Introduction
Acute myeloid leukemia (AML) is a heterogeneous
group of neoplastic hematologic disorders characterized by
differentiation arrest of hematopoiesis, which leads to
uncontrolled proliferation and accumulation of clonal cells that
are blocked at various steps of maturation (1,2). The main
clinical symptoms of AML are anemia, hemorrhage, fever,
lymphadenectasis and musculoskeletal pain (3). AML accounts for 3% of total incidences
of malignant tumors. There are ~257,000 mortalities from leukemia
annually worldwide (4). Despite
considerable advances, chemotherapy remains the primary therapeutic
approach for the treatment of leukemia, the prognosis remains poor
and the 5-year survival rate has remained at 15–30% since the 1970s
(2,5,6). It has
been reported that genetic alterations are involved in
leukemogenesis (7,8), thus manipulating myeloid
maturation-associated genes may lead to potential therapies
(9,10). Therefore, investigating the underlying
molecular mechanisms is important for a comprehensive study of the
progression of leukemia and for the identification of novel
candidate targets for therapy.
Raf kinase trapping to Golgi (RKTG) is a member of
progestin and adipoQ receptor family and also a membrane protein of
the Golgi apparatus. Previous studies have reported that RKTG
functions as a spatial regulator of Raf kinase by binding it to the
Golgi apparatus. Therefore, RKTG has an inhibitory effect on
Ras/Raf/mitogen-activated protein kinase kinase (MEK)/extracellular
signal-regulated kinase (ERK) signaling (11,12). The
Ras/Raf/MEK/ERK signaling pathway is responsible for the
extracellular signal transduction into the nucleus, therefore it
has a pivotal role in regulation of fundamental cellular functions,
including cell proliferation, apoptosis, metabolism and
differentiation (13). Studies have
demonstrated that RKTG can act as a tumor suppressor in human
malignant melanoma cells (12), renal
cell carcinoma (14), colorectal
cancer (15), laryngeal squamous cell
carcinoma (16) and osteosarcoma
(17). Deletion of RKTG markedly
increased the incidence of chemical carcinogen-induced
tumorigenesis in the mouse skin (14). However, to the best of our knowledge,
whether RKTG is implicated in the progress of leukemia remains to
be investigated.
In the present study, RKTG was exogenously expressed
in the human leukemia cell line U937. The present study
demonstrated for the first time to the best of our knowledge that
RKTG is able to markedly impair cell viability and induce apoptosis
of U937 cells. Furthermore, RKTG overexpression suppressed the
activation of the ERK and phosphoinositide 3-kinase (PI3K)/AKT
signaling pathways in the U937 cell line. In summary, the present
study uncovered a tumor-suppressive effect of RKTG in leukemia cell
lines.
Materials and methods
Cell culture
Human leukemia cell line U937 was obtained from the
Cell Bank of Type Culture Collection Center of the Chinese Academy
of Science (Shanghai, China). Cells were cultured with RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (HyClone; GE Healthcare Life
Sciences, Logan, UT, USA), 100 U/ml penicillin and 100 µg/ml
streptomycin at 37°C in a humidified incubator with 5% CO2.
Plasmid construction and cell
transfection
The coding sequence of RKTG was amplified by
quantitative polymerase chain reaction (qPCR) using a forward
primer, 5′-CGCAAGCTTATGCATCAGAAGCTGCTGA-3′ and a reverse primer,
5′-CACGCTCGAGTCAAACAAGCAAATACAGGT-3′. The products were double
digested by HindIII/XhoI restriction enzymes (Fermentas; Thermo
Fisher Scientific, Inc., Pittsburgh, PA, USA) and inserted into the
pcDNA3.1 plasmid by using UltraPower pUM-T fast clone kit (BioTek
China, Beijing, China). Positive clones were screened by 100 µg/ml
ampicillin and identified by automated sequencing. For
transfection, U937 cells were seeded into 6-well plates at a
density of 5×105 cells/well and grown to 70–80%
confluence. The U937 cells were subsequently transfected with
pcDNA3.1 vector or pcDNA3.1-RKTG using Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. G418 (600 µg/ml) was added to the medium
48 h following transfection, and the stable transfected clones were
selected for 2 weeks. Finally, RKTG expression in the stable
transfected cell line was examined by qPCR and western
blotting.
Reverse transcription (RT)-qPCR
Total RNA of the cells was extracted using RNApure
Rapid Total RNA Extraction kit (BioTek China) and reverse
transcribed by Super M-MLV Reverse Transcriptase (BioTek China).
SYBR-Green-based qPCR was performed by using 2X Power Taq PCR
MasterMix (Beijing Solarbio Science and Technology Co., Ltd.,
Beijing, China) with β-actin employed as an internal control. The
sequences of the primers used were as follows: RKTG forward,
5′-GCTTTGCTCT-GTGGGCTAT-3′ and reverse, 5′-TGCGTGAGGTAATTGGGAT-3′;
and β-actin forward, 5′-CTT-AGTTGCGTTACACCCTTTCTTG-3′ and reverse,
5′-CTGTCACCTTCACCGTTCCAGTTT-3′. The full PCR cycling conditions
were as follows: 95°C 10 min; 95°C 10 sec, 60°C 20 sec, 72°C 30
sec, for 40 cycles. The levels of RKTG were normalized to the
levels of β-actin using the 2−ΔΔCt method, as previously
described (18).
