Introduction
Lung cancer is a leading cause of cancer-associated
mortality worldwide (1). Non-small
cell lung cancer (NSCLC) accounts for 80% of all cases of lung
cancer, and ~30% of patients with NSCLC present with locally
advanced lung cancer. The standard treatment for patients with
locally advanced NSCLC (LA-NSCLC) possessing a good Eastern
cooperative oncology group performance status (ECOG-PS) (0 or 1)
and adequate organ function is thoracic radiotherapy (TRT) combined
with chemotherapy (2,3). Previous randomized trials demonstrated
that concurrent cytotoxic chemoradiotherapy (cCRT) with a
third-generation regimen, a combination of a platinum compound with
novel agents, is more effective compared with second-generation
regimens (4,5). However, the majority of treated
individuals developed disease recurrence, with a 5-year survival
rate of 15–20% (3,5). The identification of somatic gene
mutations in the tyrosine kinase domain of the epidermal growth
factor receptor (EGFR) (6,7) led to the development of a novel
treatment strategy for patients with advanced NSCLC (8–11).
Molecular profiling has become essential for the treatment of
patients with advanced NSCLC to predict the response to specific
molecular targeted agents such as EGFR tyrosine kinase inhibitors
(TKIs). Despite the developments in this area of molecular biology,
and the discovery of EGFR mutation, there have been no additional
improvement in the treatment of LA-NSCLC in the previous decade. In
this context, additional research is required to understand the
biological behavior of the population of patients with LA-NSCLC
with EGFR mutations. The objective of the present study was to
evaluate and validate the frequency of EGFR mutations among
patients with LA-NSCLC, and the clinical efficacy of cCRT in
patients with LA-NSCLC according to EGFR mutation status.
Patients and methods
Patient selection
The patients enrolled in the present retrospective
cohort study were diagnosed with unresectable LA-NSCLC and received
cisplatin-based chemotherapy with cCRT at the Kitasato University
Hospital (Sagamihara, Japan) between January 2007 and December
2013. All patients were histologically or cytologically diagnosed
with NSCLC, and received a thoracic radiotherapy (TRT) dose of
50–60 Gy. Medical records were reviewed to collect patient data and
data associated with the tumors including the age, gender, tumor
EGFR mutation status, clinical disease stage, ECOG-PS, smoking
history and presence or absence of a history of EGFR-TKI therapy in
each patient. The patients were classified according to their
smoking status as non-smokers, <100 cigarettes in a lifetime, or
current/former smokers. All patients with unknown EGFR mutation
status and patients who underwent induction chemoradiotherapy
followed by definitive surgery were excluded. The present study was
carried out with approval from the Institutional Review Board at
Kitasato University School of Medicine (Sagamihara, Japan).
Response analysis
Tumor response was classified according to the
Response Evaluation Criteria in Solid Tumors (version 1.1)
(12). Patients were evaluated to
identify the evaluable lesions prior to chemoradiotherapy. If chest
radiography suggested disease progression or recurrence, additional
detailed examination was performed by computed tomography (CT)
scans of the chest and abdomen, and other imaging techniques such
as magnetic resonance imaging of the head and fludeoxyglucose
positron emission tomography.
Analysis for detecting EGFR
mutations
Cytologic or histologic specimens were examined for
the presence or absence of EGFR mutations by the peptide nucleic
acid (PNA)-locked nucleic acid (LNA) polymerase chain reaction
(PCR) clamp method or Cycleave method as previously described
(13,14).
