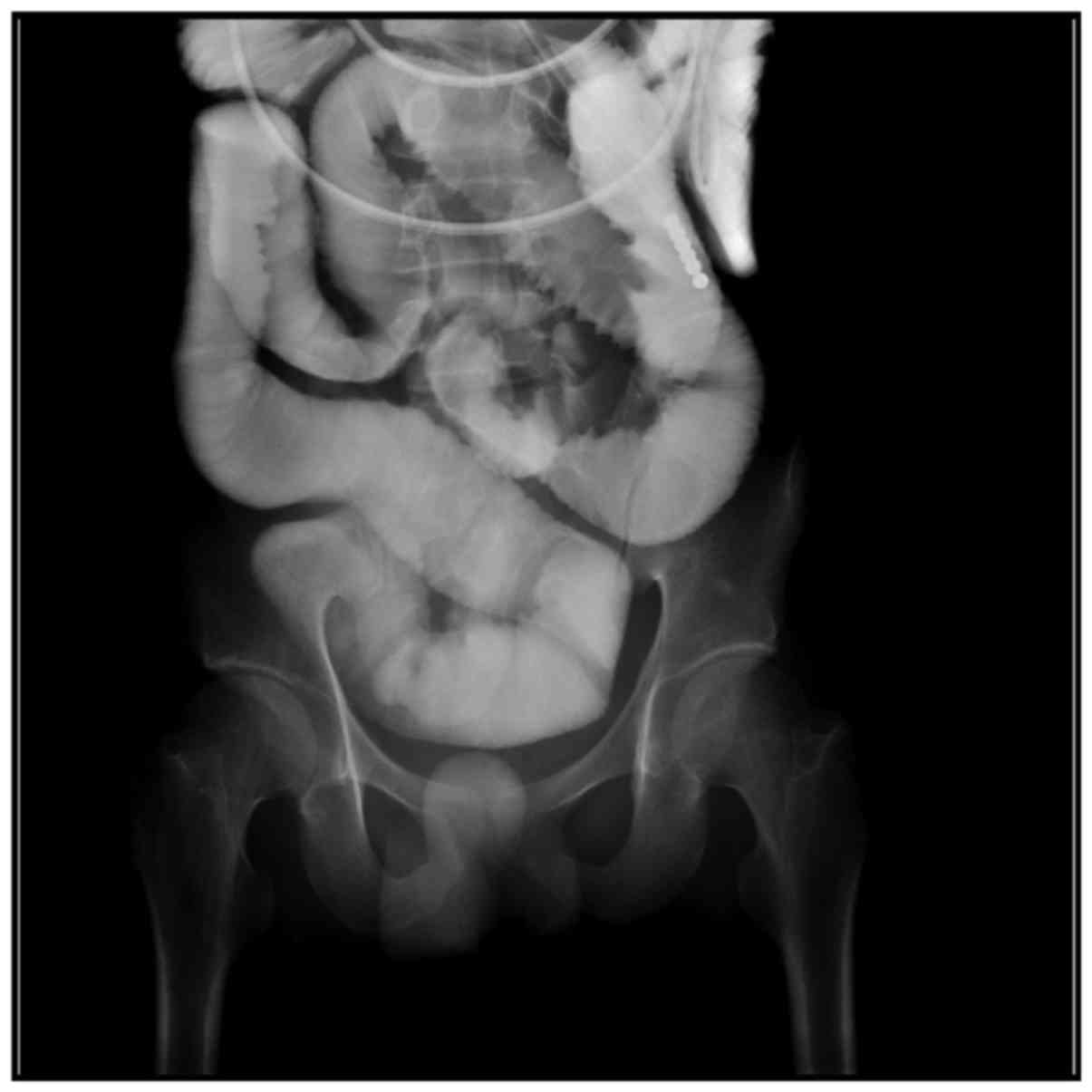
patients showed that contrast medium slowly enters the intestinal
canal at the distal tube, but it cannot clearly show the condition
of a narrowed intestinal canal segment because of the overlap of
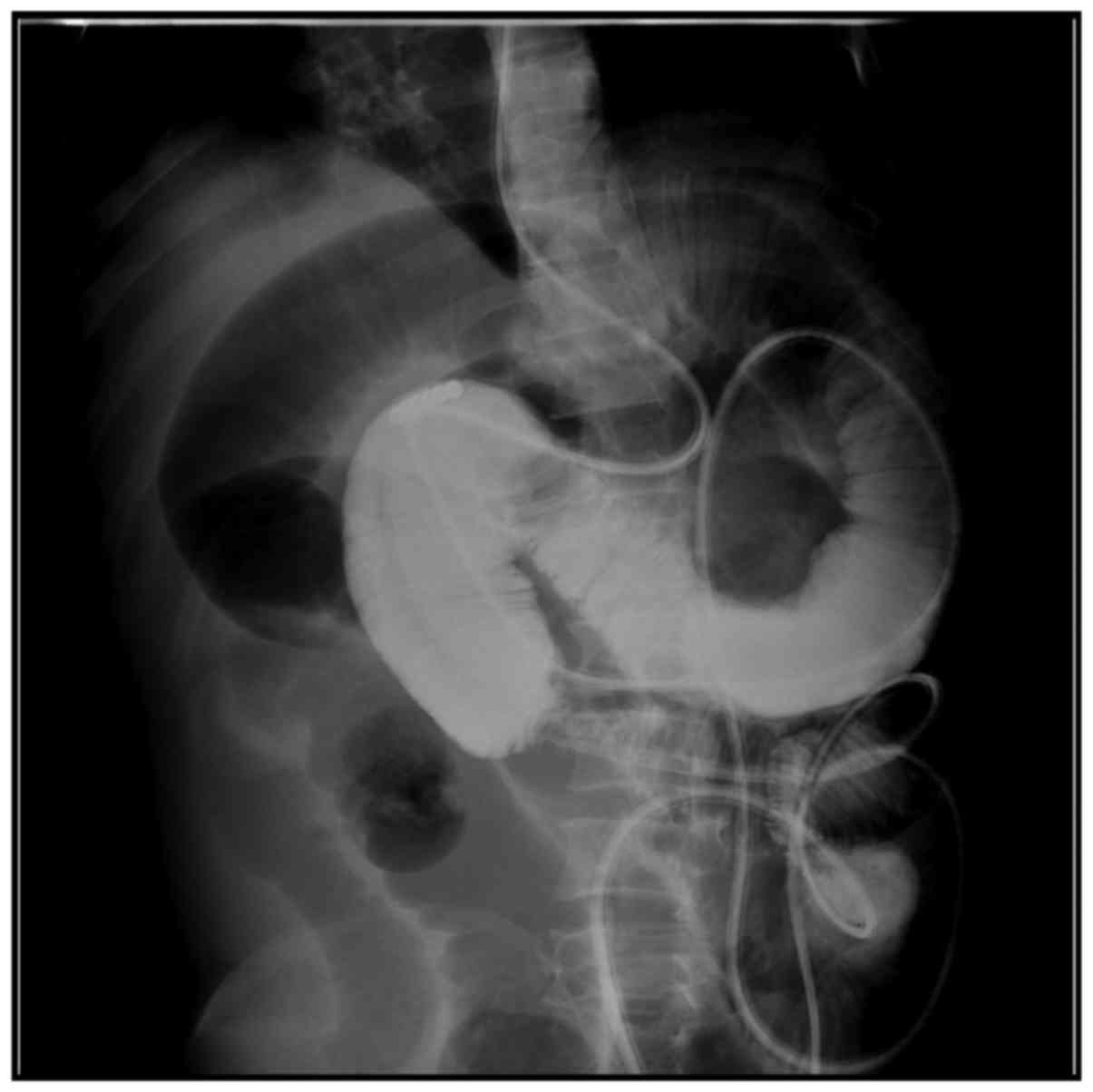
the intestinal canal (Fig. 2), among
which 3 patients had expansion of the distal intestinal canal
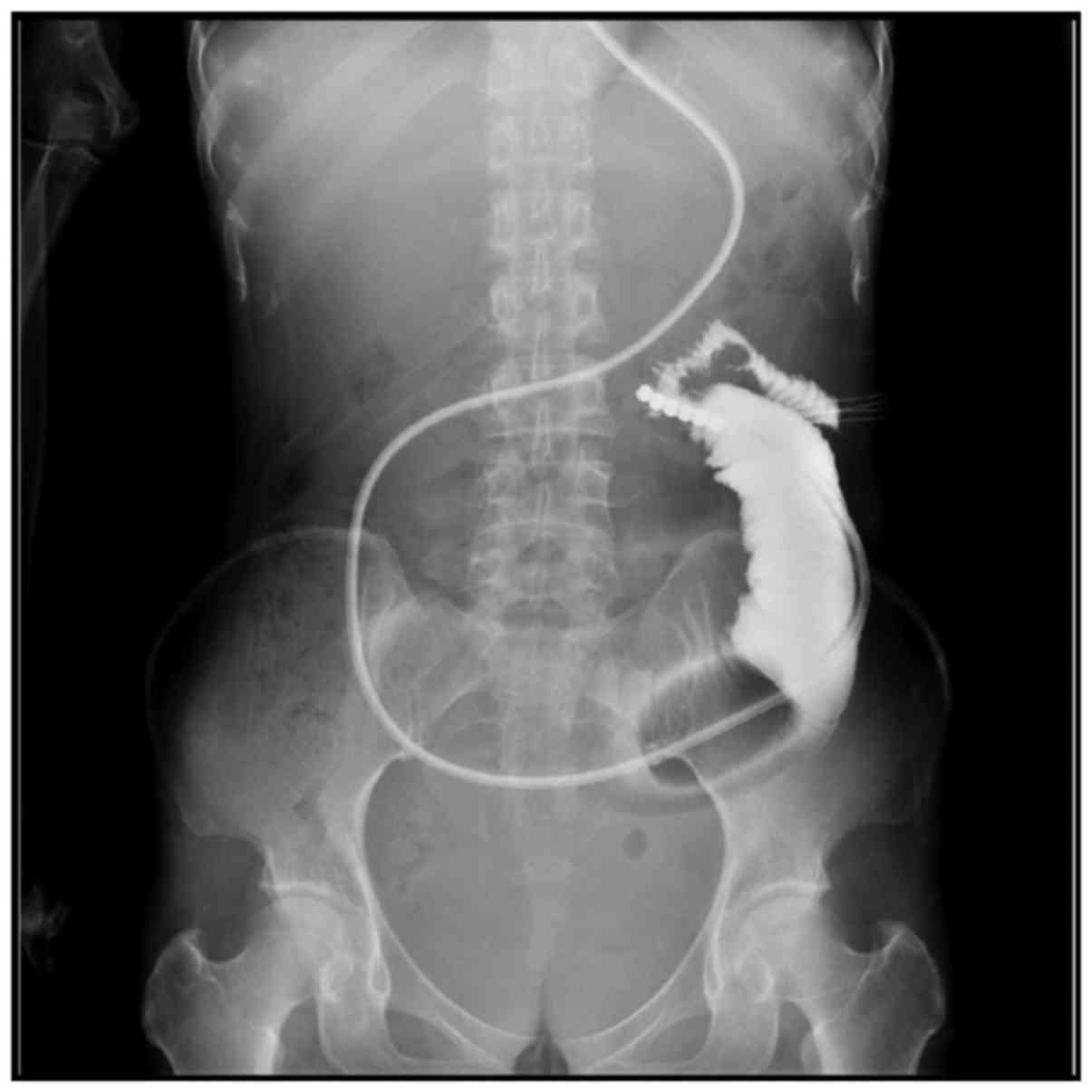
(Fig. 3). Opacification of the 3
patients showed that the small intestine at the distal end had
localized intestinal canal stenosis and the border was irregular,
which was considered a result of tumor invasion (Fig. 4). Of the 22 patients, 4 were able to
receive enteral nutrition because the position of the obstruction
was ~1 m away from the jejunum.
Application of small intestine
decompression combined with eating through the mouth
Among the 22 patients, 18 patients underwent enteral
nutrition at the same time as small intestine decompression, of
which 6 patients had moderate abdominal distension at the beginning
of oral enteral nutrition and expressed tolerance after proper
adjustments of both the time and amount. A total of 18 patients did
not show abdominal distension, abdominal pain or signs of
peritoneal activation during the treatment period of eating, and
had increased serum albumin and prealbumin without significant
weight loss after 2 weeks of treatment (Table I).
 | Table I.Nutrition index before and after
enteral nutrition. |
Table I.