Western blotting
Cells from the U937 group, pcDNA3.1 transfected
group and RKTG transfected group were lysed by
radioimmunoprecipitation assay buffer solution (Beyotime Institute
of Biotechnology, Haimen, China). The concentration of total
protein was measured using BCA protein assay kit (Beyotime
Institute of Biotechnology). A total of 40 µg protein was subjected
to 5, 10 or 13% SDS-PAGE and transferred onto polyvinylidene
fluoride membranes (EMD Millipore, Billerica, MA, USA). Following
blocking with 5% (m/v) fat-free milk, the membranes were incubated
at 4°C overnight with primary antibodies: Anti-RKTG (1:200;
sc-161992; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-cleaved caspase 3 (1:1,000; ab2302; Abcam, Cambridge, MA,
USA), anti-B-cell lymphoma 2 (anti-Bcl-2; 1:400; BA0412; Wuhan
Boster Biological Technology, Ltd., Wuhan, China),
anti-Bcl-2-associated X, apoptosis regulator (anti-Bax; BA0315;
1:400; Wuhan Boster Biological Technology, Ltd.),
anti-phosphorylated (p)-mitogen-activated protein kinase 1
(anti-p-ERK; 1:500; bs-1522R; BIOSS, Beijing, China), anti-ERK
(1:500; bs-2637R; BIOSS), anti-p-AKT serine/threonine kinase 1
(anti-p-AKT; 1:200; sc-135651; Santa Cruz Biotechnology, Inc.),
anti-AKT (1:200; sc-8312; Santa Cruz Biotechnology, Inc.),
anti-p-glycogen synthase kinase-3β (anti-p-GSK-3β; sc-11757; 1:200;
Santa Cruz Biotechnology, Inc.) or anti-GSK-3β (1:200; sc-9166;
Santa Cruz Biotechnology, Inc.). Specific bands were detected by
horseradish peroxidase conjugated goat anti-rabbit (1:5,000; A0208;
Beyotime Institute of Biotechnology) or goat anti-mouse IgG
(1:5,000; A0216; Beyotime Institute of Biotechnology) at 37°C for
45 min and visualized using enhanced chemiluminescence (ECL) kit
(Qihai Biotechnology, Shanghai, China).
Cell-counting kit-8 (CCK-8) assay
Cells from the U937 group, pcDNA3.1 transfected
group and RKTG transfected group were seeded in 96-well plates
(2×103 cells/well) and cultured in a humidified
atmosphere (5% CO2) at 37°C. The CCK-8 solution (Beyotime Institute
of Biotechnology) was added at 0, 24, 48, 72 and 96 h prior to an
additional 1 h incubation. The optical density values at 450 nm
were subsequently measured with a microplate reader (ELx-800;
BioTek Instruments, Inc., Winooski, VT, USA).
Flow cytometry
For cell cycle analysis, 1×106 cells from
each group were fixed by 70% ethanol at 4°C for 2 h. Following
washing with PBS twice, cells were re-suspended in 500 µl staining
buffer and incubated with 25 µl propidium iodide (PI) (Wanleibio,
Shenyang, China) at 37°C for 30 min in the dark. Apoptotic cells
were assessed by Annexin V-fluorescein isothiocyanate (FITC)/PI
double-staining cell apoptosis kit (Nanjing KeyGen Biotech Co.,
Ltd., Nanjing, China) according to the manufacturer's instructions.
Briefly, the cells were stained with 5 µl Annexin V-FITC and 5 µl
PI for 15 min at room temperature in the dark. The proportion of
cells in each cell cycle phase and apoptotic state was measured by
flow cytometry (BD Accuri C6; BD Biosciences, Franklin Lakes, NJ,
USA). Data were processed using matched Accuri CFlow Plus software
(version 1.0.1727; BD Accuri Cytometers Inc., MI, USA).
Hoechst staining
Hoechst staining was performed using a Hoechst 33258
staining kit (Wanleibio). Briefly, cells from each group were
plated in a 12-well plate with a coverslip at a density of
2×104 cells/well and grown at 37°C for 24 h. Following
fixation with 4% paraformaldehyde for 20 min at room temperature,
cells were stained with 2 µl/ml Hoechst staining solution and
washed with phosphate buffer solution two times. The slides were
mounted and apoptotic nuclei were observed under a fluorescence
microscope (IX53; Olympus Corporation, Tokyo, Japan).
