Introduction
α-fetoprotein (AFP)-producing gastric cancer (APGC)
is a rare subtype of gastric cancer that accounts for between 1.3
and 15% of all gastric cancer (GC) (1). APGC is characterized by a significant
increase in serum AFP and also AFP expression in tumor cells
(2). The potential underlying
molecular mechanism may be the common embryonic origin of the
stomach and liver from the foregut (3). The genes responsible for expression of
AFP are inhibited during fetal development and may be reactivated
during tumorigenesis of gastric cells (4,5). APGC is
associated with a poor prognosis in <90% of patients due to its
high propensity for lymphatic and venous invasion, and synchronous,
as well as metachronous, liver metastases. Similar to other types
of advanced GC, occurrence of complications in patients with APGC
also indicates incurability. The disease is typically diagnosed at
an advanced stage and is therefore associated with a high
recurrence rate (6). Although
surgical resection or systemic chemotherapy is used to treat liver
metastases, the benefit of a palliative gastrectomy in patients
with advanced GC is debatable (7).
Following palliative treatment, high incidences of postoperative
morbidity, prolonged hospital stay and poor quality of life have
been observed frequently in patients with APGC and a poor survival
rate compared with patients with non-APGC (8).
Conventional chemotherapy is predominantly
ineffective in APGC. However, a previous study has suggested that a
SOX (S-1 and oxaliplatin) regimen may be an effective therapy to
treat advanced GC (9). S-1 is an
orally active derivative of 5-fluorouracil (5-FU), which is a
fourth-generation oral fluoropyrimidine (10). The study indicated that S-1-based
therapy is superior compared with 5-FU in terms of overall survival
(OS), progression-free survival (PFS) and objective response rate
(10). For the treatment of GC,
oxaliplatin is typically administered with fluorouracil and
leucovorin in a combination known as FOLFOX (11). Previous studies have reported higher
response rates (between 53 and 59%) and lower toxicity with a SOX
chemotherapy regimen in the treatment of advanced GC (9,10,12). Previous phase II trials have
demonstrated the superior efficacy and safety profile of the SOX
regimen as a first-line treatment for advanced GC in Korean
(13) and Chinese (14) patients. APGC with liver metastasis
that appears to be unresectable may become resectable following
treatment with neo-adjuvant chemotherapy. However, it remains
controversial to perform surgery following adjuvant chemotherapy in
cases of advanced gastric cancer (15). Although the use of SOX chemotherapy in
gastric cancer has been established, there are limited data
reported in the literature for its use, safety and efficacy to
treat APGC, possibly due to its rarity. The efficacy and safety of
the SOX regimen with or without resection of the primary tumor was
retrospectively evaluated for the treatment of APGC.
Materials and methods
Characteristics of enrolled
patients
A total of 24 patients with APGC that satisfied the
inclusion and exclusion criteria were identified from Liaocheng
People's Hospital (Liaocheng People's Hospital) between January
2011 and December 2013. All medical data of the patients were
reviewed retrospectively and only the outcomes were updated at the
time of analysis. The inclusion criteria were: i) APGC diagnosed by
pathology or cytology; ii) liver metastasis diagnosed by computed
tomography (CT) or magnetic resonance imaging (MRI) with >3
inoperable metastatic lesions; iii) performance status score 0–2
according to the Eastern Cooperative Oncology Group (16); iv) no contraindication for oral
medication; and v) without obvious myelosuppression including
having a whole blood count of ≥3×109 cells/l (normal,
4.0–10.0 cells/l), hemoglobin ≥8.0 g/dl (normal 11.0–15.0 cells/l),
platelet count of ≥75×109 cells/l (normal, 100–300), adequate liver
function (defined as total bilirubin ≤3-fold that of the upper
limit and aspartate aminotransferase/alanine aminotransferase level
of ≤3-fold that of the standard limit) and adequate renal function
(serum creatinine ≤1.8 mg/dl). The exclusion criteria included the
following: i) History of severe allergy to study drugs; ii)
neurological disorders or physically unable to cooperate with the
present study; iii) severe dysfunction of heart, lung, liver or
kidney; and iv) extensive metastasis. The present study was
approved by the Ethics Committee of Liaocheng People's Hospital.
Written informed consent was obtained from all study
participants.
Immunohistochemistry (IHC)
analysis
All of the specimens were obtained from endoscopic
biopsy and surgical resection of gastric cancer tissues. The
sections were treated with 3% hydrogen peroxide
(H2O2) and heated in microwave for antigen
retrieval. Following blocking with 10% mouse serum (catalog no.
M1025100, Wolcavi Biotech Co., Ltd., Beijing, China), the sections
were incubated with a mouse anti-human primary antibody against AFP
(1:1,000; catalog no. MAB-0013; Fuzhou Maixin Biotech Co., Ltd.,
Fuzhou, China) overnight at 4°C. The sections were washed with
phosphate-buffered saline (PBS, Na2HPO4 8.1 mmol/l, KH2PO41.5
mmol/l, NaCl 137 mmol/l, 2.7 mmol/l; PH 7.4) and then further
incubated with secondary antibody (1:500, catalog no. KIT-5002;
MaxVision™ HRP-Polymer anti-mouse IHC kit; Fuzhou Maixin Biotech,
China). The reaction was visualized using 3,3′-diaminobenzidine
(Fuzhou Maixin Biotech, China) and counterstained with Mayer's
hematoxylin. A known AFP-positive slide was set as a positive
control, and a slide incubated with PBS instead of primary antibody
was used for a negative control. Immunostaining of AFP was mainly
located in cytoplasm or membrane, presented as yellow or brown
particles. Immunostaining of AFP was scored on the scale of
semiquantitative assessment by combining evaluation of the
intensity and percentage of positive cells. Percentage scores were
assigned as follows: 0, 0%; 1, 1–10%; 2, 11–50%; 3, 51–80%; and 4,
81–100%. The intensity of AFP staining was scored as 0 (none), 1
(yellow), 2 (light brown) and 3 (deep brown). The scores of
percentage and intensity were added to give a final score from 0 to
7: 0–1, negative; 2–3, weak positive; 4–5, positive; and above 5,
strong positive. All the slides were inspected blindly by two
independent pathologists, who solved all discordant cases by
discussion, or a mean value of the two scores was used.
Treatment regimen
Patients in the SOX group received only
chemotherapy, whereas those in the SOX plus surgery group were
treated with palliative surgical resection of the primary lesion
supplemented with pre- and post-operative SOX chemotherapy. The
specific regimen consisted of oxaliplatin intravenously on the
first day and S-1 twice daily for 14 consecutive days. The dosage
was calculated according to body surface area (BSA): BSA <1.25
m2, 80 mg/day; 1.25 m2 ≤BSA <1.5 m2, 100 mg/day; BSA >1.5 m2,
120 mg/day. This chemotherapy regime was repeated every 3 weeks
with a total of ≥4 cycles in the two treatment groups. Antiemetic
and supportive treatments were administered according to the
standard clinical practice during chemotherapy.
