Introduction
Tumor necrosis factor (TNF)-related
apoptosis-inducing ligand (TRAIL), also known as Apo2L and TNFSF10,
is a type II transmembrane protein belonging to the TNF superfamily
that is able to induce programmed cell death (1,2).
TRAIL-mediated apoptosis follows binding to its receptors: Death
receptor 4 (DR4, also known as TRAIL-R1 and TNFRSF10a) and DR5
(also known as TRAIL-R2 and TNFRSF10b), which contain an
intracellular death domain and transmit apoptotic signals to
intracellular apoptosis-associated proteins (3,4). TRAIL has
been the center of attention as it is relatively non-toxic to
normal cells, but selectively induces apoptotic cell death in
multiple types of transformed or malignant cells (1,5). However,
certain malignant cells are resistant to TRAIL (6,7).
Therefore, finding novel reagents that are able to promote
TRAIL-induced apoptosis in cancer cells is useful to increase the
effects of TRAIL-based cancer therapy, or to overcome TRAIL
resistance.
Bladder cancer is one of the most common
genitourinary malignant neoplasms and is typically observed in
older patients (8). According to the
growth of aging populations worldwide, the occurrence of bladder
cancer is expected to increase (8,9). To
develop improved treatments, multiple studies have been conducted
to detect drugs that sensitize bladder cancer to TRAIL-induced
apoptosis (10,11). Previous research has also demonstrated
that a combination of chemotherapy or natural agents with TRAIL
triggers apoptosis through the upregulation of DRs, activation of
caspases, the downregulation of survival proteins including
cellular Fas-associated death domain-like interleukin-1β-converting
enzyme inhibitory protein (cFLIP) and X-linked inhibitor of
apoptosis protein (XIAP), and the modification of B cell lymphoma-2
(Bcl-2) family proteins (12–14). Therefore, the resistance of bladder
cancer cells to TRAIL may be overcome by the use of sensitizing
chemicals that alter the dysregulated expression of DRs and
apoptosis-associated proteins (15).
Bufalin is a major component of Sum Su, which is a
traditional oriental medicine obtained from the skin and parotid
venom gland of the toad. Bufalin possesses various biological
properties, acting as a cardiotonic, an anesthetic and a blood
pressure stimulant, as well as demonstrating respiration-improving
and anti-neoplastic activities (16,17). In
terms of its anti-tumor activities, bufalin has been demonstrated
to suppress cell proliferation and to cause apoptosis in a wide
spectrum of cancer cells, including lung adenocarcinoma cells,
prostate cancer cells, ovarian cancer cells, gastric cancer cells,
bladder cancer cells, hepatocellular cells, breast cancer and
leukemia cells (16–20). On the other hand, only 2 previous
studies concerning the use of bufalin as a sensitizing reagent to
overcome TRAIL resistance in breast cancer have been conducted. Yan
et al (12,21) demonstrated that bufalin enhanced
TRAIL-induced apoptosis of breast cancer cells via upregulation of
the DRs in vitro and in vivo. However, to the best of
our knowledge, a combination treatment including bufalin and TRAIL
is yet to be attempted in bladder carcinoma cells.
Therefore, the present study investigated whether
bufalin promoted TRAIL-induced apoptotic cell death and was able to
overcome resistance to TRAIL in human bladder cancer cells, and
focused on understanding the underlying molecular mechanisms. In
the present study, bufalin was demonstrated to markedly sensitize
bladder cancer cells to TRAIL-induced apoptosis.
Materials and methods
Reagents and antibodies
MTT, propidium iodide (PI) and DAPI were purchased
from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). RPMI-1640
medium, fetal bovine serum (FBS) and penicillin/streptomycin were
purchased from Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA,
USA). TRAIL was obtained from Koma Biotech (Seoul, Korea) and
dissolved in phosphate buffered saline (PBS) to 10 mg/ml and stored
at −70°C. Bufalin was purchased from Sigma-Aldrich; Merck KGaA. The
formula weight was 386.52 g/mol and the purity was >97%, as
determined by high performance liquid chromatography. Bufalin was
dissolved in dimethyl sulfoxide (DMSO; vehicle) to a concentration
of 10 mM. Further dilutions of TRAIL and bufalin were made in RPMI
culture medium prior to use. Antibodies against Bcl-2-associated X
(Bax; cat. no. sc-493), Bcl-2 (cat. no. sc-782), BH3 interacting
domain death agonist (Bid; cat. no. sc-11423), XIAP (cat. no.
sc-11426), cFLIP (cat. no. sc-5276), DR5 (cat. no. sc-65314), DR4
(cat. no. sc-7863), caspase-3 (cat. no. sc-7272), caspase-9 (cat.
no. sc-7885), caspase-8 (cat. no. sc-7890), poly (ADP-ribose)
polymerase (PARP; cat. no. sc-1562), β-actin (cat. no. sc-1616)
(all dilutions, 1:1,000) and peroxidase-labeled goat anti-rabbit
(cat. no. sc-2004), goat anti-mouse (cat. no. sc-2005) and cow
anti-goat (cat. no. sc-2350; all dilution, 1:1,500) IgG were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
All other chemicals were purchased from Sigma-Aldrich (Merck KGaA)
unless otherwise indicated.
Cell lines and cell culture
The human bladder carcinoma T24 cell line was
obtained from the American Type Culture Collection (Manassas, VA,
USA), and cultured in RPMI-1640 medium supplemented with 10%
heat-inactivated FBS, 2 mM glutamine, 100 U/ml penicillin and 100
µg/ml streptomycin at 37°C and 5% CO2.
MTT assay and cell morphological
analysis
To measure cell viability, an MTT assay was
conducted. The T24 cells were seeded at a density of
1×105 cells/well in 6-well plates, and treated with
bufalin (0, 5 or 10 nM), TRAIL (0, 25 or 50 ng/ml) or a
combination. Following 24 h incubation at 37°C with 5% CO2, the
medium was removed, and the cells were incubated with 0.5 mg/ml MTT
solution for 2 h. Then, the supernatant was discarded and the
formazan crystals were dissolved with DMSO. The optical density was
measured by a microplate reader (Dynatech Laboratories, Inc.,
Chantilly VA, USA) at 540 nm. The cell images were taken with an
inverted light microscope (Carl Zeiss AG, Oberkochen, Germany) at a
magnification of ×50.