Statistical analysis
Statistical analysis was performed by SPSS version
16.0 software (SPSS, Inc., Chicago, IL, USA). Results are expressed
as the mean ± standard deviation. Differences between groups were
compared by one-way analysis of variance followed by the least
significant difference post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
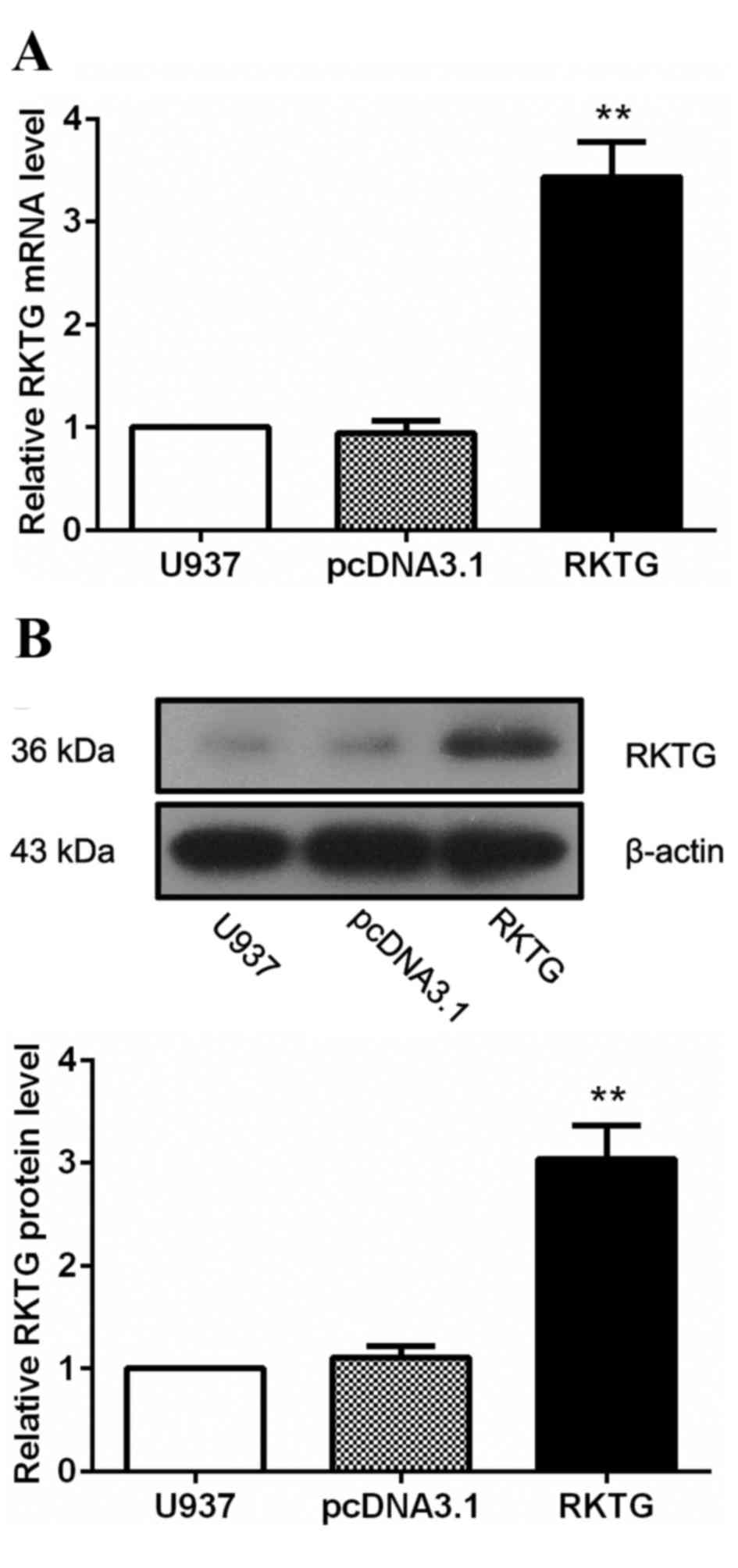
Establishment of RKTG overexpressing
human leukemia cell line
To investigate the biological function of RKTG in
the progression of leukemia, human leukemia cell line U937 was
transfected either with pcDNA3.1-RKTG that contained the
full-length coding sequence of RKTG or the vector pcDNA3.1, which
served as a negative control. The G418-resistant clones were
subsequently selected, and the expression of RKTG was determined by
RT-qPCR and western blotting. As expected, in cells transfected
with pcDNA3.1-RKTG, the level of RKTG mRNA was significantly
upregulated compared with the pcDNA3.1-transfected group (3.43±0.35
vs. 0.95±0.12; P<0.01; Fig. 1A).
In addition, the protein level of RKTG was also significantly
increased following transfection with pcDNA3.1-RKTG compared with
the pcDNA3.1-transfected group (3.04±0.33 vs. 1.10±0.11; P<0.01;
Fig. 1B). The RKTG overexpressing
leukemia cell line was subsequently maintained in culture medium
containing 300 µg/ml G418 for further investigation.