PFS was defined as the duration between initiation
of treatment and disease progression or date of mortality or loss
to follow-up. OS was defined as the duration between initiation of
treatment and date of mortality or loss to follow-up. Patient
status was monitored using enhanced CT, MRI, serum AFP levels and
gastroscopy. The treatment efficacy following two cycles of
chemotherapy was evaluated using Response Evaluation Criteria in
Solid Tumors (RECIST 1.1) (17)
following every two treatment cycles. Tumor lesions were measured 1
week before and 2 months after therapy. Gastroscopy was performed
following chemotherapy if required. All adverse events were
recorded and followed up according to the National Cancer Institute
Common Terminology Criteria for Adverse Events (NCI-CTCAE4.0;
version 4.03; http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdf.
Statistical analysis
Statistical analyses were performed using SPSS
(version 21.0; IBM SPSS, Armonk, NY, USA). The differences in
clinicopathological characteristics of patients in the SOX and SOX
plus surgery groups were analyzed using the χ2 test. PFS and OS
were compared using Kaplan-Meier estimator analysis. The
differences in prognoses between the two groups were analyzed using
a log-rank test. Quantitative data were analyzed using Fisher's
exact test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
A total of 46 patients with APGC admitted to
Liaocheng People's Hospital between January 2011 and December 2013
were identified and 24 patients (15 males, 9 females; average age,
59 years, range, 32–81 years; Table
I) among them met the inclusion and exclusion criteria and were
enrolled in the present retrospective study. A total of 10 patients
were assigned to the SOX group as they were resistant to docetaxel,
cisplatin and/or 5-FU. In total, 14 patients were assigned to the
SOX plus surgery group and underwent SOX chemotherapy prior to and
following palliative resection of the primary lesion. All patients
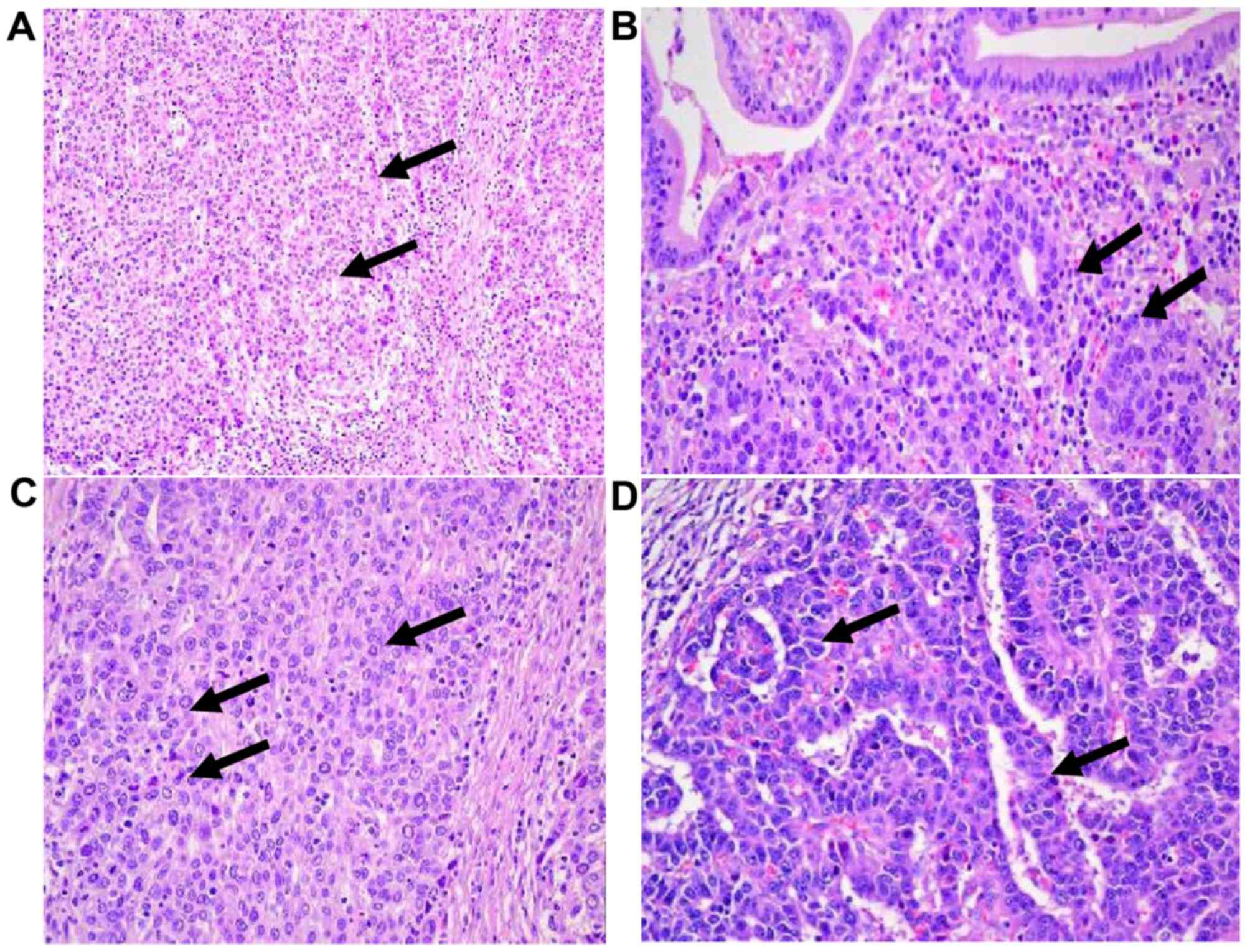
exhibited typical histopathological characteristics, including
poorly differentiated adenocarcinoma and signet ring cells
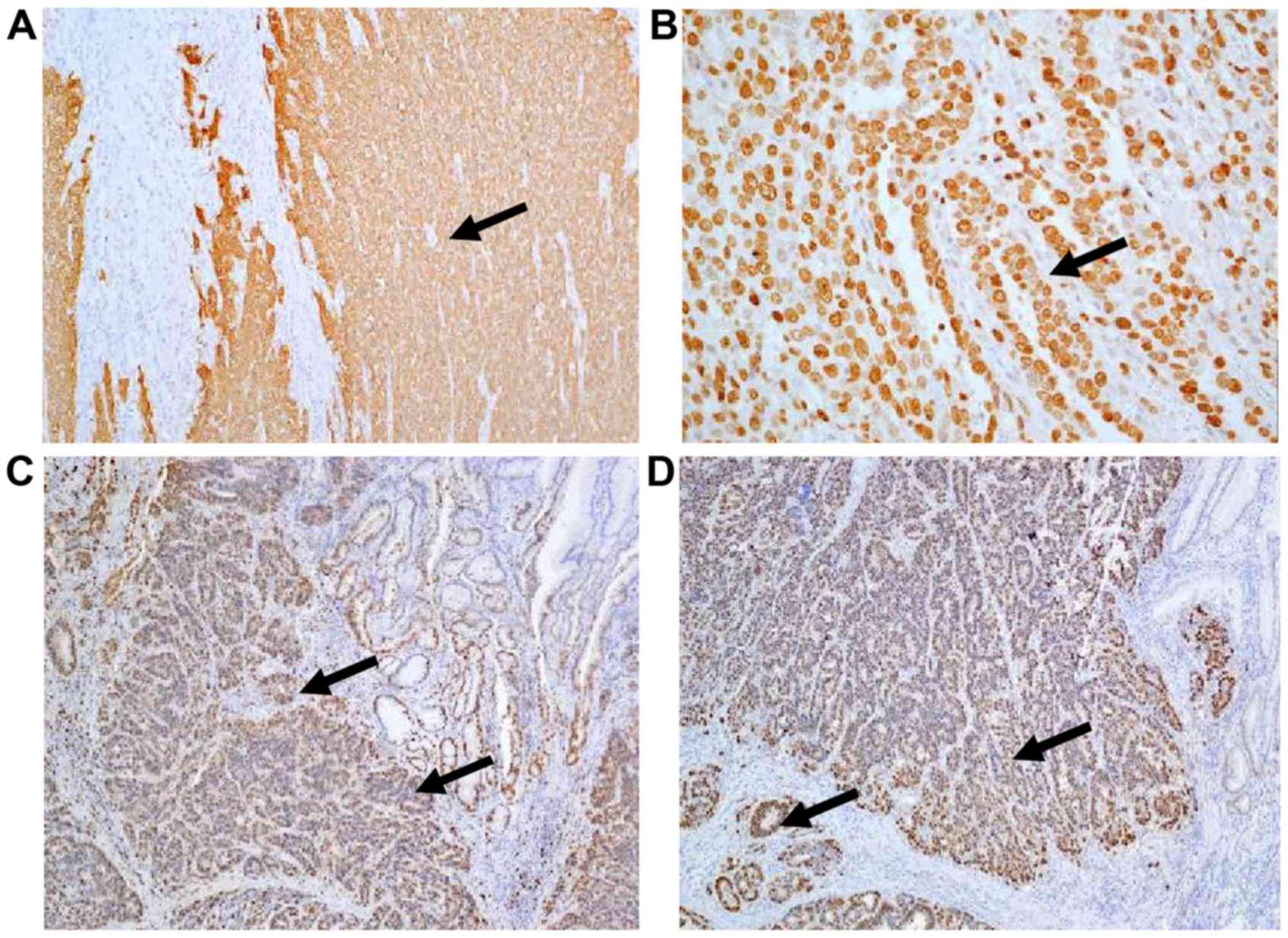
(Fig. 1). AFP was detected using
immunohistochemical staining (stained in brown; Fig. 2A and B); carcinoembryonic antigen and
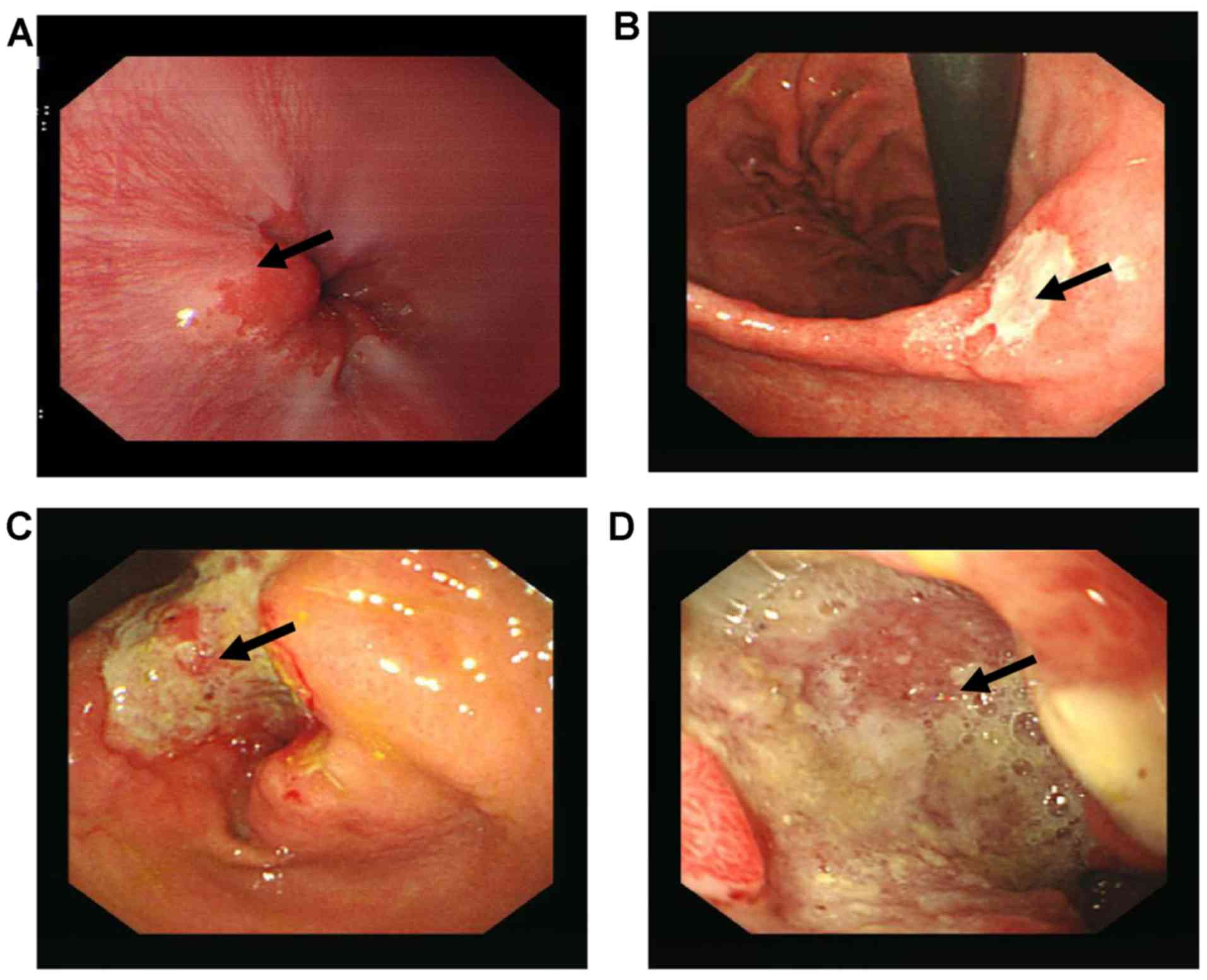
cancer antigen 125 were also positive (Fig. 2C and D). Typical characteristics of GC
were observed upon gastroscopy. However, the lesions of certain
patients were comparatively small and were frequently less
ulcerated (Fig. 3). All patients
exhibited significantly elevated AFP levels. Patients with
metastatic lesions of >5 cm in diameter exhibited AFP levels
>3,000 µg/l.
 | Table I.Characteristics of patients with APGC
in the two study groups. |
Table I.
