Introduction
Renal cell carcinoma (RCC) accounts for 2–3% of all
cancer types (1). Approximately 1/3
of patients exhibit metastatic disease at presentation and
metastatic RCC (mRCC) is highly resistant to chemotherapy (2). Thus, the prognosis of mRCC is poor, with
a 5-year survival rate of <10% (3). Previously, immunotherapy using
interleukin-2 and interferon (IFN)-α was the standard treatment
against mRCC, with a response rate of 10–20% (3). Recently, an improved understanding of
cancer biology has enabled the development of molecularly targeted
agents. Vascular endothelial growth factor (VEGF) and mammalian
target of rapamycin (mTOR) signaling pathways have proven to be
involved in the pathogenesis of mRCC, leading to the development of
tyrosine kinase inhibitors (TKIs) and mTOR inhibitors (4). Sunitinib (SU) is a multi-targeted TKI
that inhibits signaling by VEGF receptors (VEGFRs) 1–3,
platelet-derived growth factor receptors (PDGFRs) α and β, and KIT
proto-oncogene receptor tyrosine kinase (c-Kit) (5). In a previous phase III study,
progression-free survival time was longer and the response rate was
higher in patients with mRCC who received SU compared with that in
patients who received IFN-α (6), and
SU is currently the first-line agent against mRCC. However, SU
treatment is not always successful due to frequent resistance and
severe side effects (7). Combination
therapy is one of the strategies used to solve these problems. The
combinations of SU and IFN-α (8) or
an mTOR inhibitor (9) have been
investigated for the treatment of mRCC; both resulted in failure
due to dose-limiting toxicity. Therefore, the establishment of a
novel combination strategy for mRCC treatment is warranted.
In order to identify a prominent combination agent
for SU, the effects of SU with sodium butyrate (NaBu) was
investigated in the present study. NaBu is a short-chain fatty
acid, which is present in the human gut at a concentration of 2–10
mM (10). In the 1970s, NaBu was
revealed to induce the differentiation of leukemic cells, which was
dependent on histone deacetylase (HDAC) inhibition (11). Furthermore, NaBu was identified to
exhibit anti-tumor activity (12). On
the basis of these observations, clinical trials on NaBu have been
performed on a few patients with acute leukemia. However, minimal
efficacy was observed due to the rapid metabolism of NaBu and weak
HDAC inhibitory activity in vivo (13). Therefore, it is primarily used as a
study tool to elucidate the mechanism underlying the effects of
HDAC inhibitors (HDACIs) and to identify a potential enhancer of
the anticancer effects of existing agents. The effects of HDACIs
are generally pleiotropic, as HDACs modulate the acetylation status
of histones and non-histone proteins (14). For instance, NaBu inhibits the
activity of AKT serine/threonine kinase (Akt) and extracellular
signal regulated kinase (ERK), which are downstream proteins of the
receptor tyrosine kinase (RTK) signaling pathway (15). Additionally, NaBu has been reported to
suppress the transcriptional activity of hypoxia inducible factor
(HIF)-α, which is a major transcription factor of VEGF and PDGF
(16). Thus, it is expected that NaBu
could enhance the anticancer effect of SU, due to its potential for
inhibiting the RTK signaling pathway. This study was designed to
investigate the efficacy of combination therapy with SU and NaBu in
RCC cells.
Materials and methods
Reagents
All reagents were purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany), unless otherwise indicated. SU
was dissolved in dimethyl sulfoxide (DMSO; Wako Pure Chemical
Industries, Ltd., Osaka, Japan) at a concentration of 10 mM and
stored at −20°C. NaBu was dissolved in medium (McCoy's 5A medium or
Dulbecco's modified Eagle's medium; DMEM) prior to use. MTT
(Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was
dissolved in distilled water to achieve a concentration of 5 mg/ml
and stored at −20°C.
Cell culture
Three human RCC cell lines, Caki-1 (American Type
Culture Collection, Manassas, VA, USA), ACHN and 786-O (provided by
Dr Tomohiro Yano, Toyo University, Tokyo, Japan), were used. Caki-1
cells were grown in McCoy's 5A medium (Sigma-Aldrich; Merck KGaA)
supplemented with 10% fetal bovine serum (FBS; Equitech-Bio,
Kerrville, TX, USA), 100 U/ml penicillin, 100 µg/ml streptomycin
and 0.22 g/l L-glutamine (all Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). ACHN and 786-O cells were grown in DMEM
(Wako Pure Chemical Industries, Ltd.) supplemented with FBS and the
aforementioned antibiotics. All cells were incubated at 37°C in 5%
CO2. For hypoxic conditions, an AnaeroPack™ system
(Mitsubishi Gas Chemical Company, Inc., Tokyo, Japan) was used.
Cell viability assay
A total of 2.0×103 Caki-1 cells, and
8.0×102 ACHN and 786-O cells were seeded into a 96-well
plate. Following 24 h of incubation, the cells were treated with SU
(1.1–3.3 µM), NaBu (1.3–2.0 mM) or both (final concentrations
listed in Fig. 1A), followed by
culturing for 12 h, 24 h, 2 days, 3 days, 5 days, and 7 days at
37°C. Control cells were treated with 0.1% DMSO. Drug
concentrations were determined by the IC50 values for 48
h single treatment of SU or NaBu, detected in preliminary assays
(data not presented). On days 1, 3, 5 and 7, 0.25 mg/ml MTT was
added to each well and the plate was incubated for 1 h, at 37°C in
5% CO2. The supernatant was removed and DMSO was added
to each well. The absorbance at a wavelength of 540 nm and a
reference wavelength of 650 nm was read with a Multiskan JX
microplate reader (Thermo Labsystems, Santa Rosa, CA, USA). Cell
viability (%) was calculated as follows: [Optical density (OD) of
the treated wells]/(OD of the control cells)x100.