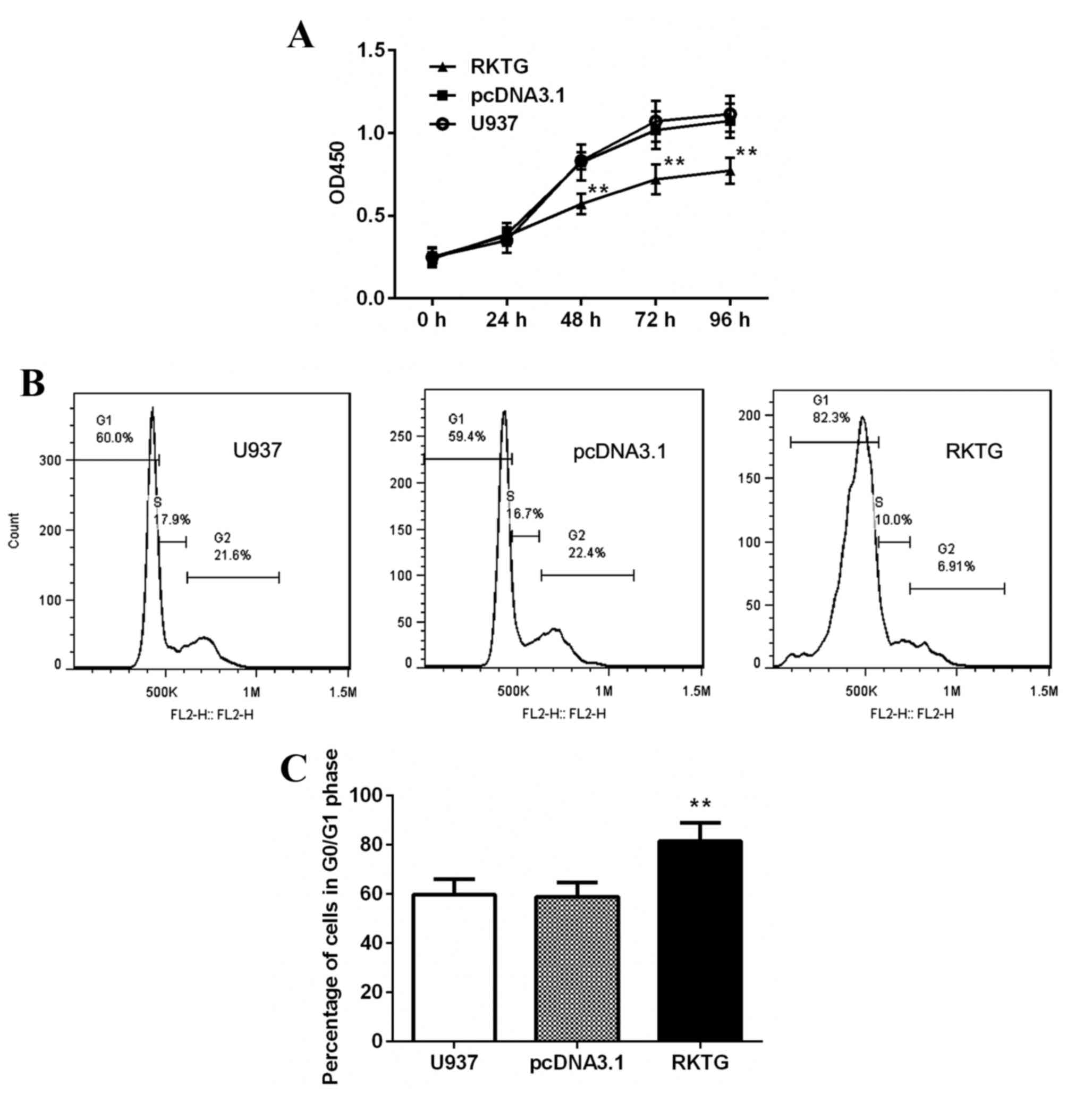
Overexpression of RKTG suppresses
proliferation of human leukemia cells
To evaluate the effect of RKTG on leukemia cell
growth, proliferation rate and cell cycle was analyzed by CCK-8
assay and flow cytometry. As shown in Fig. 2A, compared with the control group,
exogenous expression of RKTG significantly inhibited the
proliferation of leukemia cells at 48, 72 and 96 h (P<0.01).
Additionally, overexpression of RKTG significantly increased the
percentage of G0/G1 phase cells (81.43±7.44% vs. 58.87±5.82%;
P<0.05; Fig. 2B and C). However,
the proportion of cells in G2/M phase was significantly reduced
when compared with the pcDNA3.1 transfected group (7.24±5.21% vs.
23.73±2.57%; P<0.01; Fig. 2B and
C). Collectively, these results indicate that RKTG
overexpression induced cell cycle arrest and inhibited
proliferation of human leukemia cells.