Treatment methods
All patients were treated with TRT and 2 cycles of
cCRT. TRT was administered in 2 Gy daily standard fractionation
using 6 or 10 MV X-rays, depending on the position and size of the
individual tumors. The total target dose of radiation was fixed at
60 Gy. A CT-based treatment-planning system was mandatory to define
the planning target volume. Dose distribution was calculated with
tissue heterogeneity correction. The radiation field was reduced
around the primary tumor and involved the lymph nodes, subsequent
to exposure to 40 Gy using adequate fields to limit the dose to the
spinal cord, which received a maximum dose ≤50 Gy. The planned
percentages of lung volume receiving >20 Gy (V20) was <35%.
cCRT consisted of 80 mg/m2 cisplatin on day 1 and 20
mg/m2 vinorelbine on days 1 and 8. Subsequently, the
patients received 2 cycles of consolidation chemotherapy with 80
mg/m2 cisplatin on day 1 and 25 mg/m2
vinorelbine on days 1 and 8.
Statistical analysis
The differences in the response rates and recurrence
patterns according to the tumor EGFR mutation status were compared
using the χ2 test. Progression-free survival (PFS) was
measured from the date of start of cCRT to the date of
documentation of treatment failure; death, disease progression or
appearance of unacceptable toxicity, or the date of censoring at
the last follow-up examination. Overall survival (OS) was defined
as the interval between the date of start of cCRT and date of death
from any cause or the date of censoring. Post-progression survival
(PPS) was measured from the date of documentation of disease
progression to the date of death from any cause or the date of
censoring. The survival curves were plotted using the Kaplan-Meier
method, and the differences between the survival times were
analyzed using the log-rank test. The variables, including gender,
smoking status, PS, status of EGFR mutation, histology and clinical
stage were used as variables in a Cox's proportional hazards model
to determine the hazard ratios for OS and PPS. P<0.05 was
considered to indicate a statistically significant difference, and
statistical analysis was performed using SPSS, version 17.0 (SPSS,
Inc., Chicago, IL, USA) for Windows.
Results
Patient characteristics
The data of 64 patients with unresectable LA-NSCLC
who received cCRT with cisplatin-based chemotherapy were examined.
The median follow-up time was 27.4 months. The main clinical
characteristics of the patients are summarized in Table I. Amongst the 64 patients, 15 (23%)
patients possessed EGFR mutations in the tumor. EGFR mutations in
the tumors were observed predominantly in non-smokers. All the
patients possessed an ECOG-PS of 0–1, and the distribution of the
clinical stage was not statistically different between the mutant
and wild-type EGFR groups.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
| EGFR mutation
(%) |
|
|---|
|
|
|
|
|---|
| Characteristics | Mutant (n=15) | Wild type (n=49) |
|
|---|
| Age |
|
|
|
| Median,
range | 61, 52–72 | 60, 34–74 |
|
| Gender |
|
|
|
| Male | 10 (67) | 38 (78) |
|
|
Female | 5
(33) | 11 (22) |
|
| Smoking status |
|
|
|
|
Non-smoker | 8
(53) | 6
(12) |
|
|
Current/former smoker | 7
(47) | 43 (88) | P<0.001 |
| ECOG-PS |
|
|
|
| 0–1 | 15
(100) | 49 (100) |
|
| 2–4 | 0 | 0 |
|
| Clinical stage |
|
|
|
| IIIA | 7
(47) | 22 (45) |
|
| IIIB | 8
(53) | 27 (55) |
|
| Chemotherapy
regimen |
|
|
|
| Platinum
based regimen | 15
(100) | 49
(100) |
|
| Presence of EGFR-TKI
therapy | 15
(100) | 3
(0.12) | P<0.0001 |
Response and survival
The objective tumor responses are summarized in
Table II. The overall response rate
was 73.4%. The response rates (RRs) in the patient groups with
mutant and wild-type EGFR in the tumors were 66.7 and 75.5%,
respectively, and no statistically significant difference was
observed. The median time to achieve the objective response was not
significantly different between the mutant and wild-type EGFR
groups, 1.25 and 1.28 months, respectively. The crude recurrence
rates in the mutant and wild-type EGFR groups were 100 (15/15) and
89% (44/49), respectively. The frequency of distant metastasis was
significantly higher (P=0.01) in the mutant EGFR group compared
with the wild-type EGFR group (Table
III).
 | Table II.Tumor response. |
Table II.