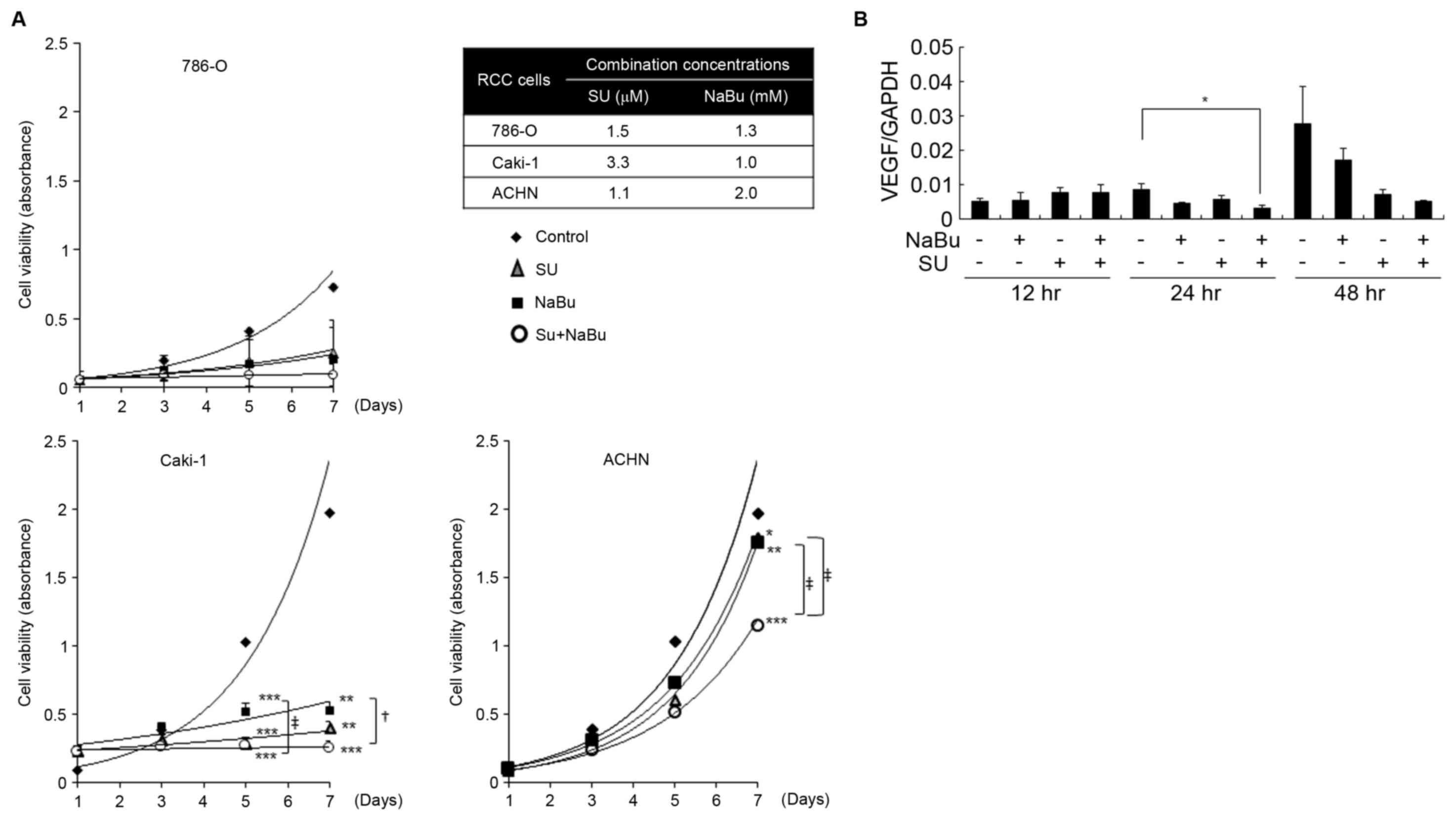 | Figure 1.Effects of the combined treatment with
SU and NaBu on the growth of human renal cancer cell lines. (A)
Cells were treated with the indicated concentrations of SU, NaBu or
both for 7 days and cell viability was determined using an MTT
assay. *P<0.05, **P<0.01, ***P<0.001, vs. the DMSO-treated
control group using the Tukey-Kramer's test. +P<0.05,
‡P<0.01 significant differences among the indicated
groups following the Tukey-Kramer's test. (B) Effects of the
combined treatment with SU and NaBu on the HIF-α target; VEGF
expression was detected using reverse transcription-quantitative
polymerase chain reaction analysis in Caki-1 cells over the course
of 48 h. All data are presented as the mean ± standard deviation
following three independent experiments. SU, sunitinib; NaBu,
sodium butyrate; cont, control; VEGF, vascular endothelial growth
factor; HIF-α, hypoxia-inducible factor-α. |
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from each cell line using
RNAzol® RT reagent (Cosmo Bio Co., Ltd., Tokyo, Japan)
according to the manufacturer's protocol. cDNA was synthesized in a
20 µl reaction mixture containing 500 ng of total RNA using
ReverTra Ace® qPCR RT Master mix (Toyobo Co., Ltd.,
Osaka, Japan). qPCR was performed using 2 µl cDNA (500 ng of each
original RNA) with the StepOne™ Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and SYBR Premix Ex Taq™
(Takara Bio Inc., Otsu, Japan), according to the manufacturer's
protocol. The thermocycling conditions maintained were as follows:
95°C for 10 sec, followed by 40 cycles at 95°C for 5 sec and 60°C
for 31 sec. The expression of VEGF was determined from the
threshold cycle values and were normalized to the internal standard
gene, GAPDH, using a standard curve based method, according to
Larionov et al (17). The
primer sequences used in the present study are described in
Table I. Each experiment had set
duplicate samples, and final data presented is the average of 3
independent experiments.
 | Table I.Primers used in reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primers used in reverse
transcription-quantitative polymerase chain reaction analysis.
| Target gene | Sequence |
|---|
| VEGF (395 bp) |
|
|
Forward |
5′-AACTTTCTGCTGTCTTGG-3′ |
|
Reverse |
5′-TTTGGTCTGCATTCACAT-3′ |
| GAPDH (180 bp) |
|
| Forward |
5′-CCAACGTGTCAGTGGTGGAC-3′ |
| Reverse |
5′-CAGCGTCAAAGGTGGAGGAG-3′ |
Western blot analysis
A total of 2×105 Caki-1 and ACHN cells
were seeded into 60-mm dishes. Following 24 h of incubation at
37°C, the cells were treated with SU, NaBu or both for each
indicated period. Control cells were treated with 0.1% DMSO.