Nuclear staining with DAPI
T24 cells were seeded at a density of
1×105 cells/well in 6-well plates and treated with
bufalin (10 nM), TRAIL (50 ng/ml), a combination, or neither, and
incubated for 24 h at 37°C with 5% CO2. The cells were then
harvested with trypsin, washed once with cold PBS and centrifuged
at 500 × g at 4°C for 5 min. The cells were fixed with 3.7%
paraformaldehyde (Sigma-Aldrich; Merck KGaA) in PBS at room
temperature for 10 min. Next, the fixed cells were washed with PBS
and stained with 2.5 µg/ml DAPI solution at room temperature for 10
min. The nuclear morphology of the cells was observed by a
fluorescence microscope (Carl Zeiss AG) at a magnification of
×200.
Cell cycle analysis
T24 cells (1×105) cells/well in 6-well
plates were treated with bufalin (10 nM), TRAIL (50 ng/ml), a
combination, or neither, and incubated for 24 h at 37°C with 5%
CO2. The cells were harvested with trypsin and washed once in PBS.
Subsequent to centrifugation at 500 × g and 4°C for 5 min, the
pellets were fixed in 70% ethanol and stored at 4°C for 1 h. Prior
to the analysis, the cells were washed once again with PBS and
suspended in 1 ml cold PI solution containing 100 µg/ml RNase A, 50
µg/ml PI, 0.1% (w/v) sodium citrate and 0.1% (v/v) nonidet p40,
then incubated on ice for 30 min in the dark. Flow cytometric
analysis was performed using a flow cytometer (FACSCalibur) with
CellQuest software (version 6; both BD Biosciences, Franklin Lakes,
NJ, USA) was used to estimate the relative DNA content based on the
presence of a red fluorescence. The sub-G1 population was
calculated to estimate the apoptotic cell population.
Protein extraction and western blot
analysis
T24 cells (5×105) were seeded in 100-mm
plates, treated with bufalin (10 nM), TRAIL (50 ng/ml), a
combination, or neither, and incubated for 24 h at 37°C with 5%
CO2. The cells were washed with ice-cold PBS and lysed with a lysis
buffer (20 mM sucrose, 1 mM EDTA, 20 µM Tris-Cl, pH 7.2, 1 mM
dithiothreitol (DTT), 10 mM KCl, 1.5 mM MgCl2, and 5 µg/ml
aprotinin) for 30 min on ice. The lysis mixture was centrifuged at
13,453 × g and 4°C for 15 min to remove cell debris. Supernatant
liquid was collected and protein concentration was quantified using
a Bio-Rad protein assay kit (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) according to the manufacturer's protocol. For western blot
analysis, equal amounts of protein (30 µg) were subjected to
electrophoresis on 8–12% of SDS-polyacrylamide gel (depending on
the molecular weight of the antibody target) and then
electrotransferred to a nitrocellulose membrane for 2 h at 4°C
(Schleicher & Schuell Bioscience, Inc., Keene, NH, USA). The
blots were probed with the primary antibodies, incubated with the
diluted enzyme-linked secondary antibodies. The blot was visualized
using an enhanced chemiluminescence western blotting detection
reagent (GE Healthcare Life Sciences, Chalfont, UK), observed with
a UV transilluminator imaging system and analyzed with CAPT 2000
software (version 12.8; both Vilber Lourmat, Collégien,
France).
Determination of caspase activity
T24 cells were seeded at a density of
1×105 cells/well in 6-well plates and treated with
bufalin (10 nM), TRAIL (50 ng/ml), a combination, or neither, and
incubated for 24 h at 37°C with 5% CO2. Caspase-3, −8 and −9
activity levels were the measured using colorimetric assay kits
(R&D Systems, Inc., Minneapolis, MN, USA) according to the
manufacturer's protocol. Briefly, the cells were lysed in the
supplied lysis buffer from the kits. The supernatant liquid was
collected and incubated with the supplied reaction buffer,
containing DTT and colorimetric tetrapeptides as follows: An
Asp-Glu-Val-Asp-p-nitroaniline (pNA) substrate for caspase-3, an
Ile-Glu-Thr-Asp-pNA substrate for caspase-8 and a
Leu-Glu-His-Asp-pNA substrate for caspase-9, respectively, at 37°C.
The extent of reaction was measured as absorbance at 405 nm using
an ELISA reader.
Statistical analysis
All data are presented as the mean ± standard
deviation. The significant differences between the groups were
analyzed using unpaired Student's t-tests. P<0.05 was considered
to indicate a statistically significant difference. The results
depicted in each of the figures were obtained from representatives
of ≥2 independent experiments.