Nutrition index before and after
enteral nutrition.
| Characteristics | Albumin, g/l | Prealbumin, mg/l | Body weight, kg |
|---|
| Before treatment | 32.0±2.7 | 34.1±2.3 | 61.2±2.4 |
| After treatment | 238±27.4 | 316.0±28.9 | 60.8±5.3 |
| P-value | P<0.05 | P<0.05 | P<0.05 |
Discussion
The increase of tumor incidence year by year leads
to increased incidence of malignant intestinal obstructions. For
malignant intestinal obstructions whereby the obstruction and
origin cannot be removed by surgery, emesis, abdominal pain and
abdominal distension occurs in patients, while they are afflicted
with the incapacity to eat. Presently, the principles and
prescription of nutrition for patients with such tumors remains the
common means of nutritional support for patients with common or
more serious gastrointestinal problems. Patients with
gastrointestinal function prefer enteral nutrition, while patients
with gastrointestinal function insufficiency or disability prefer
parenteral nutrition (4,5). It is recommended to combine enteral
nutrition and parenteral nutrition. Maintainance of the intestinal
endometrium barrier and immune function occurs by enteral
nutrition, while parenteral nutrition provides energy and
nutritional substrates. Small intestinal obstruction is a common
complication in patients with middle and late stage malignant
tumors, and may endanger life if not treated in time. In treatment,
not only is the prompt removal of intestinal obstruction needed,
but consideration must be given to diagnosis of the cause of the
obstruction and a nutrition support plan, to give advantage to
later treatment. Mortality 30 days after late period tumor
intestinal obstruction surgery is ~9–40%, and the complication
incidence is 99.0%. Therefore, late stage tumor causing mechanical
intestinal obstruction surgery can only benefit certain patients,
including those with mechanical intestinal obstruction caused by
fibrosis adhesion, localized tumor solitary obstruction and
chemotherapy insensitive tumor bearing patients, thus it is not
appropriate to commonly choose surgical treatment.
After the occurrence of malignant small intestinal
obstruction, a series of pathological and physiological changes
appear, partially in the intestine and throughout the body. The
most important pathophysiological consequences are edema of the
intestinal dissepiment and localized tumor, and the damage caused
by liquid secretion-absorption equilibrium in the intestinal canal.
Localized intestinal canal stenosis causes continuous uncoordinated
peristalsis and thus aggravates the intestine by expansion of the
proximal end of the obstruction, and leads to an increase of
intestinal cavity internal pressure, which results in intestinal
mucous membrane ischemia, anoxia and intestinal wall blood
transportation disability, and finally leads to intestinal wall
necrosis and piercing. Hence the key to treatment is to reduce
internal pressure of the intestinal cavity and efficiently drain
intestinal contents, to improve intestinal wall blood
transportation. Traditional nasogastric tube decompression can only
decompress and drain the gastral cavity, and has poor effects on
small intestine content drainage, thus is cannot reach the
requirement of clinical treatment. We inserted 3-m small intestine
decompression tubes to expand the small intestine and carry out
whole range decompression and drainage, to treat intestinal
obstruction (6). We also injected
contrast medium through the decompression tube to the small
intestine to carry out selective small intestinal opacification,
which can assist the diagnosis and treatment.
All 22 patients in the group could drain gas and
liquid (average 1,280 ml) from the small intestine per day after
insertion of the nasogastric decompression tube. After 10 h to 1
week (average ~2.2 days) of tube decompression, all patients were
relieved of their symptoms including abdominal pain, abdominal
distension and vomiting. This demonstrates the effectiveness of
using nasogastric decompression tubes to treat small intestinal
obstruction. Small intestine opacification all showed positive
results (11 patients showed that small intestine canal is
obstructed at the anterior of decompression end, contrast medium
cannot pass the obstructed intestinal canal segment, and the
proximal intestinal canal expands. Opacification of 8 patients
showed that contrast medium slowly entered the intestinal canal at
the distal tube, among which 3 patients still had an expanded
distal intestinal canal. Opacification of 3 patients showed that
the small intestine at distal tube had localized stenosis and the
border was irregular. These results are all significantly improved
over regular nasogastric tube drainage. There are also multiple
studies reporting the superiority of decompression tubes in aspects
of decompression and drainage (7,8).