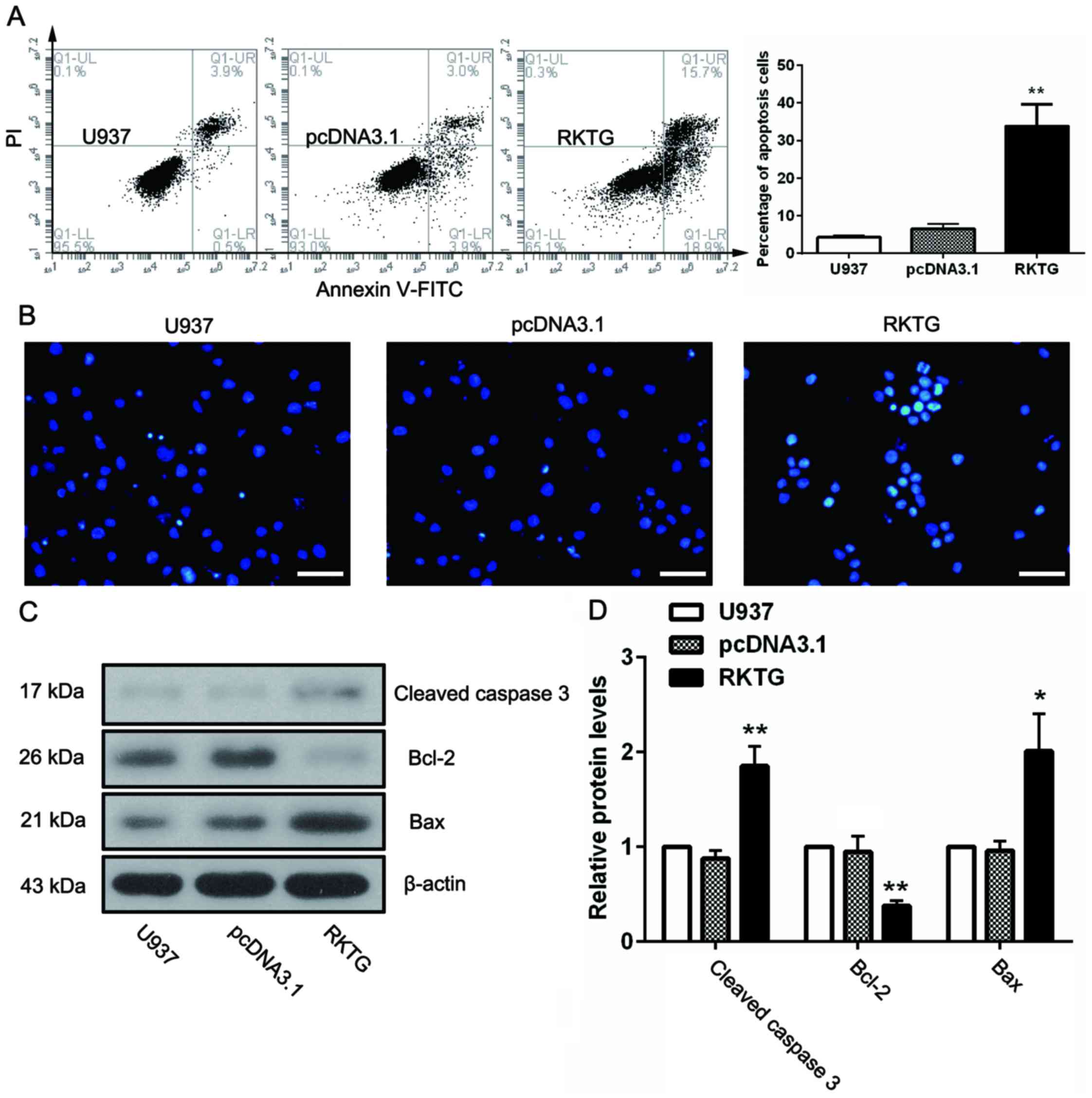
Overexpression of RKTG induces
apoptosis of human leukemia cells
To investigate the role of RKTG in cell apoptosis,
U937 cells from each experimental group were stained with Annexin
V-FITC and PI. The cells were subsequently subjected to flow
cytometry. The results demonstrated that overexpression of RKTG in
leukemia cells resulted in a marked increase in the rate of
apoptosis as compared with the negative control (33.80±5.81% vs.
6.54±1.22%; P<0.01; Fig. 3A). This
finding was further verified by Hoechst staining, (Fig. 3B). Apoptotic nuclei were observed in
the cells overexpressing RKTG, which were characterized by
brilliant blue staining of the apoptotic bodies, but not in the
other groups. Furthermore, several apoptosis-associated proteins
were also examined, as shown in Fig. 3C
and D. When compared with pcDNA3.1 group, the level of
anti-apoptotic protein Bcl-2 was markedly decreased (P<0.01),
whilst the levels of proapoptotic protein cleaved caspase 3 and Bax
were significantly upregulated by RKTG overexpression (P<0.01,
P<0.05).