Characteristics of patients with APGC
in the two study groups.
| Characteristic | SOX group n (%) | SOX plus surgery
group n (%) | χ2 | P-value |
|---|
| Total | 10 | 14 |
|
|
| Age, years |
|
|
|
|
| ≤65 | 7 (70) | 9 (64.3) | 0.105 | 0.794 |
|
>65 | 3 (30) | 5 (35.7) |
|
|
| Sex |
|
|
|
|
| Male | 6 (60) | 9 (64.3) | 2.764 | 0.486 |
|
Female | 4 (40) | 5 (35.7) |
|
|
| PS grade |
|
|
|
|
| 0 | 2 (20) | 2 (14.3) | 3.079 | 0.492 |
| 1 | 6 (60) | 9 (64.3) |
|
|
| 2 | 2 (20) | 3 (21.4) |
|
|
| Primary lesion,
cm |
|
|
|
|
| ≤6 | 4 (40) | 4 (28.5) | 1.433 | 0.517 |
|
>6 | 6 (60) | 10 (71.5) |
|
|
| Liver
metastasis |
|
|
|
|
| ≤5 | 3 (30) | 4 (28.5) | 2.527 | 0.479 |
|
>5 | 7 (70) | 10 (71.5) |
|
|
Efficacy evaluation
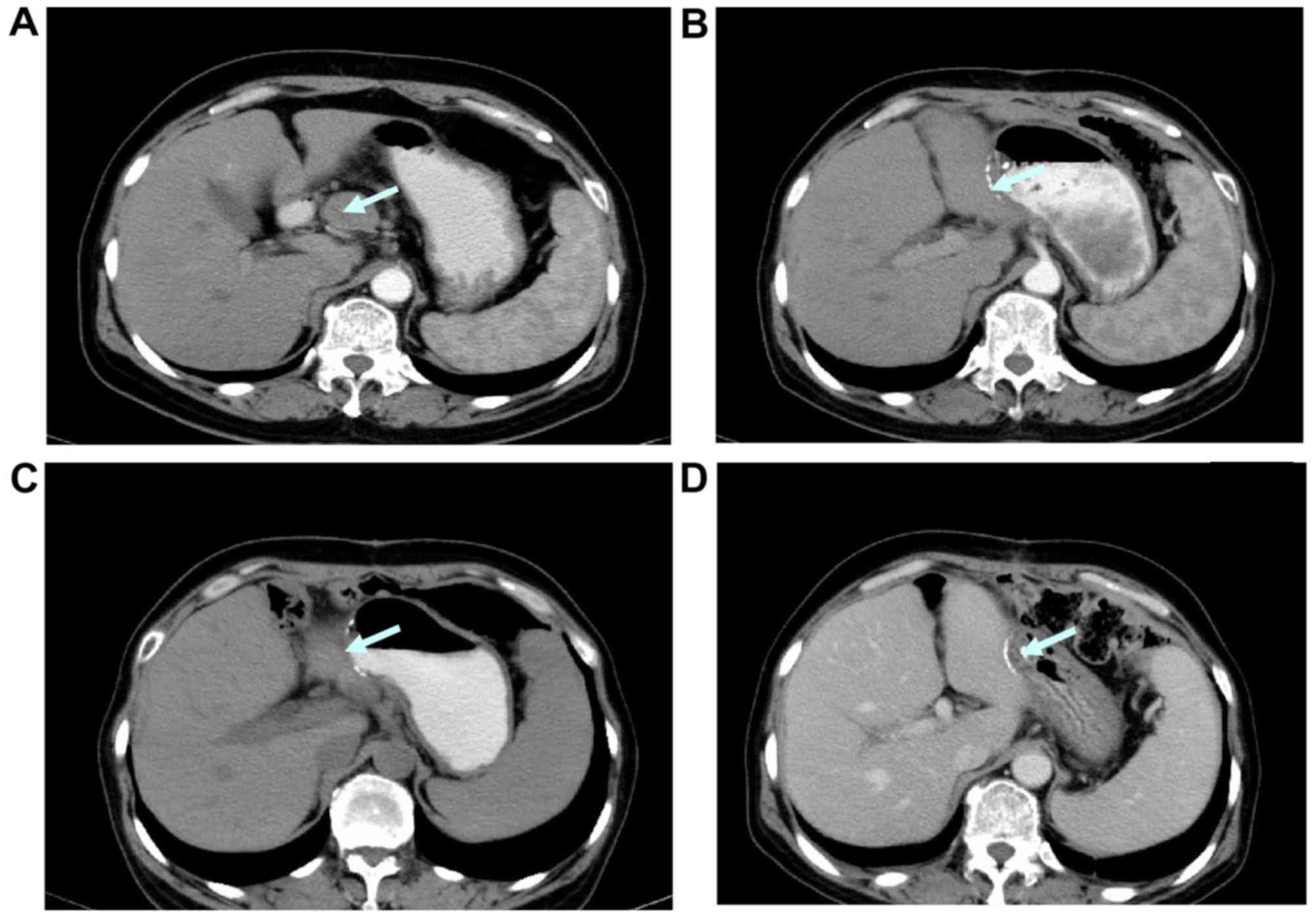
All patients were monitored using CT during and
following treatment, including alterations in the largest
horizontal diameter of the tumor multiplied by the largest vertical
diameter, the number of tumors and any newly diagnosed tumors
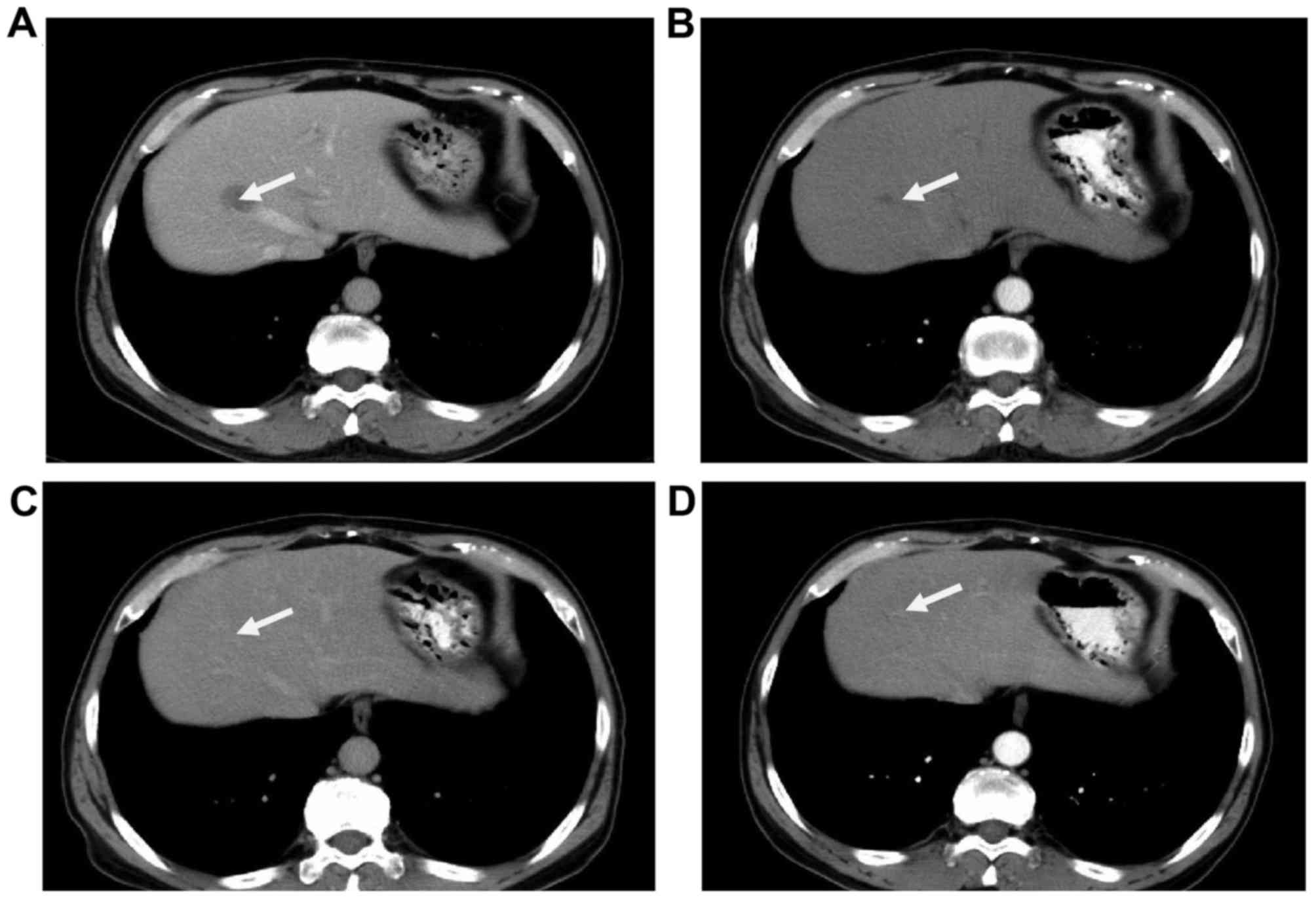
(Figs. 4–6). In the SOX group, 4 (40%) cases exhibited
a partial response; in 3 (30%) cases, the patient's condition was