Tumor response.
|
|
| EGFR mutation |
|
|---|
|
|
|
|
|
|---|
| Variables | All patients
(n=64) | Mutant (n=15) | Wild type
(n=49) |
P-valuea |
|---|
| Complete
response | 2 | 0 | 2 |
|
| Partial
response | 45 | 10 | 35 |
|
| Stable disease | 12 | 5 | 7 |
|
| Progressive
disease | 4 | 0 | 4 |
|
| Not evaluable | 1 | 0 | 1 |
|
| Response rate
(%) |
73.4 |
66.7 |
75.5 | 0.84 |
 | Table III.Recurrence rate and recurrence
pattern. |
Table III.
Recurrence rate and recurrence
pattern.
|
| EGFR mutation |
|
|---|
|
|
|
|
|---|
| Variables | Mutant (n=15) | Wild type
(n=49) | P-value |
|---|
| Crude recurrence
rate (%) | 15 (100) | 44 (89) |
|
| Loco-regional
recurrence | 1 | 15 | 0.06 |
| Distant
recurrence | 14 | 29 | 0.01 |
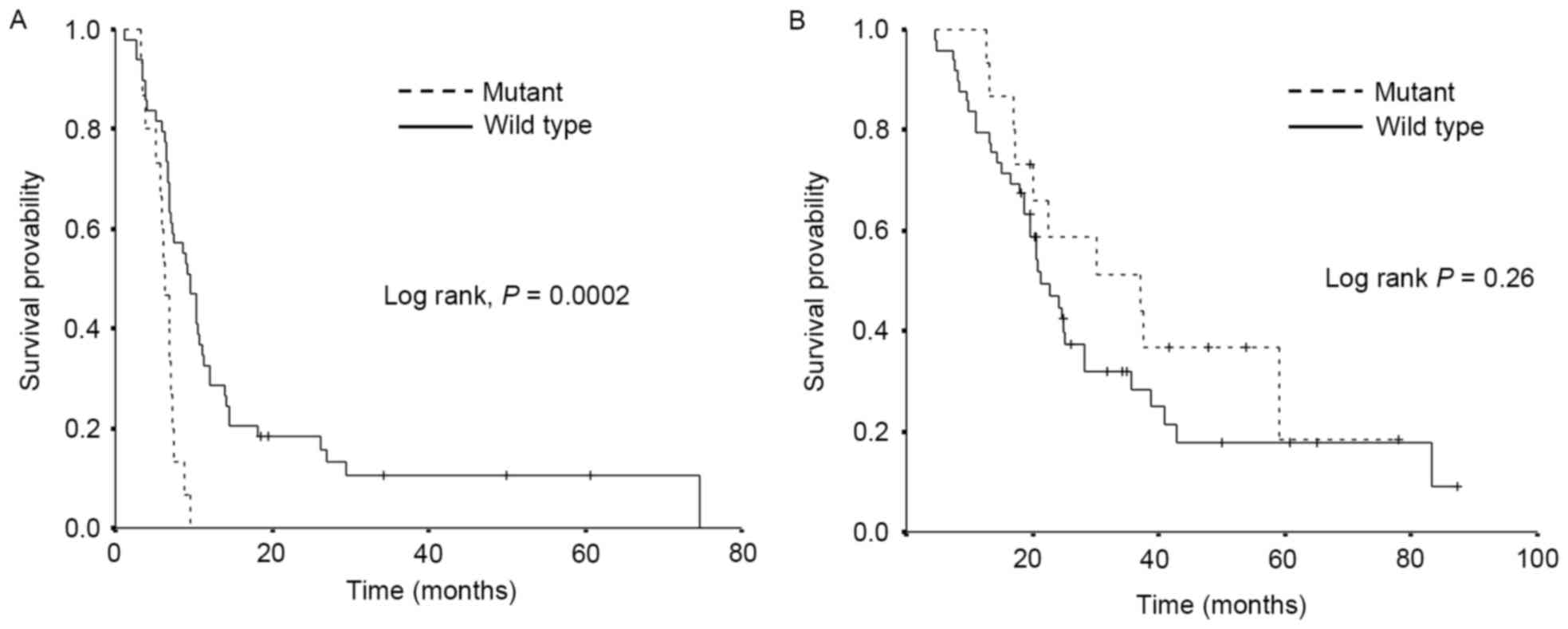
The survival data are demonstrated in Fig. 1. A significantly shorter PFS was
observed in the mutant EGFR group compared with the wild-type EGFR
group, median PFS 6.3 and 9.5 months, respectively (P<0.001).