Subsequently, cells were collected through scraping and dissolved
in ice-cold lysis buffer [50 mM Tris (pH 7.4), 150 mM sodium
chloride, 1% Triton X, 10 mM β-glycerophosphate, 1 mM sodium
orthovanadate, 1 mM ethylenediaminetetraacetic acid, 1 mM
phenylmethane sulfonyl fluoride and 1% protease inhibitor
cocktail]. Protein concentration was determined using a DC protein
assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Proteins
(20 µg/lane) were electrophoresed using 7.5–15% SDS-PAGE. SDS was
purchased from Wako Pure Chemical Industries, Ltd. and 30%
acrylamide solution was purchased from Bio-Rad Laboratories, Inc.
The separated proteins were transferred to a polyvinylidene
difluoride membrane (Atto Corporation, Tokyo, Japan). Then, the
membranes were blocked with 5% skim milk (Megmilk Snow Brand Co.,
Ltd., Tokyo, Japan) in TBS-Tween-20 (13.7 mM sodium chloride, 25 mM
Tris and 0.05% Tween-20; Wako Pure Chemical Industries, Ltd.) for 1
h at room temperature. Subsequently, the membranes were incubated
with primary antibodies overnight at 4°C, followed by incubation
with secondary horseradish peroxidase (HRP)-conjugated antibodies
(Sigma-Aldrich; Merck KGaA) for 1 h at room temperature. Antibody
information is indicated in Table
II. The detection was accomplished using an Immobilon™ Western
Chemiluminescent HRP Substrate (Merck KGaA) and a Luminescent Image
Analyzer LAS-1000 plus (Fujifilm, Tokyo, Japan). β-actin was used
as the internal standard. Data is the average or representative of
3 independent experiments.
 | Table II.Primary antibodies used in western
blot or immunoprecipitation analyses. |
Table II.
Primary antibodies used in western
blot or immunoprecipitation analyses.
| Target | Source | Cat. no. | Dilution | Species |
|---|
| Anti-phosphotyrosine
pAb | Upstate
Biotechnology, Inc. (Lake Placid, NY, USA) | 06–427 | 500 | Rabbit AH |
| PDGFR-β mAb | Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) | sc-53872 |
2,000 | Mouse AH |
| p44/42 MAPK (ERK1/2)
(137F5) mAb: ERK | Cell Signaling
Technologies, Inc. (Danvers, MA, USA) | 4695 |
2,000 | Rabbit AH |
| Phopho-p44/42 MAPK
(ERK1/2) | Cell Signaling
Technologies, Inc. | 9101 |
2,000 | Rabbit AH |
| (Thr202/Tyr204)
mAb: p-ERK Akt pAb | Rockland, Inc.
(Gilbertsville, PA, USA) | 100-401–401 |
2,000 | Rabbit AH |
| Phospho-Akt
(Ser473) mAb: p-Akt | Rockland, Inc. | 200-301-268 |
2,000 | Mouse AH |
| HIF-1α (28b)
mAb | Santa Cruz
Biotechnology, Inc. | sc-13515 |
2,000 | Mouse AH |
| HIF-2α pAb | Novus Biologicals,
LLC (Littleton, CO, USA) | NB100-122 |
2,000 | Rabbit AH |
| VEGF (147) pAb | Santa Cruz
Biotechnology, Inc. | sc-507 |
1,000 | Rabbit AH |
| β-actin mAb | Sigma-Aldrich;
Merck KGaA (Darmstadt, Germany) | A5441 | 10,000 | Mouse AH |
Immunoprecipitation analysis
A total of 2×105 786-O cells were seeded
into 60-mm dishes. Following treatment with 1.5 µM SU or 1.3 mM
NaBu or a combination for 24 h, cells were obtained according to
the aforementioned method in the western blot analysis.
Immunoprecipitation analysis was perfomed according to the
manufacturer's protocol (Bio-Rad Laboratories, Inc.; 161–4823JA,
SureBeads™ Protein G Magnetic Beads). Briefly, total cell lysates
(400 µg/ml), SureBeads™ (Bio-Rad Laboratories, Inc.) and 5 µl
anti-PDGFR-β antibody (Table II)
were incubated for 1 h at room temperature. Immunoprecipitates were
washed three times with lysis buffer. A total of 40 µl sample
buffer [250 mM Tris (pH 6.8), 40% sucrose, 20% 2-mercaptoethanol,
8% SDS (all Wako Pure Chemical Industries, Ltd.) and 0.002%
bromophenol blue (ICN Biomedicals, Eschwege, Germany)] were added,
and then heated at 70°C for 10 min. For western blotting, each
protein sample was diluted 4 times in lysis buffer and analyzed
according to the aforementioned method. To detect Tyrosine
phosphorylation (P-Tyr), the membrane was incubated with a rabbit
anti-human primary antibody directed against phosphotyrosine
(Table II) overnight at 4°C. The
detection was accomplished using an Immobilon™ Western
Chemiluminescent HRP Substrate and a Luminescent Image Analyzer
ImageQuant™ LAS 4000 (GE Healthcare Life Sciences, Chalfont,
UK).