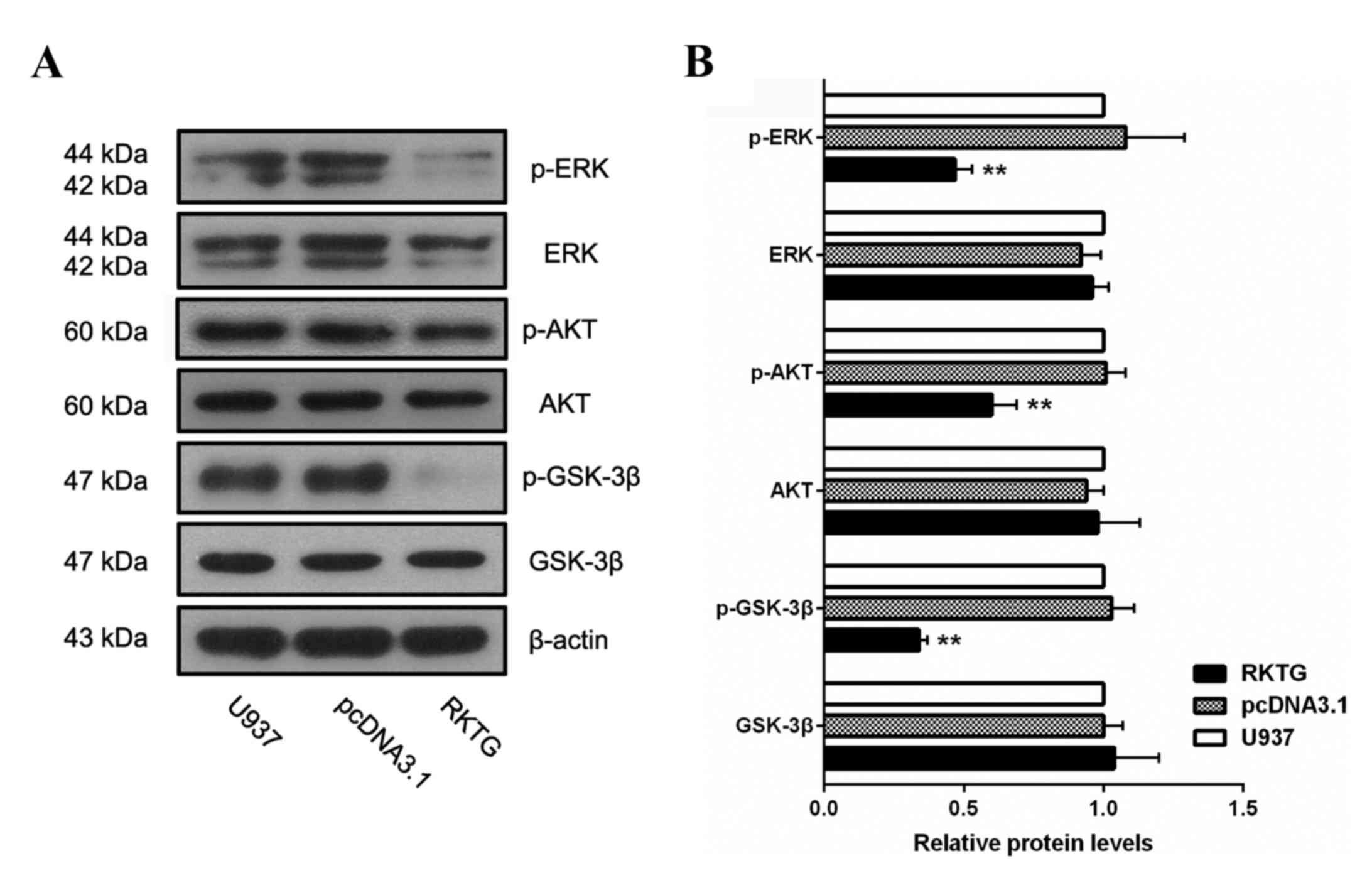
Overexpression of RKTG suppresses ERK
and PI3K/AKT signaling in human leukemia cells
To assess whether ERK or PI3K/AKT signaling pathways
are implicated in the RKTG-associated anticancer effect in leukemia
cells, the expression of the key components of these pathways was
measured by western blotting. The results demonstrated that the
levels of p-ERK, p-AKT and p-GSK-3β were all significantly reduced
in RKTG overexpression cells when compared with the cells
transfected with the vector alone (0.47±0.06 vs. 1.08±0.21;
P<0.01; 0.52±0.07 vs. 1.01±0.06; P<0.01; 0.34±0.03 vs.
1.03±0.8; P<0.05; Fig. 4).
Therefore, exogenous expression of RKTG suppressed the activation
of ERK and PI3K/AKT signaling pathways in leukemia cells, which may
be a molecular mechanism for how RKTG inhibits proliferation and
induces apoptosis of leukemia cells.