evaluated as stable and 3 (30%) cases demonstrated evidence of
disease progression. In the SOX plus surgery group, 6 (42.8%) cases
exhibited a partial response, 4 (28.6%) cases remained stable and 4
(28.6%) cases demonstrated evidence of disease progression. The
disease control rate was 70.0 and 71.4% in the SOX and SOX plus
surgery group, respectively. However, no statistically significant
difference was identified (P=0.208; Table II).
 | Table II.Comparison of treatment efficacy in
the two study groups. |
Table II.
Comparison of treatment efficacy in
the two study groups.
| Outcome | SOX group n
(%) | SOX plus surgery
group n (%) |
|---|
| CR | 0 | 0 |
| PR | 4 (40) | 6 (42.8) |
| SD | 3 (30) | 4 (28.6) |
| PD | 3 (30) | 4 (28.6) |
| DCR | 7 (70) | 4 (71.4) |
| PFS, months | 6.5 (4.6–8.4) | 7.0 (5.7–8.3) |
| OS, months | 13.5
(8.1–18.9) | 14 (11.0–17.1) |
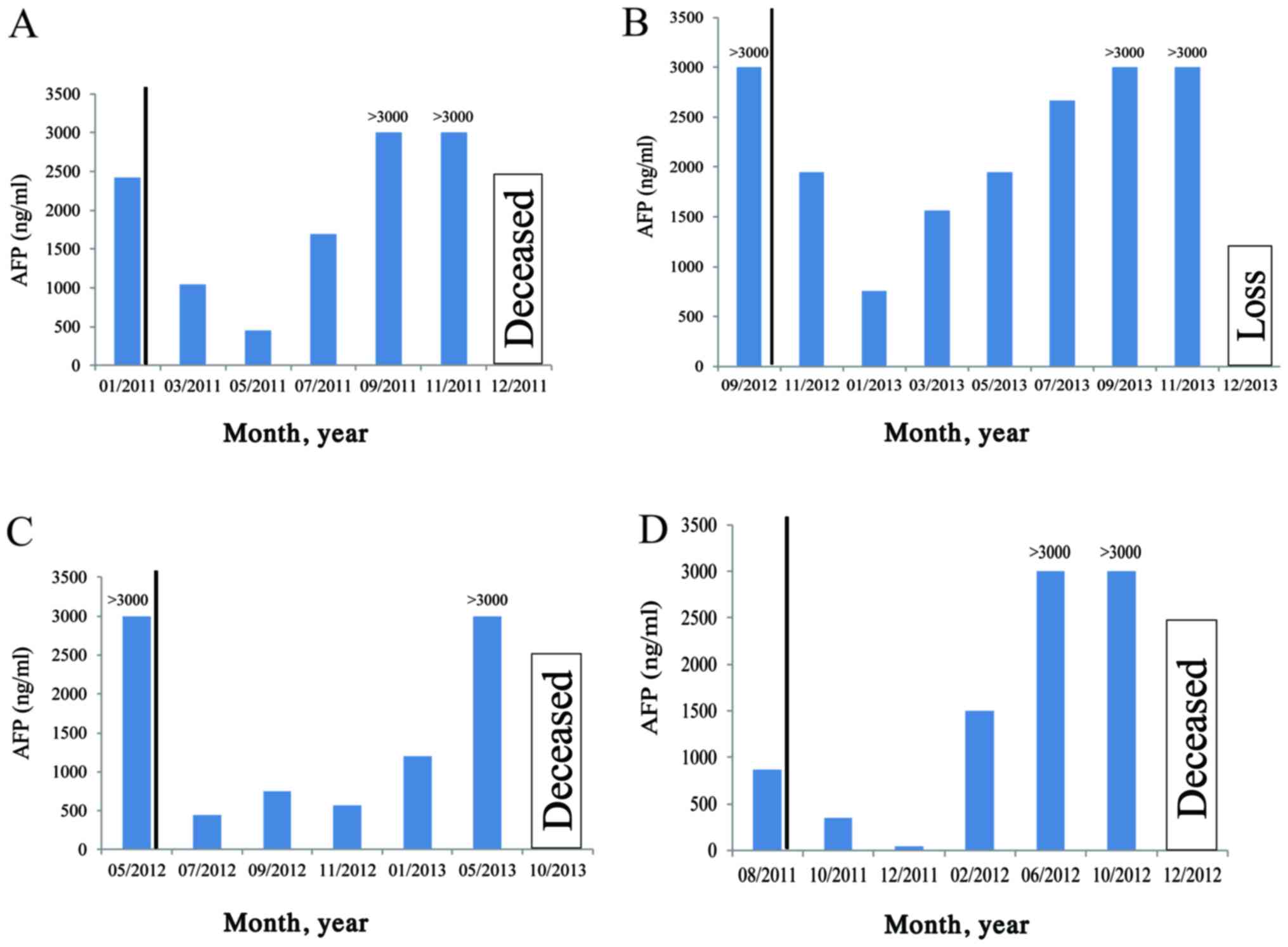
The dynamics of the plasma AFP levels were monitored
in patients during and following treatment. AFP detection in plasma
was identified as a sensitive indicator of APCG treatment efficacy
(Fig. 7).