Statistical analysis
Statistical analyses were performed using the
Dunnett's test and the Tukey-Kramer's test (SPSS, version 22, IBM
SPSS, Armonk, NY, USA). All experiments were performed >3 times.
Data were expressed as mean ± standard error. P<0.05 was
considered to indicate a statistically significant difference.
Results
Long-term combined treatment with SU
and NaBu effectively inhibits proliferation and VEGF induction in
RCC cell lines
To investigate the combined effect of SU and NaBu on
RCC cell proliferation, SU and NaBu were used at 50% growth
inhibitory concentrations (IC50) following a single
exposure to SU or NaBu, then cell viability following combination
treatment and that of the single treatment were compared. The final
combined concentrations for each cell line are depicted in Fig. 1A. Cell viability gradually increased
in a time-dependent manner over the course of 7 days in all three
RCC cell lines. Regarding Caki-1 cells, on day 5 cell viability was
significantly decreased to ~30 and 50% of the DMSO control by 1.0
mM of NaBu (P<0.001), and 3.3 µM of SU (P<0.001),
respectively (Fig. 1A). By day 7,
cell viability had slightly increased following treatment with SU
or NaBu alone, whereas the combination of these agents had
significantly suppressed this increase (P<0.001 vs. control;
P<0.001 between NaBu single group and combination group). In
ACHN cells, 1.1 µM of SU or 2.0 mM of NaBu alone had almost no
effect on proliferation. However, cell viability significantly
decreased to ~50% of that of the control cells following combined
treatment with SU and NaBu by day 7. The proliferation rate of
786-O cells was slow, as compared with Caki-1 or ACHN cells. Single
treatment with 1.5 µM of SU or 1.3 mM of NaBu sufficiently
suppressed cell growth during the experimental term, although
combined treatment demonstrated the most potent growth
inhibition.
SU was originally considered an anti-angiogenic
agent, thus one of the targets of SU is VEGF-mediated signaling
(5). VEGF is the direct target of
HIF-α protein, which servers a critical role in RCC progression.
Treatment using SU leads to a gradual development of resistance to
SU, with a corresponding unresponsiveness to elevations in VEGF
levels. In the present study, VEGF mRNA expression gradually
increased in Caki-1 cells over the course of 48 h; however, this
was significantly suppressed following 24 h combined treatment with
NaBu and SU (Fig. 1B).
Combined treatment with SU and NaBu
suppresses RTK signaling activity
The effect of SU and NaBu on RTK signaling was
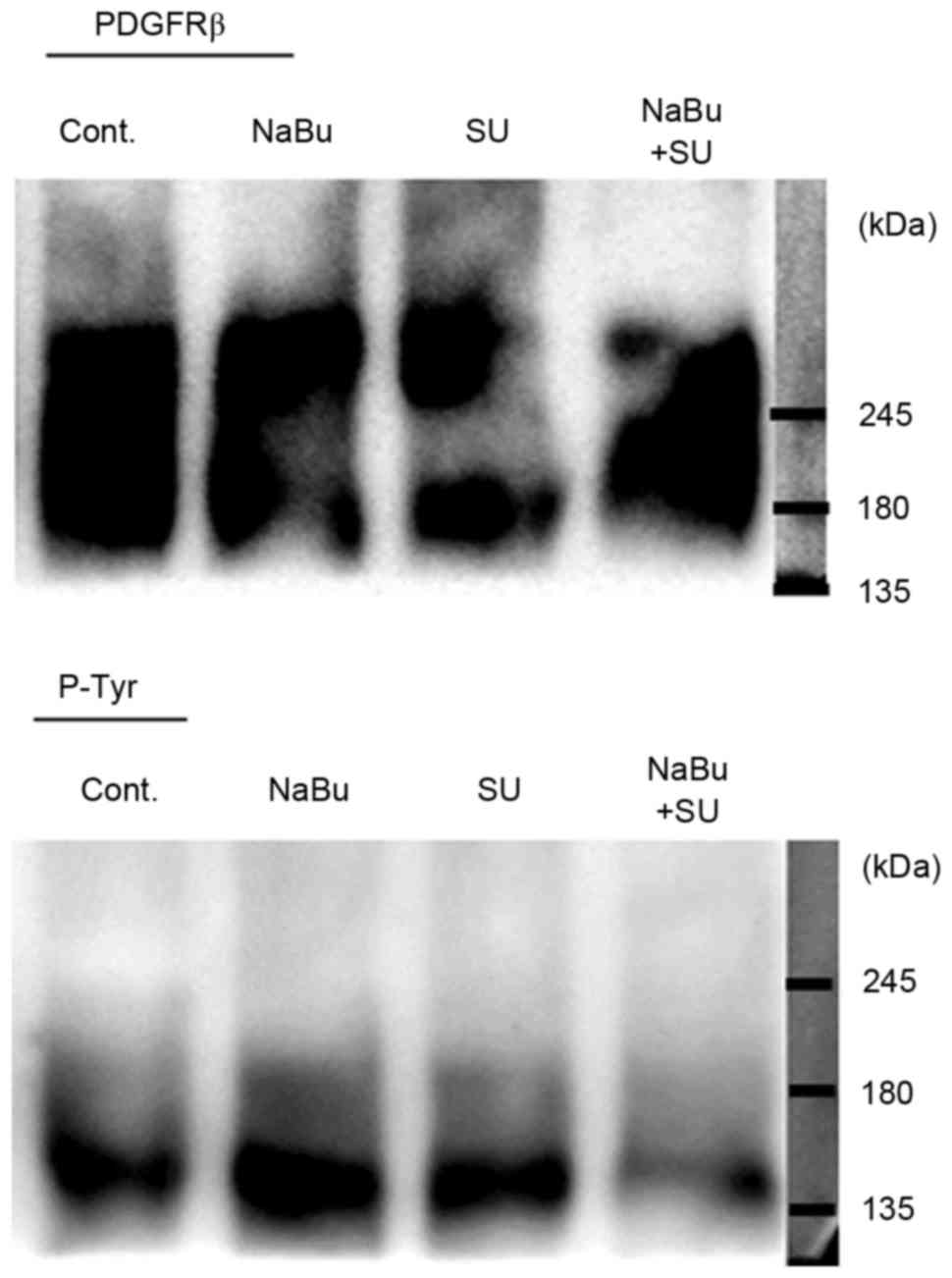
subsequently investigated. The expression levels of certain
tyrosine receptors, including the direct targets of SU c-Kit and
PDGFR-β, were detected in all the three RCC cell lines used in the
present study, particularly PDGFR-β (data not presented). The
phosphorylation status of PDGFR-β, as indicated by the
immunoprecipitation band with p-Tyr, was activated in untreated and
single treated cells. However, combined treatment with SU and NaBu
resulted in a decrease in phosphorylated PDGFR-β levels, as
compared with those in the untreated control cells (Fig. 2).