Results
Combined bufalin and TRAIL treatment
significantly inhibits T24 cell viability
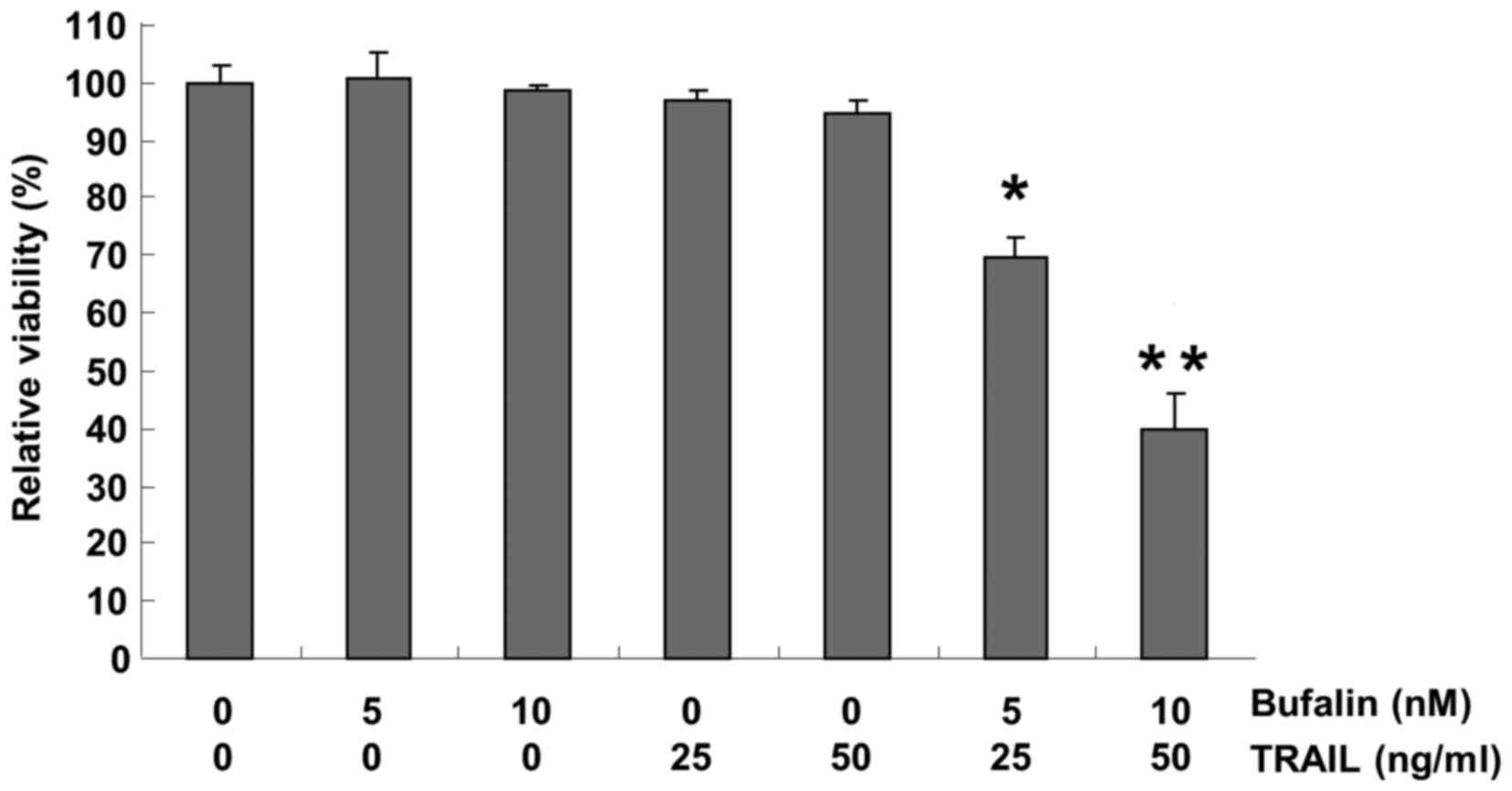
Initially, the present study sought to determine
whether a combination of bufalin and TRAIL treatment inhibited the
viability of T24 cells using the MTT assay. For the present study,
the cells were treated with bufalin (5 or 10 nM), TRAIL (25 or 50
ng/ml), both agents (bufalin 5 nM with TRAIL 25 ng/ml or 10 nM with
50 ng/ml), or neither, for 24 h. Treatment with bufalin and TRAIL
alone did not significantly affect cell viability; however, the
combination of bufalin with TRAIL significantly reduced the
viability of T24 cells (Fig. 1)
compared with the negative control. Collectively, these results
verified that a combination of bufalin and TRAIL was more effective
together than either agent alone in decreasing the viability of
bladder cancer cells.
Bufalin sensitizes TRAIL-resistant T24
cells to TRAIL-mediated apoptosis
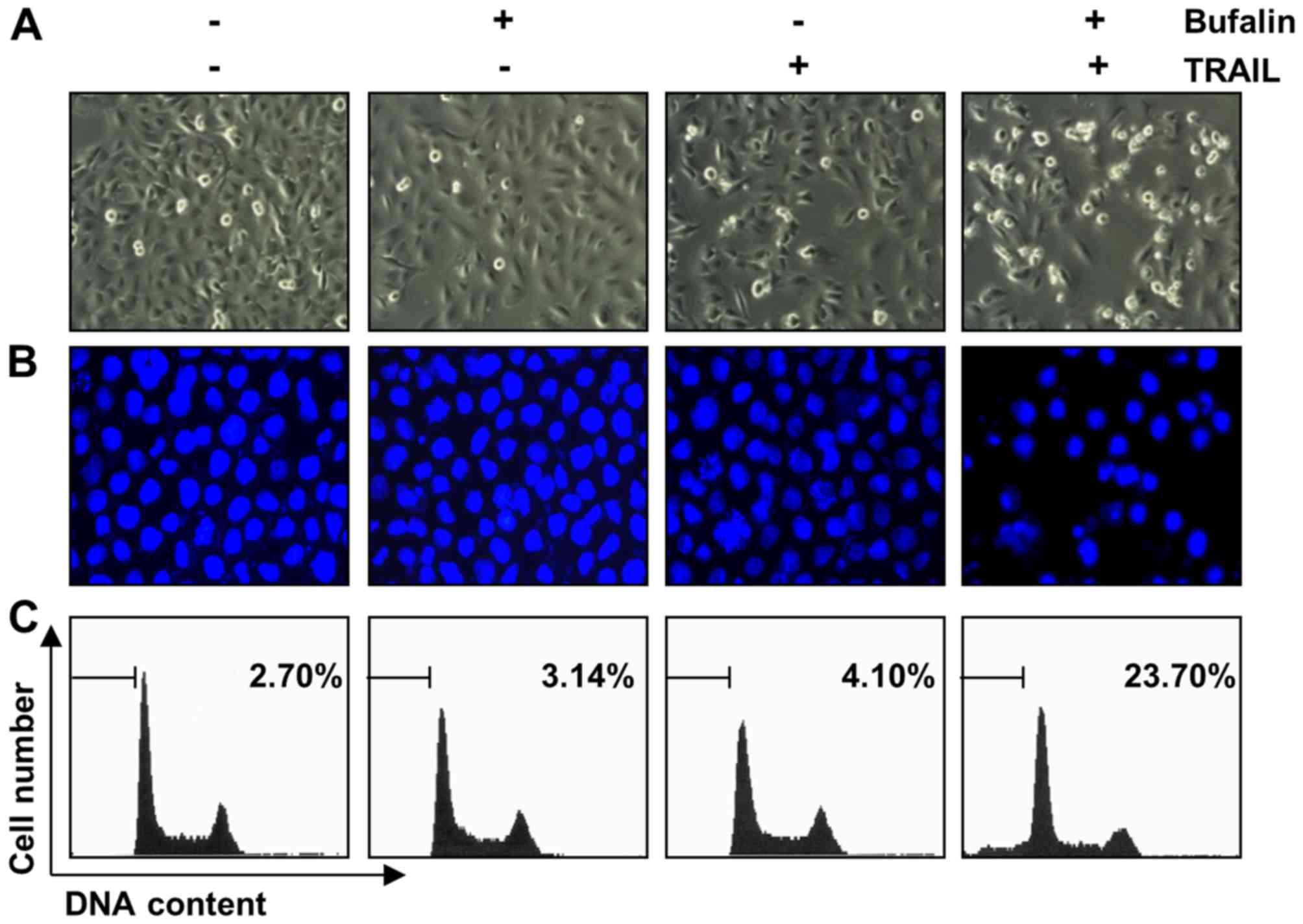
Next, the present study investigated whether the
inhibition of cell viability as a result of a combined treatment of
bufalin and TRAIL was associated with apoptosis. Specific apoptotic
features were observed by microscopy and flow cytometry, and T24
cells were treated with bufalin (10 nM), TRAIL (50 ng/ml), or a
combination of the two for 24 h. Direct observation using inverted
microscopy revealed that morphological changes and decreased cell
density compared with the negative control occurred in only the
group treated with a combination of bufalin and TRAIL (Fig. 2A). In addition, while there was little