Conversely, there was no significant difference in OS observed
between the two groups, although OS tended to be longer in the
mutant EGFR group, median OS 37.1 and 21.1 months, respectively
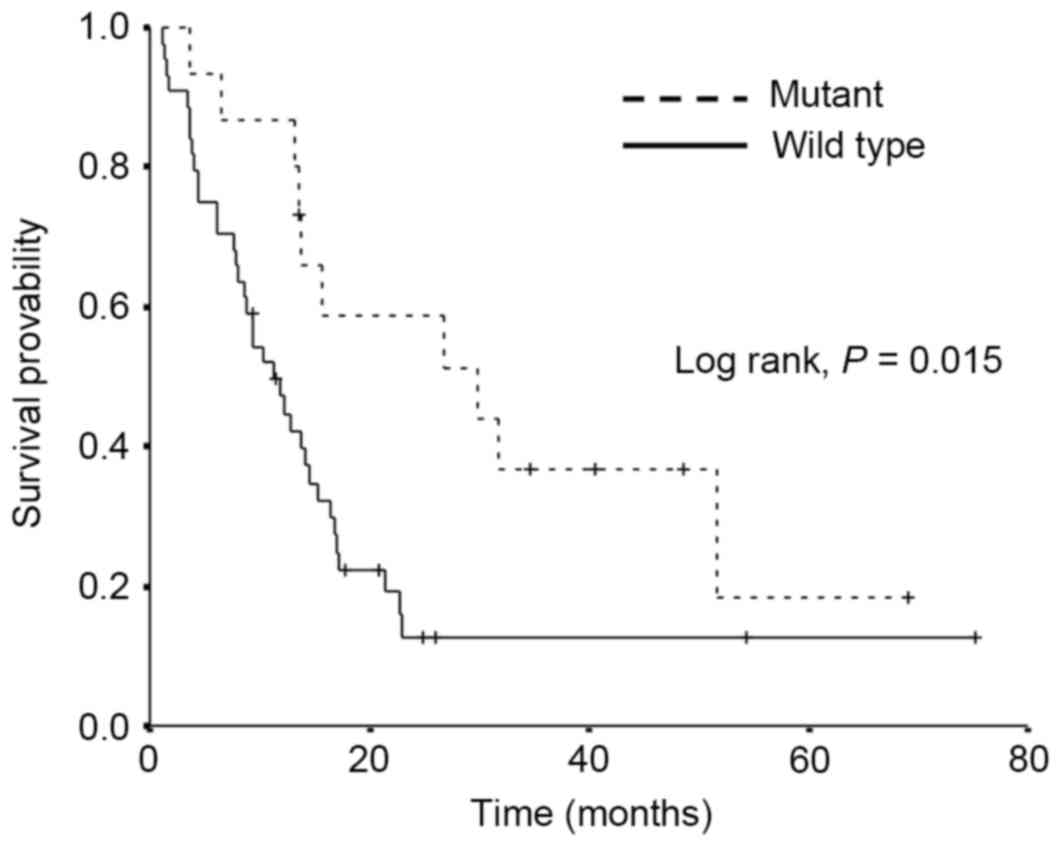
(P=0.26). The PPS data are demonstrated in Fig. 2. Amongst the patients who exhibited
disease relapse, the PPS was significantly longer in the mutant
EGFR group compared with the wild-type EGFR group, median PPS 29.9
and 11.2 months, respectively (P=0.015).