The central region of solid tumors is generally
exposed to hypoxic conditions, which promote tumor angiogenesis and
progression by activating the expression of pro-angiogenic factors
(16). An important factor in this
process is HIF-α. Specifically, HIF-1α is an important
transcription factor involved in acute hypoxia, whereas HIF-2α
drives the cell response to chronic hypoxia (18). Therefore, the expression of HIF-α
proteins, Akt, ERK and their phosphorylated/active forms in ACHN
cells was investigated under normoxic and hypoxic conditions. The
expression of HIF-1α increased following 4 h of hypoxic exposure,
compared with the levels observed under normoxic conditions,
whereas the expression levels of HIF-2α were increased following 6
h and this was maintained until 24 h following exposure (Fig. 3A). The change in the expression levels
of HIF-α proteins was larger for HIF-2α compared with HIF-1α under
normoxic and hypoxic conditions. The levels of HIF-2α increased
12-fold following 10 h incubation in hypoxic conditions, as
compared with the levels in normoxic conditions (Fig. 3B). This increase was suppressed by the
combined treatment with SU and NaBu. Furthermore, this combination
suppressed the levels of phosphorylated Akt and ERK to almost the
same as those present in control cells, although the phosphorylated
status of these factors were also increased by a single treatment
with SU or NaBu following 10 h of exposure. Notably, SU monotherapy
under hypoxic conditions appeared to elevate the phosphorylation of
Akt and ERK. VEGF is an essential growth factor that is directly
transcribed by HIF-α (16). Under
hypoxic conditions, single treatment with SU markedly decreased
VEGF expression levels (to ~70% of hypoxic control), but the range
of the expression levels of VEGF induced by hypoxia or NaBu
treatment appeared to be small (50–70% of normoxic control)
compared with that observed for other RTK signaling factors
(2–5-fold compared with control; Fig.
3B).
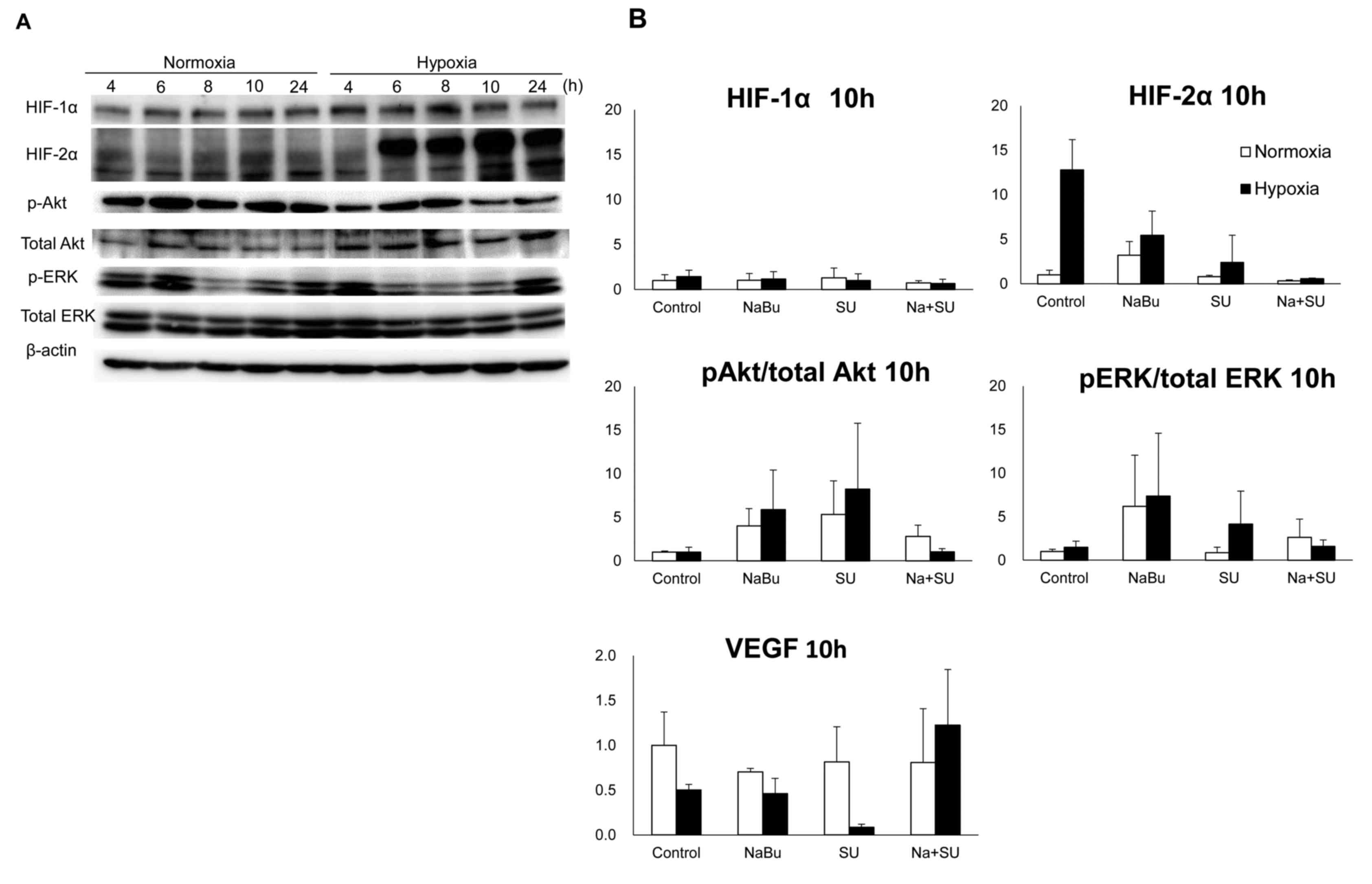 | Figure 3.Effects of the combined treatment with
SU and NaBu on the protein expression of HIFs, VEGF and downstream
RTK signaling proteins Akt and ERK, and their phosphorylated
variants. Intact ACHN cell culture dishes were exposed to normal
oxygen tension (normoxia, 21% oxygen tension) or <0.1% oxygen
tension (hypoxia) for each indicated period. (A) Representative
images of three independent experiments. (B) Each column represents
the mean ± standard error following three independent experiments.