change when cells were treated with either bufalin or TRAIL, cells
demonstrated visibly increased chromatin condensation in the nuclei
and increased formation of apoptotic bodies following combined
treatment compared with the negative control (Fig. 2B). To further examine apoptosis, the
size of the sub-G1 cell pool was measured. A total of 3.14% sub-G1
cells were measured following bufalin treatment, 4.10% following
TRAIL treatment and 23.70% following combined treatment in T24
cells (Fig. 2C). These experiments
indicated that bufalin and TRAIL synergistically stimulated
TRAIL-mediated apoptosis in T24 cells.
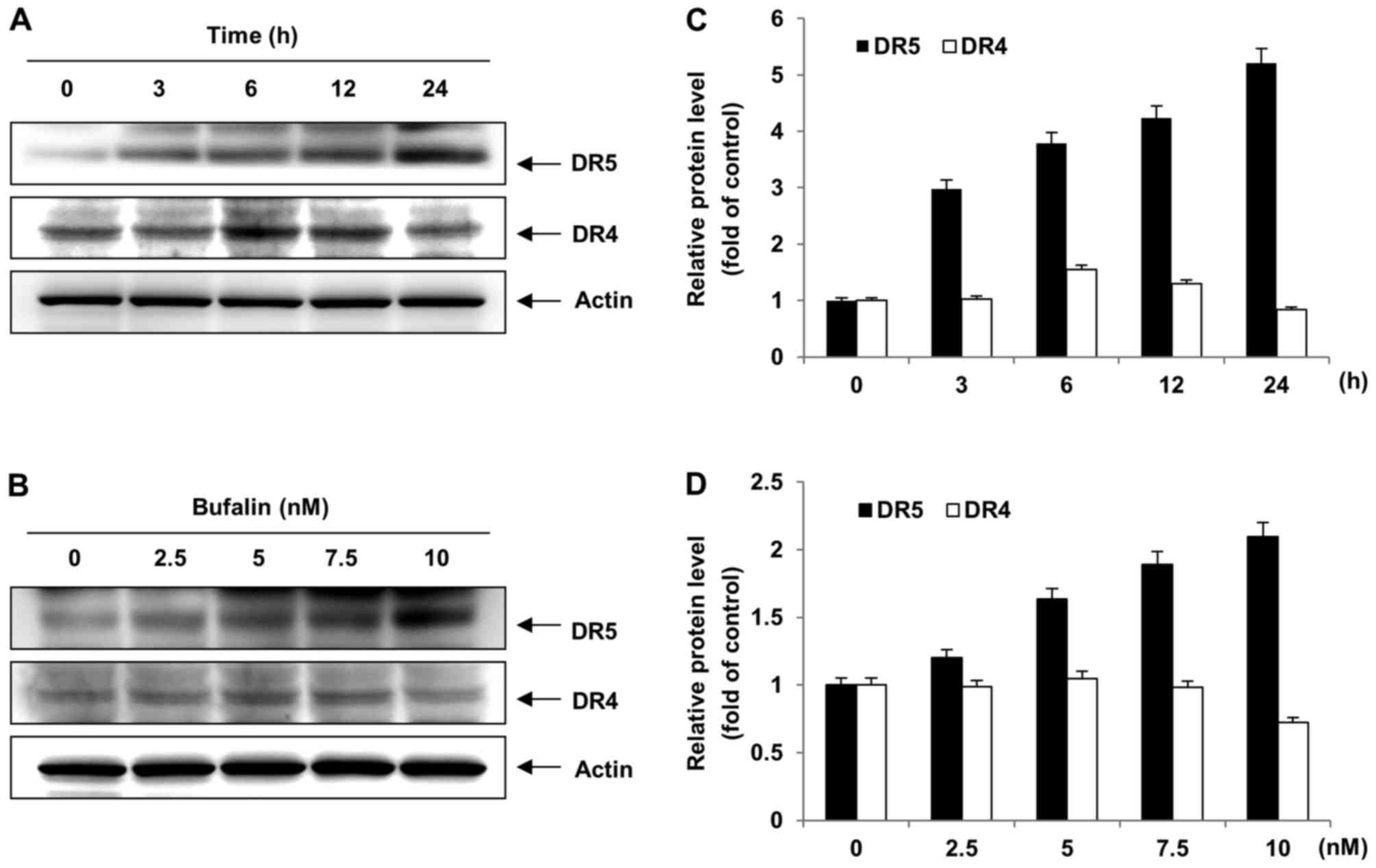
Bufalin upregulates the expression of
DR5 in T24 cells
DR4 and DR5 are expressed in the membrane of
multiple cancer cell lines, and the ligation of TRAIL to DR4 or DR5
allows the initiation of the apoptosis pathway (22). To certify the mechanism of bufalin as
a single agent capable of inducing apoptosis on the expression of
DR4 and DR5 in T24 cells, T24 cells were exposed to 0, 2.5, 5, 7.5
or 10 nM bufalin, or 10 nM bufalin for 0, 3, 6, 12 or 24 h, and
western blot analysis was performed. Bufalin treatment visibly
increased DR5 and DR4 protein levels in T24 cells (Fig. 3). Consistently, treatment with bufalin
enhanced DR5 protein levels, but not DR4 protein levels, following
treatment with the indicated concentrations (Fig. 3) Taken together, these results
suggested that bufalin-induced apoptosis was involved in the
upregulation of DR5 protein levels in T24 cells.