The results of multivariate analysis are summarized
in Table IV. The presence of EGFR
mutation and diagnosis of clinical stage were independent
prognostic factors of short PFS. Additionally, the presence of EGFR
mutation tended to be a predictor of a long PPS.
 | Table IV.Multivariate analysis using a Cox's
proportional hazards regression model. |
Table IV.
Multivariate analysis using a Cox's
proportional hazards regression model.
| A, PFS |
|---|
|
|---|
|
| Multivariate
analysis |
|---|
|
|
|
|---|
| Variables | HR (95% CI) | P-value |
|---|
| Gender | 1.07
(0.50–2.25) | 0.87 |
| ECOG-PS (0 vs.
1) | 0.95
(0.51–1.77) | 0.87 |
| Smoking
(current/former smoker vs. non-smoker) | 0.79
(0.35–1.76) | 0.56 |
| EGFR (mutant vs.
wild type) | 3.23
(1.51–6.88) | <0.01 |
| Clinical stage
(IIIA vs. IIIB) | 1.94
(1.07–3.52) | 0.03 |
| Histology (Sq. vs.
Non-Sq.) | 1.06
(0.48–2.30) | 0.89 |
|
| B, PPS |
|
|
| Multivariate
analysis |
|
|
|
| Variables | HR (95% CI) | P-value |
|
| Gender | 1.45
(0.64–3.26) | 0.38 |
| ECOG-PS (0 vs.
1) | 1.15
(0.55–2.40) | 0.72 |
| Smoking
(current/former smoker vs. non-smoker) | 0.93
(0.35–2.46) | 0.89 |
| EGFR (mutant vs.
wild type) | 0.41
(0.16–1.03) | 0.06 |
| Clinical stage
(IIIA vs. IIIB) | 0.79
(0.43–1.45) | 0.44 |
| Histology (Sq. vs.
Non-Sq.) | 1.17
(0.50–2.78) | 0.71 |
Discussion
The present study evaluated the effect of EGFR
mutation in patients with LA-NSCLC who underwent cCRT with
definitive radiotherapy. In the present study, PFS was revealed to
be significantly shorter in the EGFR mutant group compared with the
wild type group. Disease recurrence was revealed to occur more
frequently in the EGFR mutant group compared with the wild type
group, particularly more frequently in the distant site from the
primary region.
Other studies have reported on the association
between EGFR status and treatment outcomes subsequent to cCRT in
patients with LA-NSCLC. Amongst these studies, the majority of
groups reported that PFS and recurrence rate were not statistically
different (15–17) between the two groups. In the present
study PFS was shorter and recurrence rate was higher in the EGFR
mutant group, which was inconsistent with data from previous
studies. Only one study, Tanaka et al (18), demonstrated that the PFS was shorter
and 2-year recurrence-free survival rate was poorer in the EGFR
mutant group.
Notably, the recurrence patterns in these studies
subsequent to cCRT in LA-NSCLC setting were similar to those of the
present study, which demonstrated that the EGFR mutant groups
exhibited lower loco-regional recurrence rate (15,16,18).
Preclinical studies have demonstrated that NSCLC cell lines with
EGFR mutations exhibit greater radiosensitivity compared with those
without EGFR mutation. The assumed mechanism underlying this
observation is the delayed repair of radiation-induced DNA damage
in these cells (19,20). These preclinical studies may measure
the aforementioned clinical observations, better local control of
the cCRT for EGFR mutant patients, as the anticancer effect for
irradiated field is superior for the EGFR mutant tumors.
Although these observations promote the expectation
that cCRT is a potentially more favorable treatment in patients
with LA-NSCLC that possess EGFR mutations, these studies do not
demonstrate that cCRT is more beneficial compared with PFS.