Traditionally, enteral nutrition treatment is only
started after full recovery of gastrointestinal function, that is
the ability to pass flatus. Before the full recovery of
gastrointestinal function, complete fasting is needed and patients
are given total parenteral alimentation. However, an obvious
weakness of this approach is that long fasting periods can cause
intestinal mucous membrane atrophy, damage of barrier function,
displacement of bacteria, and requires expensive long-term use of
parenteral nutrition. Therefore, it is worthy of attention to carry
out predigested short peptide enteral nutrition under the
protection of small intestine compression and drainage, using
anterior intestinal canal segment, having absorption and digestion
function (9). Under the circumstance
of having drainage tubes in the small intestine, 18 patients in the
group had increased serum albumin, prealbumin without significant
reduction of body weight after orally taking predigested short
peptide nutrition, highlighting the value of eating and drawing at
the same time when suffering from middle and late neoplasm related
small intestinal obstructions. According to our experience, we
consider patients clearly diagnosed with malignant neoplasm induced
intestinal obstruction and accompanied by the following conditions
are suitable for treatment with eating and drawing at the same
time: i) Verified widespread metastasis in the abdominal cavity by
iconography check, ii) magnanimous ascites that recurs after
drainage, iii) open necrosectomy and certified that further surgery
cannot be carried out, iv) previous abdominal surgery demonstrating
widespread metastasis, v) widespread abdominal contents, vi)
uncontrolled symptoms caused by outer abdominal cavity metastasis,
vii). previous abdominal cavity or cavitas pelvis radiotherapy, and
viii) poor common condition and advanced age.
To summarize, the comprehensive treatment based on
small intestine decompression combined with enteral nutrition is
worthy of clinical application.
Acknowledgements
This study was supported by the Science and
Technology Bureau topic (Xuzhou, Jiangsu, China), no. XZZD1353.
References
|
1
|
Beltran MA and Cruces KS: Primary tumors
of jejunum and ileum as a cause of intestinal obstruction: A case
control study. Int J Surg. 5:183–191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roeland E and von Gunten CF: Current
concepts in malignant bowel obstruction management. Curr Oncol Rep.
11:298–303. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abdulzhalilov MK: Ways of raising efficacy
of nasointestinal drainage in patients with intestinal obstruction
and general peritonitis. Khirurgiia (Mosk). 4:39–41. 2003.(In
Russian).
|
|
4
|
Eren T, Bayraktar B, Celik Y, Boluk S and
Adali G: Acute malignant intestinal obstruction accompanied by
synchronous multifocal intestinal cancer in Peutz-Jeghers syndrome:
Report of a case. Surg Today. 42:1125–1129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li J: Rationality of the preference to
enteral nutrition. J Parenter Enteral Nutr. 20:321–322. 2013.
|
|
6
|
Toskin KD and Pak AN: Use of tube
decompression enterography in the diagnosis of acute obstruction of
the small intestine. Klin Khir. 2:22–24. 1988.(In Russian).
|
|
7
|
Yao HW, Fu W, Wang DC, Yuan J, Zhang TL
and Xiu DR: Long naso-intestinal tube decompression versus
octreotide in the treatment of early post-operative inflammatory
ileus. Zhonghua Wai Ke Za Zhi. 48:564–568. 2010.(In Chinese).
PubMed/NCBI
|
|
8
|
Li de C, Li RH and Tian Q: Efficacy of
intestinal decompression with long nasointestinal tube and
selective contrast radiography in the treatment of small bowel
obstruction in elderly patients. Minerva Chir. 71:85–90.
2016.PubMed/NCBI
|
|
9
|
Zhang L, Gong JF, Ni L, Chen QY, Guo Z,
Zhu WM, Li N and Li JS: Influence of preoperative nutritional
support on surgical outcomes of chronic radiation enteritis
patients complicated with intestinal obstruction. Zhonghua Wei
Chang Wai Ke Za Zhi. 16:340–344. 2013.(In Chinese). PubMed/NCBI
|