Combination treatment with bufalin and
TRAIL modifies the expression of apoptosis-related proteins
In order to confirm the molecular mechanisms
underlying these effects, the modulation of apoptosis-associated
proteins was evaluated with western blot analysis, to examine
whether a combined bufalin and TRAIL treatment induced apoptosis by
modulating the expression of Bcl-2 family members, which are
important proteins for a wide array of diverse upstream survival
and distress signals (23). Treatment
with bufalin or TRAIL alone, and the combination thereof, did not
affect the expression levels of Bax or Bcl-2 proteins in T24 cells
(Fig. 4). In contrast, the expression
of Bid, a pro-apoptotic protein, was visibly decreased in response
to combined treatment in T24 cells compared with the control
(Fig. 4). The caspase inhibitor
proteins XIAP and cFLIP were downregulated in T24 cells following
combined bufalin and TRAIL treatment (Fig. 4). Consistently, the combination
treatment increased DR5 protein levels, but not DR4 protein levels,
compared with the control (Fig. 4).
As a result, bufalin was demonstrated to sensitize T24 cells to
TRAIL-mediated apoptosis via modulation of Bid, cFLIP, XIAP, and
DR5 protein levels.
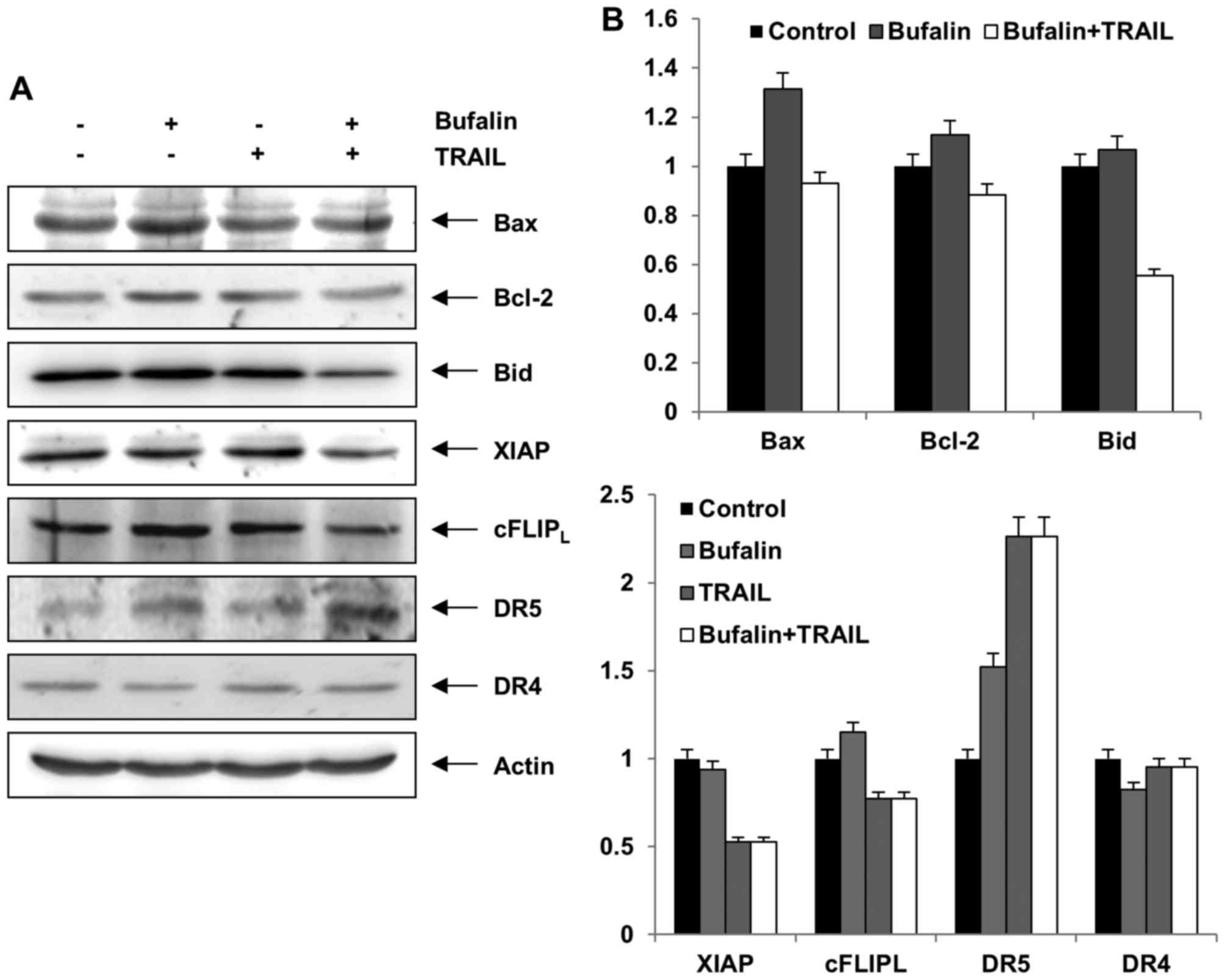 | Figure 4.Effects of bufalin and TRAIL on the
expression of apoptosis related proteins, as assessed by western
blot. (A) T24 cells were incubated with bufalin (10 nM), TRAIL (50
ng/ml) or a combination for 24 h. Actin expression was used as a
loading control. The blots are representative of three experiments.