Additionally, the PFS was significantly shorter in the EGFR mutant
group in the present study. To explain this discrepancy, the
recurrence rate in the distant site was a focus of the present
study. It was significantly higher in the EGFR mutant group
compared with the wild type (P=0.01). Therefore, it may be
hypothesized that the EGFR mutant tumors were more likely to result
in distant metastasis. These data regarding recurrence patterns are
considered important as it suggests that the improvement of the
treatment strategy for patients with EGFR mutant LA-NSCLC is
required.
Regarding OS, the present study demonstrated a
tendency towards a longer median OS and a significantly longer
median PPS in the mutant EGFR group compared with the wild-type
EGFR group. Previous studies have revealed that in patients with
metastatic NSCLC who possess EGFR mutations, EGFR-TKI therapy was
associated with higher response rates and a longer PFS compared
with standard chemotherapy (8–11).
Therefore, the present study hypothesized that the addition of
EGFR-TKI therapy alongside standard chemotherapy may account for
the longer OS and PPS in the mutant EGFR group compared with the
wild-type EGFR group in the groups of the present study. The
multivariate analysis of PPS performed in the present study
supports this hypothesis. Several studies have reported that EGFR
inhibition enhances the antitumor activity of ionizing radiation
in vitro (21–23). Accordingly, it may be appropriate to
suggest that a combined therapy of EGFR inhibitor and TRT may
improve the probability of a cure in patients with LA-NSCLC who
possess an EGFR mutation. However, few clinical trials have been
able to demonstrate a clear benefit of combined EGFR-TKI therapy
with TRT in patients with LA-NSCLC (24–29). In an
attempt to obtain an answer to this question, the WJOG6911L trial
is currently in progress in Japan, which is a multicenter phase II
trial of gefitinib administration in combination with radiotherapy
in patients with LA-NSCLC who possess sensitizing EGFR mutations
(trial no., UMIN000008366).
The data of the present study demonstrated a higher
recurrence rate in the EGFR mutant and wild type groups compared
with historical control data of phase III studies of cCRT (3,4). It is
hypothesized that two of the eligibility criteria of the patients
may have caused this difference. Firstly, the result of the EGFR
mutation analysis was required for the present study, which was
possibly absent if a patient survived without disease recurrence.
Secondly, patients who were treated with induction
chemoradiotherapy followed by definitive surgery (CRT+S) were
excluded. In Kitasato University Hospital (Sagamihara, Japan),
CRT+S was performed for the patients in which the tumors were
nearly resectable stage IIIA tumors that had been successfully
downgraded in stage, and had become resectable with a good response
for the induction chemoradiotherapy. It is suggested that exclusion
of these patient groups who exhibited better prognoses caused the
higher recurrence rate.
There were several limitations to the present study.
First, as a retrospective study the results cannot be regarded as
definitive. Secondly, the sample size may not have been sufficient.
Thirdly, there was no pharmacokinetic validation for the
differences in the efficacy of chemotherapy according to the tumor
EGFR mutation status in the present study. In addition, the
eligibility criteria of the patients included a result of the EGFR
mutation status, which may have introduced some bias during patient
selection. The absence of EGFR mutation analysis was most commonly
due to the histological diagnosis of the patients, such as squamous
cell carcinoma.
In conclusion, the present study confirmed that
conventional cCRT using platinum based regimen may not be the most
effective type of treatment for patients with LA-NSCLC who possess
EGFR mutations. The results of ongoing studies including the
WJOG6911L trial are required to identify novel strategies for
improving the efficacy of cCRT, with special consideration given to
the potential use of EGFR-TKIs.
Acknowledgements
The authors would like to thank the staff members in
the Department of Respiratory Medicine, Kitasato University School
of Medicine (Sagamihara, Japan) for their suggestion and
assistance. The present study was supported by Grant-in-Aid for
Scientific Research (C) (grant no. 26461896) from the Japan Society
for the Promotion of Science (Tokyo, Japan).