Cells were exposed to each drug for 10 h under normoxic or hypoxic
conditions. SU, sunitinib; NaBu, sodium butyrate; cont, control;
VEGF, vascular endothelial growth factor; HIF, hypoxia-inducible
factor; AKT, AKT serine/threonine kinase; ERK, extracellular signal
regulated kinase; p, phosphorylated. |
Discussion
The present study was designed to investigate
whether the novel combination therapy with SU and NaBu could
overcome drug resistance in RCC cells. This combination is aimed as
vertical blockage of the RTK signaling pathway. The results of the
present study demonstrated that the combination treatment
effectively suppressed the growth of RCC cells following long-term
exposure. To confirm the involvement of the RTK signaling pathway
in the combination effect, hypoxic conditions were established,
which mimicked the intra-tumor environment with confirmation of
increased HIF-α protein expression. Such a hypoxic adaptation
should occur prior to the initiation of tumor growth. Thus, we
investigated in the shortest amount of time for capturing HIF-α
increase in the present study.
It was confirmed that receptors of RTK signaling,
including c-Kit and PDGFRβ, were present in RCC cells used in the
current study. VEGF was originally considered a specific stimulator
of vascular endothelial cells, but it has been revealed to affect
other cells in addition, including hematopoietic precursor cells,
macrophages and hepatic sinusoid cells. At first, the effect of SU
was thought to be exerted only via angiogenesis, which can induce
tumor shrinkage. In a previous study, the growth of endothelial
cells (HUVEC) was suppressed by the VEGF receptor tyrosine kinase
inhibitor, PTK787/ZK222584, following VEGF stimulation (19). Although, VEGF has been reported to
induce bladder cancer growth (20).
Additionally, a VEGF antibody suppressed the growth of human cancer
cells in melanoma, pancreatic cancer, cervical cancer and Kaposi
sarcoma (21). The VEGFR2 antibody
also suppressed leukemic cell growth (22). These reports indicate that VEGF could
stimulate the growth of a cancer cell via the VEGFR autocrine loop,
which is expressed on the cancer cell itself. PDGFR signaling is
also a promising target for RCC (23). Activation of the c-Kit receptor has
been known to induce transduction signaling, including induction of
the mitogen-activated protein kinase and phosphatidylinositol
3-kinase (PI3K)/Akt signaling pathways in RCC, which in turn leads
to mast cell activation (24).
In the present study, the effects of combined
treatment with SU and NaBu on RTK signaling factors were examined.
Among the RTK downstream factors, ERK and Akt exist at branching
points that determine the downstream direction. Cell-dependent
MEK/ERK signaling and PI3K/Akt signaling pathways can collaborate
with each other to maintain cell survival (25). The expression of phosphorylated ERK,
the activated form of ERK, was notably variable each hour (Fig. 3A), which may be dependent on cell
cycle progression. This trend was the same under normoxic and
hypoxic conditions, although the peak time point was slightly
shifted, whereby hypoxic exposure appeared to shift its peak
earlier compared with under normoxic conditions. Akt expression was
almost constant during the experimental periods in all conditions.
Additionally, HIF-α protein was confirmed to be increased following
hypoxic exposure compared with normoxic conditions. Hypoxic
conditions are universally observed in the tumor environment and
are the result of fragile development of the vascular system
accompanied with a high growth rate. To survive in severe
conditions, a cancer cell induces HIF-α expression, which is
normally degraded by proteasomes under normoxic/aerobic conditions
(26). Stable expression of HIF-α
induces VEGF and PDGF transcription. Notably, the results of the
present study revealed that HIF-2α induction was stronger compared
with that of HIF-1α. Three HIF-α subtypes have been identified:
HIF-1α, HIF-2α, and HIF-3α. Although HIF-1α is the most ubiquitous
and well-studied subtype, HIF-2α is considered to serve an
essential role during stable hypoxic conditions in RCC (18,27).
Furthermore, it has been reported that VEGF expression is
maintained through HIF-2α in RCC cells lacking HIF-1α (28).
NaBu has been reported to inhibit the activation of
ERK and Akt (15); however single
treatment with NaBu appeared to induce these factors compared with
non-treatment control following 10 h exposure in the current study.