(B) Relative levels of the indicated proteins. TRAIL, tumor
necrosis factor-related apoptosis-inducing ligand; Bax, BCL2
associated X, apoptosis regulator; Bcl-2, B-cell lymphoma-2; Bid,
BH3 interacting domain death agonist; XIAP, X-linked inhibitor of
apoptosis protein; cFLIP, cellular Fas-associated death domain-like
interleukin-1β-converting enzyme inhibitory protein; DR, death
receptor. |
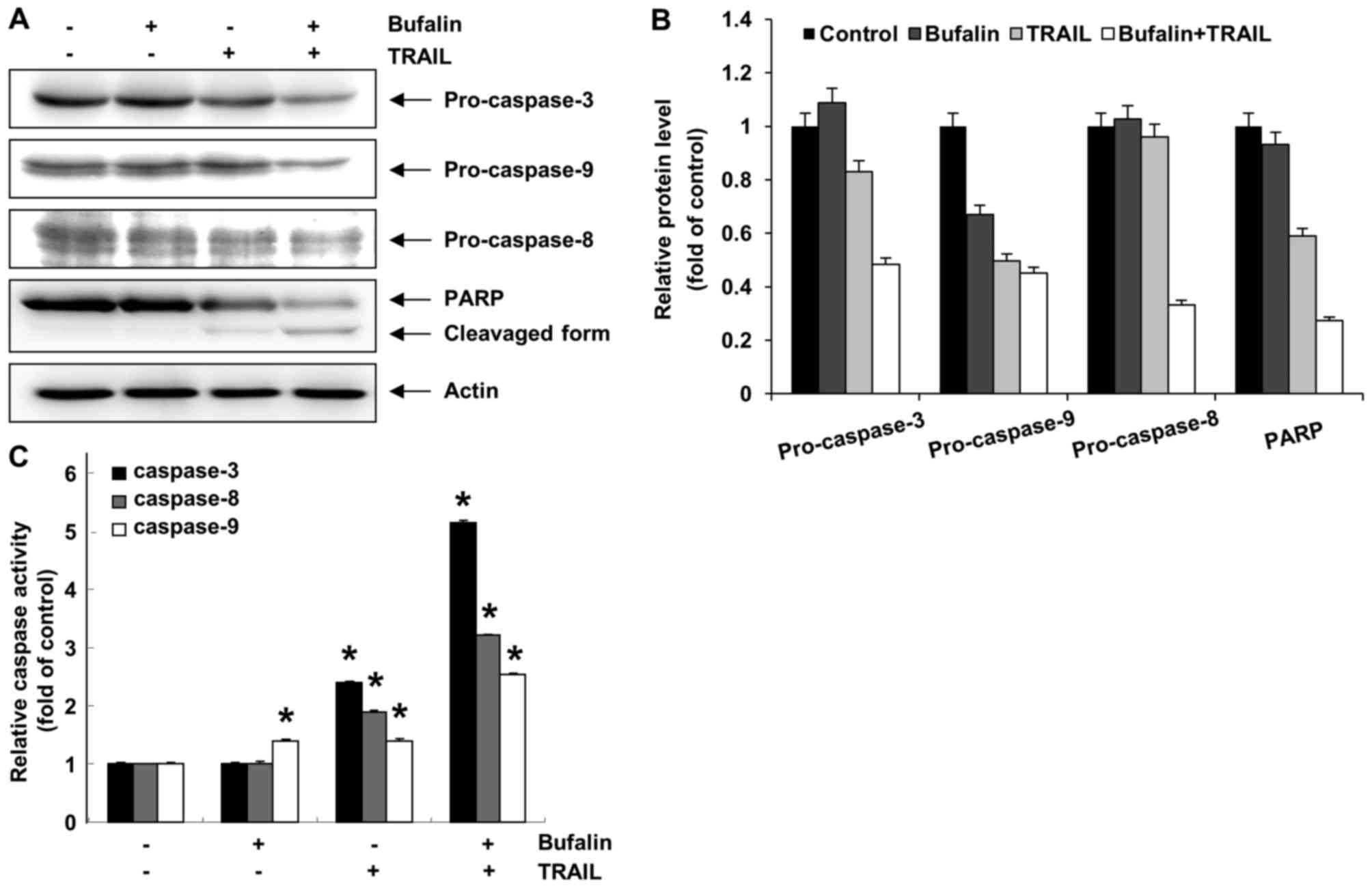
Activation of the caspases is
synergistically induced by bufalin and TRAIL combination treatment
in T24 cells
Activation of caspases works as a key regulator in
the apoptosis signaling pathway (24). Thus, the involvement of various
caspases during the bufalin and TRAIL-mediated apoptosis was
assessed using a western blot analysis and an in vitro
caspase activity test. Although treatment with bufalin or TRAIL did
not induce proteolytic processing of caspases, a combination of
bufalin and TRAIL effectively decreased pro-caspase-3, −8 and −9
levels in T24 cells (Fig. 5). PARP,
which is a substrate protein of caspase-3, was visibly degraded
following combined treatment in T24 cells. Next, cell lysates
containing equal amounts of total protein from the cells treated
with bufalin and TRAIL were analyzed for their in vitro
caspase activity. Combined treatment with bufalin and TRAIL
synergistically promoted caspase-3, −8 and −9 activity in T24
cells. Treatment with bufalin or TRAIL alone did not affect
caspase-3 or −8 activity; 10 nM bufalin treatment significantly
altered caspase-9 activity, whereas treatment with TRAIL alone did
not significantly affect caspase-9 (Fig.
5). These results indicated that caspases were involved in
bufalin and TRAIL-mediated apoptosis in T24 cells.