Discussion
RKTG has been reported to be closely associated with
the progression of different types of malignant cancer cells
(14,19). However, the role of RKTG in the
proliferation and apoptosis of leukemia cells remains to be fully
elucidated. In the present study, the exogenous overexpression of
RKTG significantly inhibited cell proliferation, blocked cell cycle
progression, induced apoptosis and suppressed the activation of ERK
and PI3K/AKT signaling pathways in leukemia cells. These results
have provided evidence that RKTG may be a tumor suppressor in human
leukemia cells.
RKTG is a spatial regulator of Raf-1 (11) and acts as an anticancer factor due to
its inhibitory activity on Raf/MEK/ERK signaling (12,14,20). RKTG
expression in the colorectal cancer samples was significantly
reduced compared with adjacent normal tissues. Furthermore, RKTG
expression was inversely correlated with the tumor grade in
colorectal cancer (15). The
proliferation rate of colon cancer cells was inhibited by RKTG
overexpression. By contrast, lentiviral-mediated knockdown of RKTG
markedly increased the proliferation rate of colon cancer cells
(15). In addition, overexpression of
RKTG in A375 melanoma cells resulted in a reduction in growth rate
and colony-forming activity (12). In
accordance with these studies, the present study demonstrated that
exogenous expression of RKTG suppressed the proliferation of human
leukemia cells. RKTG overexpression resulted in an increase in the
number of cells in the G0/G1 phase. Therefore, overexpression of
RKTG may inhibit leukemia cell proliferation by arresting the cell
cycle in G0/G1 phase.
Xie et al (20)
reported that, when treated with chemical carcinogens, the number
of apoptotic cells in RKTG-deficient mice was significantly reduced
compared with the number of cells in wild-type mice. The apoptotic
status of RKTG overexpressing leukemia cells was also examined in
the present study. The results demonstrated that upregulation of
RKTG markedly promoted the apoptosis of leukemia cells. To further
elucidate the potential mechanisms of RKTG-mediated apoptosis, the
level of apoptosis-associated proteins was also determined.
Caspase-3 is a pivotal executor of apoptosis. The activation and
cleavage of caspase-3 is a marker of irreversible apoptosis
(21). The antiapoptotic protein
Bcl-2 and proapoptotic protein Bax are key regulators of
mitochondrial-mediated apoptosis (22). In the present study, exogenous
overexpression of RKTG strongly increased the levels of cleaved
caspase 3 and Bax, and decreased the expression of Bcl-2,
suggesting that RKTG is able to induce apoptosis in leukemia
cells.
The activation of intracellular signaling pathways
is a critical determinant of the biological outcome of cancer cells
(23). Aberrant upregulation of the
Raf/MEK/ERK and PI3K/AKT pathways is frequently observed in
leukemia patients, and these changes are closely associated with
poor prognosis (24–27). The inhibitory activity of RKTG on
Raf/MEK/ERK signaling has been reported in several tumor cell lines
(11,12,14,15,20).
Additionally, the activation of the PI3K/AKT signaling pathway
induced by Gβlγ2 overexpression in monkey kidney fibroblast cells
COS7 has been previously reported to be markedly abrogated by RKTG
co-overexpression (28). The present
study demonstrated that upregulation of RKTG significantly
suppressed the activation of ERK and PI3K/AKT signaling pathways.
Since the blockade of ERK or PI3K/AKT pathways is able to induce
apoptosis and increase drug sensitivity of leukemia cells (29,30), the
present authors speculate that RKTG may inhibit proliferation and
induce apoptosis of leukemia cells, partly by the suppression of
ERK and PI3K/AKT signaling pathways.
In summary, the results of the present study
demonstrate that overexpression of RKTG is able to inhibit cell
proliferation, induce cell cycle arrest and cause apoptosis of
leukemia cells. These effects of RKTG in leukemia cells may be
associated with the inhibition of ERK and activation of PI3K/AKT
signaling. These findings indicate that overexpression of RKTG may
serve as a promising strategy for the treatment of leukemia.
References
|
1
|
Estey E and Döhner H: Acute myeloid
leukaemia. Lancet. 368:1894–1907. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smith M, Barnett M, Bassan R, Gatta G,
Tondini C and Kern W: Adult acute myeloid leukaemia. Crit Rev Oncol
Hematol. 50:197–222. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lowenberg B, Downing JR and Burnett A:
Acute myeloid leukemia. N Engl J Med. 341:1051–1062. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tallman MS, Gilliland DG and Rowe JM: Drug
therapy for acute myeloid leukemia. Blood. 106:1154–1163. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferrara F: Unanswered questions in acute
myeloid leukaemia. Lancet Oncol. 5:443–450. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Look AT: Oncogenic transcription factors
in the human acute leukemias. Science. 278:1059–1064. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Castilla LH, Garrett L, Adya N, Orlic D,
Dutra A, Anderson S, Owens J, Eckhaus M, Bodine D and Liu PP: The
fusion gene Cbfb-MYH11 blocks myeloid differentiation and
predisposes mice to acute myelomonocytic leukaemia. Nat Genet.
23:144–146. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Falini B, Nicoletti I, Martelli MF and
Mecucci C: Acute myeloid leukemia carrying cytoplasmic/mutated
nucleophosmin (NPMc+ AML): Biologic and clinical features. Blood.