In addition, single treatment with SU induced their activation,
particularly under hypoxic conditions. This opposes the results of
a previous study that demonstrated that single treatment with SU
(0.5–3 µM) did not alter the expression of phosphorylated ERK and
Akt in ACHN, and Caki-1 cells (29).
Possible reasons for the differences in results may involve the
treatment concentrations of drugs and exposure periods used. In the
present study, cells were exposed to drugs for 10 h and HIF-2α
protein was confirmed to be upregulated, which may be involved in
the drug resistance. HIF-2α has been suggested to cause resistance
to sorafenib, a RTK inhibitor, in hepatocellular carcinoma cells by
activating the transforming growth factor-α/epidermal growth factor
receptor signaling pathway (30). In
the present study, single treatment with NaBu or SU was not
observed to be effective at inhibiting RTK signaling in RCC cells
under the conditions that the cells were maintained in. However,
the results demonstrated that combined treatment suppressed the
activation of ERK and Akt, as compared with each single
treatment.
VEGF is an important protein for the induction of
angiogenesis in cancerous cells and is essential for their survival
(4,5,7). NaBu and
SU have been known to decrease VEGF expression and signaling
(5,16). However, in the present study, no
significant differences were observed in the protein expression of
VEGF under hypoxic exposure. A limitation of the current study was
the use of a cell culture system to evaluate angiogenic capacity.
Thus, further studies using in vivo or a 3D cell culture
models are warranted in which the development of vessels and
angiogenic factors can be detected.
In the present study, the growth rate of 786-O was
dissimilar to that of the other two RCC cell lines, Caki-1 and
ACHN. The latter cells demonstrated a mutually similar growth rate.
This difference may be attributable to a genetic factor, including
the presence of wild type von-Hippel-Lindau (VHL) protein present
in Caki-1 and ACHN cells (31), or
mutant VHL, which is present in 786-O cells (32). The significantly synergistic effect
was only identified in Caki-1 and ACHN cells. VHL is an essential
protein for the degradation of HIF-α under normoxic conditions
(33). Thus, HIF-α expression remains
stable in 786-O cells, even under normoxic conditions. It has been
challenging to control the VHL-mutant type of cancer. However, a
previous study indicated that inhibiting certain class II HDACs was
effective for inducing the degradation of HIF-α in a
VHL-independent manner (33).
Although the growth-inhibiting effect of NaBu is small when
combined with SU in 786-O cells, another HDACI, such as
trichostatin A, may have a more potent inhibitory effect.
In conclusion, combination treatment with NaBu and
SU has demonstrated a promising efficacy against RCC, a type of
cancer known to develop SU resistance. Further studies examining
whether the combination is effective in a clinical environment and
studies on other HDACIs, due to the limitations of NaBu, may help
to clarify whether the combination is effective for the treatment
of RCC.
Acknowledgements
The authors would like to thank Dr Akihiro Hisaka
(Chiba University, Chiba, Japan) for reviewing the present study.
The current study was partially supported by the Grant-in-Aid for
Young Scientists (B; grant nos. 24790529 and 15K19161) from the
Japan Society for the Promotion of Sciences, and a research grant
from the Takeda Science Foundation (2013–2015).
References
|
1
|
Pal SK, Hossain DM Sakib, Zhang Q, Frankel
PH, Jones JO, Carmichael C, Ruel C, Lau C and Kortylewski M:
Pazopanib as third-line therapy for metastatic renal cell
carcinoma: Clinical efficacy and temporal analysis of cytokine
profile. J Urol. 193:1114–1121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mattei J, da Silva RD, Sehrt D, Molina WR
and Kim FJ: Targeted therapy in metastatic renal carcinoma. Cancer
Lett. 343:156–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Motzer RJ and Russo P: Systemic therapy
for renal cell carcinoma. J Urol. 163:408–417. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mulders P: Vascular endothelial growth
factor and mTOR pathways in renal cell carcinoma: Differences and
synergies of two targeted mechanisms. BJU Int. 104:1585–1589. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chow LQ and Eckhardt SG: Sunitinib: From
rational design to clinical efficacy. J Clin Oncol. 25:884–896.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik
C, Kim ST, et al: Sunitinib versus interferon alfa in metastatic
renal-cell carcinoma. N Engl J Med. 356:115–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Domblides C, Gross-Goupil M, Quivy A and
Ravaud A: Emerging antiangiogenics for renal cancer. Expert Opin
Emerg Drugs. 18:495–511. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Motzer RJ, Hudes G, Wilding G, Schwartz
LH, Hariharan S, Kempin S, Fayyad R and Figlin RA: Phase I trial of
sunitinib malate plus interferon-alpha for patients with metastatic
renal cell carcinoma. Clin Genitourin Cancer. 7:28–33. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patel PH, Senico PL, Curiel RE and Motzer
RJ: Phase I study combining treatment with temsirolimus and
sunitinib malate in patients with advanced renal cell carcinoma.
Clin Genitourin Cancer. 7:24–27. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mork CN, Faller DV and Spanjaard RA: A
mechanistic approach to anticancer therapy: Targeting the cell
cycle with histone deacetylase inhibitors. Curr Pharm Des.
11:1091–1104. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Witt O and Lindemann R: HDAC inhibitors:
Magic bullets, dirty drugs or just another targeted therapy. Cancer
Lett. 280:123–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Novogrodsky A, Dvir A, Ravid A, Shkolnik
T, Stenzel KH, Rubin AL and Zaizov R: Effect of polar organic
compounds on leukemic cells. Butyrate-induced partial remission of
acute myelogenous leukemia in a child. Cancer. 51:9–14. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Patnaik A, Rowinsky EK, Villalona MA,
Hammond LA, Britten CD, Siu LL, Goetz A, Felton SA, Burton S,
Valone FH and Eckhardt SG: A phase I study of pivaloyloxymethyl
butyrate, a prodrug of the differentiating agent butyric acid, in
patients with advanced solid malignancies. Clin Cancer Res.