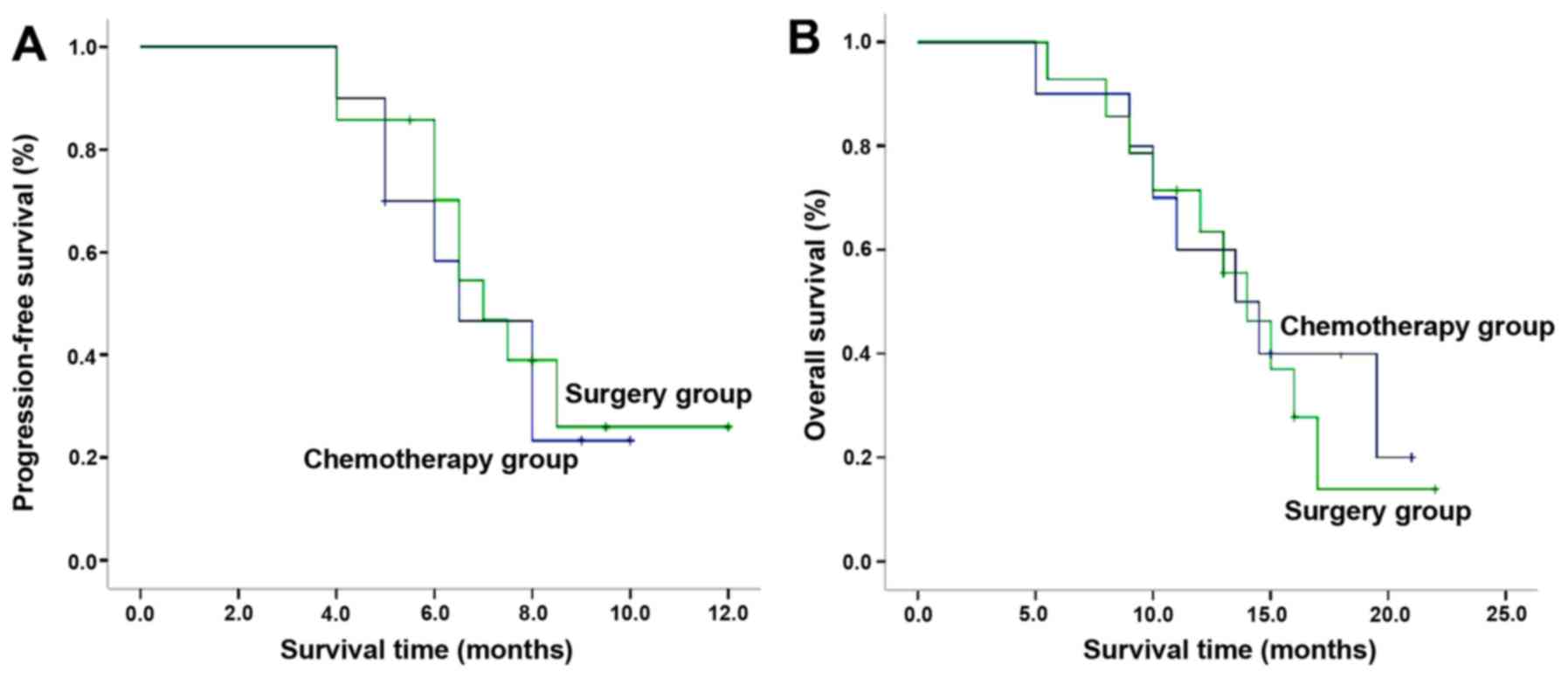
The PFS in the two groups was 6.5 [95% confidence
interval (CI), 4.6–8.4] and 7 (95% CI, 5.7–8.3) months,
respectively. The observed difference between groups was not
significant (P=0.703; Table II;
Fig. 8A). The OS of the two groups
was 13.5 (95% CI, 8.1–18.9) and 14 (95% CI, 11.0–17.1) months,
respectively. No statistically significant difference between
groups was identified (P=0.710; Table
II; Fig. 8B).
Safety profile
Adverse events in the study population are
summarized in Table III. Level 3 to
4 adverse effects included leucopenia, granulocytopenia, anemia and
diarrhea with no significant difference identified between the two
groups (P>0.05). In the SOX plus surgery group, 1 case of each
surgery-associated event (gastroplegia, pancreatic fistula,
pulmonary infection and refractory ascites) was reported. However,
all patients improved with expectant treatment with no
treatment-associated mortality.
 | Table III.Comparison of adverse effects between
SOX and SOX plus surgery groups. |
Table III.
Comparison of adverse effects between
SOX and SOX plus surgery groups.
|
| SOX group n
(%) | SOX plus surgery
group n (%) |
|---|
|
|
|
|
|---|
| Adverse effect | Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 |
|---|
| Hematology |
|
|
|
|
|
Leucopenia | 4 (40.0) | 1 (10.0) | 6 (42.9) | 2 (14.3) |
|
Granulocytopenia | 5 (50.0) | 1 (10.0) | 6 (42.9) | 1 (7.1) |
|
Thrombocytopenia | 4 (40.0) | 0 | 5 (35.7) | 0 |
|
Anemia | 5 (50.0) | 1 (10.0) | 8 (57.1) | 2 (14.3) |
|
Non-hematological |
|
|
|
|
|
Nausea | 2 (20.0) | 0 | 3 (21.4) | 0 |
|
Vomiting | 4 (40.0) | 0 | 6 (42.9) | 0 |
|
Diarrhea | 4 (40.0) | 1 (10.0) | 5 (35.7) | 1 (7.1) |
|
Constipation | 1 (10.0) | 0 | 1 (7.1) | 0 |
|
Anorexia | 6 (60.0) | 0 | 9 (64.3) | 0 |
|
Hypodynamia | 6 (60.0) | 0 | 8 (57.1) | 0 |
|
Peripheral neuropathy | 3 (30.0) | 0 | 4 (28.6) | 0 |
Discussion
GC is the fourth most frequent cancer globally
(18). In total, ~84% of patients
with GC develop advanced disease, with a median survival without
chemotherapy of between 3 and 4 months. For advanced-stage GC,
chemotherapy is the preferred treatment option (19). Samaratunga et al have suggested
that neoadjuvant chemotherapy with at least two cycles of the SOX
regimen may induce a relatively high number of pathologically
complete responses without increasing morbidity and mortality
(20). Consequently, the SOX regimen
is considered to be one of the most effective chemotherapy
treatments for GC, particularly in China and Japan (it is not
available in the majority of other countries) (9–12). The SOX
regimen is now considered as a chemotherapeutic option for the
treatment of GC. However, its efficacy in patients with APGC has
not been conclusively demonstrated.