Discussion
The purpose of the present study was to certify the
potential of bufalin to overcome TRAIL-resistance in human bladder
cancer cells. The T24 bladder cancer cell line was selected. In a
previous sensitivity examination of three human bladder
transitional cell cancer lines to TRAIL-mediated apoptosis, T24
cells (poorly differentiated) and 647V cells (moderately
differentiated) demonstrated greater resistance than SW780 cells
(well differentiated) (4). In another
study, T24 cells and J82 cells demonstrated resistance to TRAIL,
but 5637 cells were sensitive to TRAIL (25). From these previous studies, T24 cells
have a well-established resistance to TRAIL. The data from the
present study revealed that treatment consisting of a non-toxic
dose of bufalin significantly promoted TRAIL-mediated apoptosis in
T24 cell (Fig. 2).
Multiple mechanisms for bladder cancer cell escape
from TRAIL-mediated apoptosis have been reported (4,22,26,27). To
determine the mechanisms by which bufalin mediated the
susceptibility of bladder cancer cells to TRAIL, the expression of
DRs, anti-apoptotic and pro-apoptotic proteins were investigated.
In previous experimental studies, the restoration of sensitization
to TRAIL-induced apoptosis in cancer cells has been associated with
the downregulation of DRs (4,22). In a clinical study, patients with
bladder cancer with either a high DR4 or DR5 expression
demonstrated evidence of a longer recurrence-free rate following
operation than those with a low expression of the two (27). In particular, a number of studies have
demonstrated that upregulating the expression of DR5 may be a more
attractive target than DR4 to sensitize cancer cells to TRAIL
(2,28). In the present study, a combination of
bufalin and TRAIL treatment significantly upregulated the
expression of DR5 protein in T24 bladder cancer cells (Fig. 4). On the other hand, the expression of
DR4 proteins remained almost unchanged, suggesting that increasing
DR5 levels using anti-cancer drugs would lead to sensitization of
the resistant tumor cells.
When TRAIL binds to DRs it forms the death-inducing
signaling complex and leads to the activation of initiator
caspases, including caspase-8, which is known as the death receptor
pathway. This in turn activates the executioner caspases, including
caspase-3, resulting in cell death (28,29). In
the present study, a combination of bufalin and TRAIL significantly
induced the activation of caspase-3, −8 and −9 following the
cleavage of PARP in T24 cells (Fig.
5). Furthermore, the activity of the three caspases was
different. The activation of caspase-3 stood out as being more
important than the rest in T24 cells treated with bufalin and
TRAIL. The activation levels of caspase-8 and caspase-3 were higher
than caspase-9. These data suggested that the death receptor
pathway was more closely associated with bufalin/TRAIL-induced
apoptosis than the mitochondrial pathway. In addition, cFLIP is
known to be an important anti-apoptotic regulator and resistance
factor via suppressing death receptor-mediated apoptosis in
multiple types of cancer cell (30,31).
Therefore, the effect of bufalin/TRAIL on cFLIP expression was
evaluated. Treatment with bufalin and TRAIL downregulated the
expression of cFLIP among the other anti-apoptotic and
pro-apoptotic proteins, indicating that downregulation of cFLIP may
have resulted in caspase-8 activation and apoptosis (Fig. 4) as previously described (30).
Taken together, the data from the present study
demonstrated that a combination treatment of bufalin and TRAIL
increased the level of death receptor DR5 and diminished the level
of the anti-apoptotic protein cFLIP, which contributed to the
activation of caspase-8. The pro-apoptotic protein Bid, through
activated caspase-8, amplifies the apoptotic signal from DR5, but
this combination is provided that a low dose of bufalin was able to
overcome the resistance to TRAIL-mediated apoptosis in bladder
cancer cells. Collectively, bufalin may be a novel anticancer drug
and provide a different treatment option for patients with
TRAIL-resistant bladder cancer.
Acknowledgements
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea,
funded by the Korean government (grant nos. 2013R1A1A2065537,
2015R1A2A2A01004633 and 2016R1C1B2016529).
References
|
1
|
Trivedi R and Mishra DP: Trailing TRAIL
resistance: Novel targets for TRAIL sensitization in cancer cells.
Front Oncol. 5:692015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thorburn A, Behbakht K and Ford H: TRAIL
receptor-targeted therapeutics: Resistance mechanisms and
strategies to avoid them. Drug Resist Updat. 11:17–24. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dai X, Zhang J, Arfuso F, Chinnathambi A,
Zayed ME, Alharbi SA, Kumar AP, Ahn KS and Sethi G: Targeting
TNF-related apoptosis-inducing ligand (TRAIL) receptor by natural
products as a potential therapeutic approach for cancer therapy.
Exp Biol Med (Maywood). 240:760–773. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Szliszka E, Mazur B, Zydowicz G, Czuba ZP
and Król W: TRAIL-induced apoptosis and expression of death
receptor TRAIL-R1 and TRAIL-R2 in bladder cancer cells. Folia
Histochem Cytobiol. 47:579–585. 2009.PubMed/NCBI
|
|
5
|
Lim B, Allen JE, Prabhu VV, Talekar MK,
Finnberg NK and El-Deiry WS: Targeting TRAIL in the treatment of
cancer: New developments. Expert Opin Ther Targets. 19:1171–1185.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen S, Fu L, Raja SM, Yue P, Khuri FR and
Sun SY: Dissecting the roles of DR4, DR5 and c-FLIP in the
regulation of Geranylgeranyltransferase I inhibition-mediated
augmentation of TRAIL-induced apoptosis. Mol Cancer. 9:232010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang S and El-Deiry WS: TRAIL and
apoptosis induction by TNF-family death receptors. Oncogene.
22:8628–8633. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guancial EA, Roussel B, Bergsma DP, Bylund
KC, Sahasrabudhe D, Messing E, Mohile SG and Fung C: Bladder cancer
in the elderly patient: Challenges and solutions. Clin Interv
Aging. 10:939–949. 2015.PubMed/NCBI
|
|
9
|
Shamseddine A, Saleh A, Charafeddine M,
Seoud M, Mukherji D, Temraz S and Sibai AM: Cancer trends in
Lebanon: A review of incidence rates for the period of 2003–2008
and projections until 2018. Popul Health Metr. 12:42014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ahmed SM, Wu X, Jin X, Zhang X, Togo Y,
Suzuki T, Li Y, Kanematsu A, Nojima M, Yamamoto S, et al:
Synergistic induction of apoptosis by mapatumumab and
anthracyclines in human bladder cancer cells. Oncol Rep.