8:2142–2148. 2002.PubMed/NCBI
|
|
14
|
Chen S and Sang N: Histone deacetylase
inhibitors: The epigenetic therapeutics that repress
hypoxia-inducible factors. J Biomed Biotechnol. 2011:1979462011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jung JW, Cho SD, Ahn NS, Yang SR, Park JS,
Jo EH, Hwang JW, Jung JY, Kim SH, Kang KS and Lee YS: Ras/MAP
kinase pathways are involved in Ras specific apoptosis induced by
sodium butyrate. Cancer Lett. 225:199–206. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim SH, Kim KW and Jeong JW: Inhibition of
hypoxia-induced angiogenesis by sodium butyrate, a histone
deacetylase inhibitor, through hypoxia-inducible factor-1alpha
suppression. Oncol Rep. 17:793–797. 2007.PubMed/NCBI
|
|
17
|
Larionov A, Krause A and Miller W: A
standard curve based method for relative real time PCR data
processing. BMC Bioinformatics. 6:622005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koh MY, Lemos R Jr, Liu X and Powis G: The
hypoxia-associated factor switches cells from HIF-1α- to
HIF-2α-dependent signaling promoting stem cell characteristics,
aggressive tumor growth and invasion. Cancer Res. 71:4015–4027.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Qian DZ, Wang X, Kachhap SK, Kato Y, Wei
Y, Zhang L, Atadja P and Pili R: The histone deacetylase inhibitor
NVP-LAQ824 inhibits angiogenesis and has a greater antitumor effect
in combination with the vascular endothelial growth factor receptor
tyrosine kinase inhibitor PTK787/ZK222584. Cancer Res.
64:6626–6634. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakanishi R, Oka N, Nakatsuji H, Koizumi
T, Sakaki M, Takahashi M, Fukumori T and Kanayama HO: Effect of
vascular endothelial growth factor and its receptor inhibitor on
proliferation and invasion in bladder cancer. Urol Int. 83:98–106.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Masood R, Cai J, Zheng T, Smith DL, Hinton
DR and Gill PS: Vascular endothelial growth factor (VEGF) is an
autocrine growth factor for VEGF receptor-positive human tumors.
Blood. 98:1904–1913. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dias S, Hattori K, Zhu Z, Heissig B, Choy
M, Lane W, Wu Y, Chadburn A, Hyjek E, Gill M, et al: Autocrine
stimulation of VEGFR-2 activates human leukemic cell growth and
migration. J Clin Invest. 106:511–521. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang W, Qi L, Tan M, Zhang Z, Du J, Wei X
and Yao X: Effect of platelet-derived growth factor-B on renal cell
carcinoma growth and progression. Urol Oncol. 33:168.e17–e27. 2015.
View Article : Google Scholar
|
|
24
|
Marech I, Gadaleta CD and Ranieri G:
Possible prognostic and therapeutic significance of c-Kit
expression, mast cell count and microvessel density in renal cell
carcinoma. Int J Mol Sci. 15:13060–13076. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dent P: Crosstalk between ERK, AKT and
cell survival. Cancer Biol Ther. 15:245–246. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Epstein AC, Gleadle JM, McNeill LA,
Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI,
Dhanda A, et al: C. elegans EGL-9 and mammalian homologs define a
family of dioxygenases that regulate HIF by prolyl hydroxylation.
Cell. 107:43–54. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sowter HM, Raval RR, Moore JW, Ratcliffe
PJ and Harris AL: Predominant role of hypoxia-inducible
transcription factor (Hif)-1alpha versus Hif-2alpha in regulation
of the transcriptional response to hypoxia. Cancer Res.
63:6130–6134. 2003.PubMed/NCBI
|
|
28
|
Shinojima T, Oya M, Takayanagi A, Mizuno
R, Shimizu N and Murai M: Renal cancer cells lacking hypoxia
inducible factor (HIF)-1alpha expression maintain vascular
endothelial growth factor expression through HIF-2alpha.
Carcinogenesis. 28:529–536. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bai L, Yang JC, Ok JH, Mack PC, Kung HJ
and Evans CP: Simultaneous targeting of Src kinase and receptor
tyrosine kinase results in synergistic inhibition of renal cell
carcinoma proliferation and migration. Int J Cancer. 130:2693–2702.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao D, Zhai B, He C, Tan G, Jiang X, Pan
S, Dong X, Wei Z, Ma L, Qiao H, et al: Upregulation of HIF-2α
induced by sorafenib contributes to the resistance by activating
the TGF-α/EGFR pathway in hepatocellular carcinoma cells. Cell
Signal. 26:1030–1039. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tanaka T, Torigoe T, Hirohashi Y, Sato E,
Honma I, Kitamura H, Masumori N, Tsukamoto T and Sato N:
Hypoxia-inducible factor (HIF)-independent expression mechanism and
novel function of HIF prolyl hydroxylase-3 in renal cell carcinoma.
J Cancer Res Clin Oncol. 140:503–513. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lieubeau-Teillet B, Rak J, Jothy S,
Iliopoulos O, Kaelin W and Kerbel RS: von Hippel-Lindau
gene-mediated growth suppression and induction of differentiation
in renal cell carcinoma cells grown as multicellular tumor
spheroids. Cancer Res. 58:4957–4962. 1998.PubMed/NCBI
|
|
33
|
Qian DZ, Kachhap SK, Collis SJ, Verheul
HM, Carducci MA, Atadja P and Pili R: Class II histone deacetylases
are associated with VHL-independent regulation of hypoxia-inducible
factor 1 alpha. Cancer Res. 66:8814–8821. 2006. View Article : Google Scholar : PubMed/NCBI
|