Elevated serum AFP levels are frequently observed in
patients with hepatocellular carcinoma and yolk sac tumors
(21). According to certain studies,
AFP may be produced by gastrointestinal tract organs, rectal
carcinoma, gallbladder carcinoma and lung carcinoma (22). Bourreille et al (21) reported the first case of APGC with
liver metastasis in 1970. Liu et al (1,23) reported
>100 cases of patients with APGC since 1970. APGC is established
to be an aggressive tumor with a higher propensity for liver
metastasis. APGC is associated with immunosuppression due to
increased levels of AFP expression and a poorer prognosis as
compared with other subtypes of GC (24). Key factors affecting the prognosis of
APGC include primary lesion progression, histopathological
characteristics of cancer cells and immune status of the patient
(21).
As the precise underlying mechanism of APGC remains
to be elucidated, the optimal treatment approach requires further
consideration. Patients with APGC and liver metastasis may be
eligible for resection of the primary if neo-adjuvant chemotherapy
is administered. Studies have suggested that obstruction,
perforation or bleeding may be eliminated when the primary tumor is
resected in patients with advanced APGC and multiple organ
metastases (25–27). It may also decrease tumor burden and
the energy demand of patients. Studies have also demonstrated a
decrease in cytokine levels and significant immunosuppression in
these patients (24). Patients with
APGC and liver metastasis exhibit an average survival time of ≤14
months despite treatment with adjuvant therapy (14). It has been suggested that percutaneous
ethanol injection may be an effective treatment for liver
metastasis and is associated with an average survival time of 18
months, which is longer than that associated with other treatment
modalities, including partial hepatic resection and systemic
chemotherapy (the latter having a survival time of ≤7 months)
(8). Another previous study initially
proposed that patients with a solitary liver metastatic lesion
should be treated with surgical resection, whereas patients with
multiple liver metastases should undergo a partial hepatectomy
followed by transarterial continuous-infusion chemotherapy as the
preferred therapeutic approach (28).
Notably, all patients in this study did not survive for >8
months, demonstrating that hepatic resection should be considered
carefully (24). In the present
study, the efficacy and safety profile of SOX chemotherapy alone
was retrospectively compared with the combined approach of SOX plus
surgical resection in patients with APGC.
The efficacy of the SOX regimen to treat APGC was
positive in the present study compared with previous studies
involving patients with other types of GC, and those treatments had
a significantly lower efficacy (9–11). The
majority of metastatic lesions were decreased to half of their
original size following one cycle of chemotherapy (Figs. 4–6). The
plasma AFP level also decreased, but the same response was not
observed in the primary tumor in the stomach using gastroscopy. The
present study investigated whether unresectable primary tumors may
become resectable through the administration of neo-adjuvant
chemotherapy. The surgery was performed following between 2 and 3
cycles of chemotherapy, or following significant reduction in
metastatic lesions and decreased AFP levels. Serious complications
were more frequently observed in the SOX plus surgery group,
including fever of unknown origin, pulmonary infection, pancreatic
fistula and refractory ascites. The complications were associated
with decreased tolerability of further chemotherapy, which in the
majority of cases was delayed by >3 months after surgery. This
delay resulted in disease progression in certain cases and was
possibly responsible for increased drug resistance in these
patients. The patients who exhibited resistance to drugs typically
did not survive due to complications within 1 year of surgery. The
results of the present study suggest that the advantage that may be
gained by palliative resection of the primary tumor may be offset
by the disadvantage from chemotherapy interruption. The palliative
surgery may be performed following the administration of planned
chemotherapy cycles or following the attainment of plateau response
to chemotherapy.
In the SOX group, 4 patients demonstrated a partial
response to treatment, 3 cases remained in a stable condition and 3
cases exhibited evidence of disease progression. In the SOX plus
surgery group, 6 cases demonstrated a partial response, 4 cases
remained stable and in 4 cases there was evidence of disease
progression. The disease control rate was 70.0 and 71.4% in SOX and
SOX plus surgery group, respectively, with no significant
difference identified between the two groups (P=0.208), which
suggested that resection of the primary lesion may not lower the
tumor burden or affect metastatic lesions. The PFS in the SOX and
SOX plus surgery groups was 6.5 (95% CI, 4.6–8.4) and 7 (95% CI,
5.7–8.3) months, respectively. The corresponding OS was 13.5 (95%
CI, 8.1–18.9) and 14 (95% CI, 11.0–17.1) months, respectively. No
significant difference in OS was identified between the two groups
(P=0.710). These results suggested that there is no additional
benefit from surgery to the PFS or OS rate. Additionally, primary
lesion surgery did not appear to confer any prognostic advantage on
patients with APGC and liver metastasis. The results of the present
study differ from those of certain earlier studies (29,30),
possibly as all subjects in the present study were patients with
APGC.
The adverse events rates in the two groups were
comparable. However, only patients in the SOX plus surgery group
experienced severe adverse events, including gastroplegia,
pancreatic fistula, pulmonary infection and refractory ascites.
Although adjuvant chemotherapy prior to surgery aided in the
surgical resection of the primary lesion, the post-surgical
complications affected the continuity of chemotherapy leading to
tumor resistance to chemotherapy. This phenomenon may contribute to
the decreased efficacy of surgery observed in the present study. A
robust evaluation of the SOX plus surgery regimen in patients with
APGC is required.
In conclusion, the results of the present
single-center retrospective study demonstrates the specific
efficacy of SOX regimen in patients with APGC and provide evidence
that irregular chemotherapy with SOX may lead to the development of
tumor resistance. Future randomized, double-blind, large-scale
clinical trials are required to conclusively establish the role of
the SOX regimen in patients with APGC.
References
|
1
|
Liu X, Cheng Y, Sheng W, Lu H, Xu Y, Long
Z, Zhu H and Wang Y: Clinicopathologic features and prognostic
factors in alpha-fetoprotein-producing gastric cancers: Analysis of
104 cases. J Surg Oncol. 102:249–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chun H and Kwon SJ: Clinicopathological
characteristics of alpha-fetoprotein-producing gastric cancer. J
Gastric Cancer. 11:23–30. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ooi A, Nakanishi I, Sakamoto N, Tsukada Y,
Takahashi Y, Minamoto T and Mai M: Alpha-fetoprotein
(AFP)-producing gastric carcinoma. Is it hepatoid differentiation?