33:566–572. 2015.PubMed/NCBI
|
|
11
|
Ploeg M, Aben KK and Kiemeney LA: The
present and future burden of urinary bladder cancer in the world.
World J Urol. 27:289–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan S, Qu X, Xu L, Che X, Ma Y, Zhang L,
Teng Y, Zou H and Liu Y: Bufalin enhances TRAIL-induced apoptosis
by redistributing death receptors in lipid rafts in breast cancer
cells. Anticancer Drugs. 25:683–689. 2014.PubMed/NCBI
|
|
13
|
White-Gilbertson SJ, Kasman L, McKillop J,
Tirodkar T, Lu P and Voelkel-Johnson C: Oxidative stress sensitizes
bladder cancer cells to TRAIL mediated apoptosis by down-regulating
anti-apoptotic proteins. J Urol. 182:1178–1185. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
LeBlanc H, Lawrence D, Varfolomeev E,
Totpal K, Morlan J, Schow P, Fong S, Schwall R, Sinicropi D and
Ashkenazi A: Tumor-cell resistance to death receptor-induced
apoptosis through mutational inactivation of the proapoptotic Bcl-2
homolog Bax. Nat Med. 8:274–281. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi YL, Feng S, Chen W, Hua ZC, Bian JJ
and Yin W: Mitochondrial inhibitor sensitizes non-small-cell lung
carcinoma cells to TRAIL-induced apoptosis by reactive oxygen
species and Bcl-X(L)/p53-mediated amplification mechanisms. Cell
Death Dis. 5:e15792014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yin PH, Liu X, Qiu YY, Cai JF, Qin JM, Zhu
HR and Li Q: Anti-tumor activity and apoptosis-regulation
mechanisms of bufalin in various cancers: New hope for cancer
patients. Asian Pac J Cancer Prev. 13:5339–5343. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qi F, Li A, Inagaki Y, Kokudo N, Tamura S,
Nakata M and Tang W: Antitumor activity of extracts and compounds
from the skin of the toad Bufo bufo gargarizans Cantor. Int
Immunopharmacol. 11:342–349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang WW, Yang JS, Pai SJ, Wu PP, Chang
SJ, Chueh FS, Fan MJ, Chiou SM, Kuo HM, Yeh CC, et al: Bufalin
induces G0/G1 phase arrest through inhibiting the levels of cyclin
Dcyclin E, CDK2 and CDK4, and triggers apoptosis via mitochondrial
signaling pathway in T24 human bladder cancer cells. Mutat Res.
732:26–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takai N, Ueda T, Nishida M, Nasu K and
Narahara H: Bufalin induces growth inhibition, cell cycle arrest
and apoptosis in human endometrial and ovarian cancer cells. Int J
Mol Med. 21:637–643. 2008.PubMed/NCBI
|
|
20
|
Yeh JY, Huang WJ, Kan SF and Wang PS:
Effects of bufalin and cinobufagin on the proliferation of androgen
dependent and independent prostate cancer cells. Prostate.
54:112–124. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan S, Qu X, Xu C, Zhu Z, Zhang L, Xu L,
Song N, Teng Y and Liu Y: Down-regulation of Cbl-b by bufalin
results in up-regulation of DR4/DR5 and sensitization of
TRAIL-induced apoptosis in breast cancer cells. J Cancer Res Clin
Oncol. 138:1279–1289. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kelley RF, Totpal K, Lindstrom SH, Mathieu
M, Billeci K, Deforge L, Pai R, Hymowitz SG and Ashkenazi A:
Receptor-selective mutants of apoptosis-inducing ligand 2/tumor
necrosis factor- related apoptosis-inducing ligand reveal a greater
contribution of death receptor (DR) 5 than DR4 to apoptosis
signaling. J Biol Chem. 280:2205–2212. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yin XM: Signal transduction mediated by
Bid, a pro-death Bcl-2 family proteins, connects the death receptor
and mitochondria apoptosis pathways. Cell Res. 10:161–167. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shalini S, Dorstyn L, Dawar S and Kumar S:
Old, new and emerging functions of caspases. Cell Death Differ.
22:526–539. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jin CY, Park C, Hwang HJ, Kim GY, Choi BT,
Kim WJ and Choi YH: Naringenin up-regulates the expression of death
receptor 5 and enhances TRAIL-induced apoptosis in human lung
cancer A549 cells. Mol Nutr Food Res. 55:300–309. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hong SH and Choi YH: Bufalin induces
apoptosis through activation of both the intrinsic and extrinsic
pathways in human bladder cancer cells. Oncol Rep. 27:114–120.
2012.PubMed/NCBI
|
|
27
|
Li Y, Jin X, Li J, Jin X, Yu J, Sun X, Chu
Y, Xu C, Li X, Wang X, et al: Expression of TRAIL, DR4, and DR5 in
bladder cancer: Correlation with response to adjuvant therapy and
implications of prognosis. Urology. 79:968.e7–e15. 2012. View Article : Google Scholar
|
|
28
|
Wu GS: TRAIL as a target in anti-cancer
therapy. Cancer Lett. 285:1–5. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Z, Sangwan V, Banerjee S, Chugh R,
Dudeja V, Vickers SM and Saluja AK: Triptolide sensitizes
pancreatic cancer cells to TRAIL-induced activation of the death
receptor pathway. Cancer Lett. 348:156–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Safa AR: c-FLIP, a master anti-apoptotic
regulator. Exp Oncol. 34:176–184. 2012.PubMed/NCBI
|
|
31
|
Ewald F, Ueffing N, Brockmann L, Hader C,
Telieps T, Schuster M, Schulz WA and Schmitz I: The role of c-FLIP
splice variants in urothelial tumours. Cell Death Dis. 2:e2452011.
View Article : Google Scholar : PubMed/NCBI
|