References
|
1
|
Siegel R, DeSantis C, Virgo K, Stein K,
Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pritchard RS and Anthony SP: Chemotherapy
plus radiotherapy compared with radiotherapy alone in the treatment
of locally advanced, unresectable, non-small-cell lung cancer. A
meta-analysis. Ann Intern Med. 125:723–729. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Furuse K, Fukuoka M, Kawahara M, Nishikawa
H, Takada Y, Kudoh S, Katagami N and Ariyoshi Y: Phase III study of
concurrent versus sequential thoracic radiotherapy in combination
with mitomycin, vindesine, and cisplatin in unresectable stage III
non-small-cell lung cancer. J Clin Oncol. 17:2692–2699. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Segawa Y, Kiura K, Takigawa N, Kamei H,
Harita S, Hiraki S, Watanabe Y, Sugimoto K, Shibayama T, Yonei T,
et al: Phase III trial comparing docetaxel and cisplatin
combination chemotherapy with mitomycin, vindesine, and cisplatin
combination chemotherapy with concurrent thoracic radiotherapy in
locally advanced non-small-cell lung cancer: OLCSG 0007. J Clin
Oncol. 28:3299–3306. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamamoto N, Nakagawa K, Nishimura Y,
Tsujino K, Satouchi M, Kudo S, Hida T, Kawahara M, Takeda K,
Katakami N, et al: Phase III study comparing second- and
third-generation regimens with concurrent thoracic radiotherapy in
patients with unresectable stage III non-small-cell lung cancer:
West Japan Thoracic Oncology Group WJTOG0105. J Clin Oncol.
28:3739–3745. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): An open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagai Y, Miyazawa H, Huqun, Tanaka T,
Udagawa K, Kato M, Fukuyama S, Yokote A, Kobayashi K, Kanazawa M
and Hagiwara K: Genetic heterogeneity of the epidermal growth
factor receptor in non-small cell lung cancer cell lines revealed
by a rapid and sensitive detection system, the peptide nucleic
acid-locked nucleic acid PCR clamp. Cancer Res. 65:7276–7282. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yatabe Y, Hida T, Horio Y, Kosaka T,
Takahashi T and Mitsudomi T: A rapid, sensitive assay to detect
EGFR mutation in small biopsy specimens from lung cancer. J Mol
Diagn. 8:335–341. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Akamatsu H, Kaira K, Murakami H, Serizawa
M, Koh Y, Ono A, Shukuya T, Tsuya A, Nakamura Y, Kenmotsu H, et al:
The impact of clinical outcomes according to EGFR mutation status
in patients with locally advanced lung adenocarcinoma who recieved
concurrent chemoradiotherapy. Am J Clin Oncol. 37:144–147. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yagishita S, Horinouchi H, Taniyama T
Katsui, Nakamichi S, Kitazono S, Mizugaki H, Kanda S, Fujiwara Y,
Nokihara H, Yamamoto N, et al: Epidermal growth factor receptor
mutation is associated with longer local control after definitive
chemoradiotherapy in patients with stage III nonsquamous
non-small-cell lung cancer. Int J Radiat Oncol Biol Phys.
91:140–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hayashi H, Okamoto I, Kimura H, Sakai K,
Nishimura Y, Nishio K and Nakagawa K: Clinical outcomes of thoracic
radiotherapy for locally advanced NSCLC with EGFR mutations or
EML4-ALK rearrangement. Anticancer Res. 32:4533–4537.
2012.PubMed/NCBI
|
|
18
|
Tanaka K, Hida T, Oya Y, Oguri T, Yoshida
T, Shimizu J, Horio Y, Hata A, Kaji R, Fujita II, et al: EGFR
mutationimpact on definitiveconcurrent chemoradiation therapy for
inoperable stage III adenocarcinoma. J Thorac Oncol. Sep
2–2015.(Epub ahead of print). View Article : Google Scholar
|
|
19
|
Das AK, Sato M, Story MD, Peyton M, Graves
R, Redpath S, Girard L, Gazdar AF, Shay JW, Minna JD and Nirodi CS:
Non-small-cell lung cancers with kinase domain mutations in the
epidermal growth factor receptor are sensitive to ionizing
radiation. Cancer Res. 66:9601–9608. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Das AK, Chen BP, Story MD, Sato M, Minna
JD, Chen DJ and Nirodi CS: Somatic mutations in the tyrosine kinase
domain of epidermal growth factor receptor (EGFR) abrogate
EGFR-mediated radioprotection in non-small cell lung carcinoma.