Cancer. 65:1741–1747. 1990.PubMed/NCBI
|
|
4
|
Jia Y, Liu D, Xiao D, Ma X, Han S, Zheng
Y, Sun S, Zhang M, Gao H, Cui X and Wang Y: Expression of AFP and
STAT3 is involved in arsenic trioxide-induced apoptosis and
inhibition of proliferation in AFP-producing gastric cancer cells.
PLoS One. 8:e547742013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu DP, He QJ and Liu CG: Correlations
among Helicobacter pylori infection and the expression of
cyclooxygenase-2 and vascular endothelial growth factor in gastric
mucosa with intestinal metaplasia or dysplasia. J Gastroenterol
Hepatol. 25:795–799. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim T and Yu W: Prognostic value of
preoperative serum alpha- fetoprotein level in resectable gastric
cancer. J Korean Gastric Cancer Assoc. 3:33–37. 2003.(In Korean).
View Article : Google Scholar
|
|
7
|
Lim JH, Lee DH, Shin CM, Kim N, Park YS,
Jung HC and Song IS: Clinicopathological features and surgical
safety of gastric cancer in elderly patients. J Korean Med Sci.
29:1639–1645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sato Y, Nishimaki T, Date K, Shirai Y,
Kurosaki I, Saito Y, Watanabe T and Hatakeyama K: Successful
resection of metachronous liver metastasis from
alpha-fetoprotein-producing gastric cancer: Report of a case.
Surgery Today. 29:1075–1088. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhong DT, Wu RP, Wang XL, Huang XB, Lin
MX, Lan YQ and Chen Q: Combination chemotherapy with S-1 and
oxaliplatin (SOX) as first-line treatment in elderly patients with
advanced gastric cancer. Pathol Oncol Res. 21:867–873. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu GF, Tang D, Li P, Wang S, Xu YX, Long
AH, Zhou NL, Zhang LL, Chen J and Xiang XX: S-1-based combination
therapy vs S-1 monotherapy in advanced gastric cancer: A
meta-analysis. World J Gastroenterol. 20:310–318. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi C, Chen Q, Shen S, Wu R, Yang B, Liu Q
and Xu Q: Paclitaxel combined with oxaliplatin as first-line
chemotherapy for locally advanced or metastatic gastric cancer.
Expert Rev Anticancer Ther. 15:595–601. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang L, Yang YI, Qin Q, Zhou A, Zhao J,
Wang J, Shu C, Yuan X and Hu S: Evaluation of the optimal dosage of
S-1 in adjuvant SOX chemotherapy for gastric cancer. Oncol Lett.
9:1451–1457. 2015.PubMed/NCBI
|
|
13
|
Oh SY, Kwon HC, Jeong SH, Joo YT, Lee YJ,
Cho Sh, Kang MH, Go SI, Lee GW, Kim Hg and Kang JH: A phase II
study of S-1 and oxaliplatin (SOx) combination chemotherapy as a
first-line therapy for patients with advanced gastric cancer.
Invest New Drugs. 30:350–356. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu B, Ying J, Luo C, Xu Q, Zhu L and
Zhong H: S-1 combined with oxaliplatin as first line chemotherapy
for Chinese advanced gastric cancer patients.
Hepatogastroenterology. 59:6492012.PubMed/NCBI
|
|
15
|
Nishikawa K, Fujitani K, Inagaki H,
Akamaru Y, Tokunaga S, Takagi M, Tamura S, Sugimoto N, Shigematsu
T, Yoshikawa T, et al: Randomised phase III trial of second-line
irinotecan plus cisplatin versus irinotecan alone in patients with
advanced gastric cancer refractory to S-1 monotherapy: TRICS trial.
Eur J Cancer. 51:808–816. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feng D, Leong M and Li T, Chen L and Li T:
Surgical outcomes in patients with locally advanced gastric cancer
treated with S-1 and oxaliplatin as neoadjuvant chemotherapy. World
J Surg Oncol. 13:112015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Samaratunga H, Samaratunga D, Dunglison N,
Perry-Keene J, Nicklin J and Delahunt B:
Alpha-fetoprotein-producing carcinoma of the renal pelvis
exhibiting hepatoid and urothelial differentiation. Anticancer Res.
32:4987–4991. 2012.PubMed/NCBI
|
|
21
|
Bourreille J, Metayer P, Sauger F, Matray
F and Fondimare A: Existence of alpha feto protein during
gastric-origin secondary cancer of the liver. Presse Med.
78:1277–1278. 1970.(In French). PubMed/NCBI
|
|
22
|
Lunghi A, Petreni P, Romanelli RG,
Vizzutti F, Marra F, Tarquini R and Laffi G: Aggressive gastric
carcinoma producing alpha-fetoprotein: A case report and review of
the literature. Case Rep Oncol. 7:92–96. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu X, Yang M, Gao J, Zhang S and Xi Y:
Clinicopathologic features and prognosis of 51 patients with
α-fetoprotein-producing gastric cancer. Zhonghua Zhong Liu Za Zhi.
37:231–234. 2015.(In Chinese). PubMed/NCBI
|
|
24
|
Yamashita T, Nakane A, Watanabe T, Miyoshi
I and Kasai N: Evidence that alpha-fetoprotein suppresses the
immunological function in transgenic mice. Biochem Biophys Res
Commun. 201:1154–1159. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iida M, Imura J, Furuichi T, Sawada T,
Nagawa H and Fujimori T: Alteration of the AT motif binding
factor-1 expression in alpha-fetoprotein producing gastric cancer:
Is it an event for differentiation and proliferation of the tumors?
Oncol Rep. 11:3–7. 2004.PubMed/NCBI
|
|
26
|
Tiberio GA, Roviello F, Donini A and de
Manzoni G; Italian Research Group for Gastric Cancer, : Hepatic
metastases from gastric cancer: A surgical perspective. World J
Gastroenterol. 21:11489–11492. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kerkar SP, Kemp CD and Avital I: Liver
resections in metastatic gastric cancer. HPB (Oxford). 12:589–596.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu SF, Lu CR, Cheng HD, Xi HQ, Cui JX, Li
JY, Shen WS and Chen L: Comparison of therapeutic efficacy between
gastrectomy with transarterial chemoembolization plus systemic
chemotherapy and systemic chemotherapy alone in gastric cancer with
synchronous liver metastasis. Chin Med J (Engl). 128:2194–2201.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Z, Fan B, Shan F, Tang L, Bu Z, Wu A,
Zhang L, Wu X, Zong X, Li S, et al: Gastrectomy in comprehensive
treatment of advanced gastric cancer with synchronous liver
metastasis: A prospectively comparative study. World J Surg Oncol.
13:2122015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kinoshita T, Kinoshita T, Saiura A, Esaki
M, Sakamoto H and Yamanaka T: Multicentre analysis of long-term
outcome after surgical resection for gastric cancer liver
metastases. Br J Surg. 102:102–107. 2015. View Article : Google Scholar : PubMed/NCBI
|