109:874–885. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Christiansen DH, Andersen MK, Desta F and
Pedersen-Bjergaard J: Mutations of genes in the receptor tyrosine
kinase (RTK)/RAS-BRAF signal transduction pathway in
therapy-related myelodysplasia and acute myeloid leukemia.
Leukemia. 19:2232–2240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng L, Xie X, Ding Q, Luo X, He J, Fan F,
Liu W, Wang Z and Chen Y: Spatial regulation of Raf kinase
signaling by RKTG. Proc Natl Acad Sci USA. 104:pp. 14348–14353.
2007; View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan F, Feng L, He J, Wang X, Jiang X,
Zhang Y, Wang Z and Chen Y: RKTG sequesters B-Raf to the Golgi
apparatus and inhibits the proliferation and tumorigenicity of
human malignant melanoma cells. Carcinogenesis. 29:1157–1163. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cano E and Mahadevan LC: Parallel signal
processing among mammalian MAPKs. Trends Biochem Sci. 20:117–122.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Jiang X, Qin X, Ye D, Yi Z, Liu
M, Bai O, Liu W, Xie X, Wang Z, et al: RKTG inhibits angiogenesis
by suppressing MAPK-mediated autocrine VEGF signaling and is
downregulated in clear-cell renal cell carcinoma. Oncogene.
29:5404–5415. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang X, Li X, Fan F, Jiao S, Wang L, Zhu
L, Pan Y, Wu G, Ling ZQ, Fang J and Chen Y: PAQR3 plays a
suppressive role in the tumorigenesis of colorectal cancers.
Carcinogenesis. 33:2228–2235. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu Q, Zhuang K and Li H: PAQR3 plays a
suppressive role in laryngeal squamous cell carcinoma. Tumour Biol.
37:561–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ma Z, Wang Y, Piao T, Li Z, Zhang H, Liu Z
and Liu J: The tumor suppressor role of PAQR3 in osteosarcoma.
Tumour Biol. 36:3319–3324. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ling ZQ, Guo W, Lu XX, Zhu X, Hong LL,
Wang Z, Wang Z and Chen Y: A Golgi-specific protein PAQR3 is
closely associated with the progression, metastasis and prognosis
of human gastric cancers. Ann Oncol. 25:1363–1372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xie X, Zhang Y, Jiang Y, Liu W, Ma H, Wang
Z and Chen Y: Suppressive function of RKTG on chemical
carcinogen-induced skin carcinogenesis in mouse. Carcinogenesis.
29:1632–1638. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Porter AG and Jänicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programmed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marshall CJ: Specificity of receptor
tyrosine kinase signaling: Transient versus sustained extracellular
signal-regulated kinase activation. Cell. 80:179–185. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao S, Konopleva M, Cabreira-Hansen M,
Xie Z, Hu W, Milella M, Estrov Z, Mills GB and Andreeff M:
Inhibition of phosphatidylinositol 3-kinase dephosphorylates BAD
and promotes apoptosis in myeloid leukemias. Leukemia. 18:267–275.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gregorj C, Ricciardi MR, Petrucci MT,
Scerpa MC, De Cave F, Fazi P, Vignetti M, Vitale A, Mancini M,
Cimino G, et al: ERK1/2 phosphorylation is an independent predictor
of complete remission in newly diagnosed adult acute lymphoblastic
leukemia. Blood. 109:5473–5476. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Milella M, Konopleva M, Precupanu CM, Tabe
Y, Ricciardi MR, Gregorj C, Collins SJ, Carter BZ, D'Angelo C,
Petrucci MT, et al: MEK blockade converts AML differentiating
response to retinoids into extensive apoptosis. Blood.
109:2121–2129. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Milella M, Kornblau SM, Estrov Z, Carter
BZ, Lapillonne H, Harris D, Konopleva M, Zhao S, Estey E and
Andreeff M: Therapeutic targeting of the MEK/MAPK signal
transduction module in acute myeloid leukemia. J Clin Invest.
108:851–859. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang Y, Xie X, Zhang Y, Luo X, Wang X,
Fan F, Zheng D, Wang Z and Chen Y: Regulation of G-protein
signaling by RKTG via sequestration of the G betagamma subunit to
the Golgi apparatus. Mol Cell Biol. 30:78–90. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu J, Wong WW, Khosravi F, Minden MD and
Penn LZ: Blocking the Raf/MEK/ERK pathway sensitizes acute
myelogenous leukemia cells to lovastatin-induced apoptosis. Cancer
Res. 64:6461–6468. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Martelli AM, Nyakern M, Tabellini G,
Bortul R, Tazzari PL, Evangelisti C and Cocco L: Phosphoinositide
3-kinase/Akt signaling pathway and its therapeutical implications
for human acute myeloid leukemia. Leukemia. 20:911–928. 2006.
View Article : Google Scholar : PubMed/NCBI
|