Cancer Res. 67:5267–5274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
She Y, Lee F, Chen J, Haimovitz-Friedman
A, Miller VA, Rusch VR, Kris MG and Sirotnak FM: The epidermal
growth factor receptor tyrosine kinase inhibitor ZD1839 selectively
potentiates radiation response of human tumors in nude mice, with a
marked improvement in therapeutic index. Clin Cancer Res.
9:3773–3778. 2003.PubMed/NCBI
|
|
22
|
Chinnaiyan P, Huang S, Vallabhaneni G,
Armstrong E, Varambally S, Tomlins SA, Chinnaiyan AM and Harari PM:
Mechanisms of enhanced radiation response following epidermal
growth factor receptor signaling inhibition by erlotinib (Tarceva).
Cancer Res. 65:3328–3335. 2005.PubMed/NCBI
|
|
23
|
Raben D, Helfrich B, Chan DC, Ciardiello
F, Zhao L, Franklin W, Barón AE, Zeng C, Johnson TK and Bunn PA Jr:
The effects of cetuximab alone and in combination with radiation
and/or chemotherapy in lung cancer. Clin Cancer Res. 11:795–805.
2005.PubMed/NCBI
|
|
24
|
Koh PK, Faivre-Finn C, Blackhall FH and De
Ruysscher D: Targeted agents in non-small cell lung cancer (NSCLC):
Clinical developments and rationale for the combination with
thoracic radiotherapy. Cancer Treat Rev. 38:626–640. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Okamoto I, Takahashi T, Okamoto H,
Nakagawa K, Watanabe K, Nakamatsu K, Nishimura Y, Fukuoka M and
Yamamoto N: Single-agent gefitinib with concurrent radiotherapy for
locally advanced non-small cell lung cancer harboring mutations of
the epidermal growth factor receptor. Lung Cancer. 72:199–204.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Center B, Petty WJ, Ayala D, Hinson WH,
Lovato J, Capellari J, Oaks T, Miller AA and Blackstock AW: A phase
I study of gefitinib with concurrent dose-escalated weekly
docetaxel and conformal three-dimensional thoracic radiation
followed by consolidative docetaxel and maintenance gefitinib for
patients with stage III non-small cell lung cancer. J Thorac Oncol.
5:69–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rothschild S, Bucher SE, Bernier J,
Aebersold DM, Zouhair A, Ries G, Lombrieser N, Lippuner T, Lütolf
UM, Glanzmann C and Ciernik IF: Gefitinib in combination with
irradiation with or without cisplatin in patients with inoperable
stage III non-small cell lung cancer: A phase I trial. Int J Radiat
Oncol Biol Phys. 80:126–132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ready N, Jänne PA, Bogart J, Dipetrillo T,
Garst J, Graziano S, Gu L, Wang X, Green MR and Vokes EE: Cancer,
Leukemia Group B, Chicago, IL: Chemoradiotherapy and gefitinib in
stage III non-small cell lung cancer with epidermal growth factor
receptor and KRAS mutation analysis: Cancer and leukemia group B
(CALEB) 30106, a CALGB-stratified phase II trial. J Thorac Oncol.
5:1382–1390. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Choong NW, Mauer AM, Haraf DJ, Lester E,
Hoffman PC, Kozloff M, Lin S, Dancey JE, Szeto L, Grushko T, et al:
Phase I trial of erlotinib-based multimodality therapy for
inoperable stage III non-small cell lung cancer. J Thorac Oncol.
3:1003–1011. 2008. View Article : Google Scholar : PubMed/NCBI
|