Introduction
Lung cancer is the leading cause of
cancer-associated mortality worldwide. Lung cancer is classified
into non-small cell lung cancer (NSCLC) and small-cell lung cancer,
and 80% of lung cancers are NSCLC (1). Patients with early stage NSCLC are
candidates for curative thoracic surgery, whereas those with
advanced stage NSCLC are usually treated with systemic chemotherapy
or chemoradiotherapy. In total, ~50% of patients with NSCLC are
diagnosed with stage IIIB or IV cancers, and morbidity is higher
among elderly patients compared with non-elderly patients (1). Previous meta-analyses have revealed that
platinum-based combination chemotherapy slightly improves survival
compared with best supportive care (2,3). A
previous study compared the survival time of elderly patients with
NSCLC who received single-agent treatment, with that of patients
who underwent a platinum-based regimen, and median overall survival
was 10.3 months for platinum-based chemotherapy and 6.2 months for
monotherapy (P<0.0001). The 1-year survival rate was 44.5% and
25.4%, respectively (4). The
incidence of lung cancer has been increasing among elderly
individuals, and single agents including vinorelbine and docetaxel
have been widely used as first-line treatments against NSCLC in
elderly patients with advanced disease in Japan. In a phase III
study, Abe et al (5) reported
that the survival time following docetaxel monotherapy was
significantly increased compared with combination therapy with
docetaxel plus cisplatin in elderly patients with advanced NSCLC.
Therefore, it remains unclear whether combination chemotherapy
improves survival compared with monotherapy in these patients.
Further studies evaluating the clinical significance of combination
chemotherapy against advanced NSCLC in elderly patients are
warranted.
S-1 (Taiho Pharmaceutical Co., Ltd., Tokyo, Japan)
is an oral anticancer agent composed of tegafur, 5-chloro-2,
4-dihydroxypyridine, and potassium oxonate, in a molar ratio of
1:0.4:1 (6). Tegafur, a prodrug of
5-fluorouracil (5-FU), is gradually converted to 5-FU, and is
rapidly catabolized by dihydropyrimidine dehydrogenase in the
liver. In several studies, S-1 has been reported to actively reduce
tumor growth in various human cancers, and a combination of
platinum-based regimens with S-1 has been the standard treatment
regimen against advanced NSCLC (7–12).
Gemcitabine, an anticancer drug that structurally resembles
cytosine arabinoside, has been demonstrated to exhibit high
anti-tumor activity with minimal adverse effects (13). A previous study revealed that a
combination of gemcitabine and uracil-tegafur was effective and
tolerable in patients with advanced NSCLC (14). It was demonstrated that a combination
of gemcitabine and S-1 was also useful in patients with advanced
pancreatic cancer (15). Therefore,
S-1 and gemcitabine are considered to be antimetabolites with
minimal toxicities and promising efficacies.
In a previous phase I study, a combination of
gemcitabine and S-1 was observed to be an effective and feasible
treatment against NSCLC in elderly patients (16). Seto et al (17) also reported the clinical benefit of
gemcitabine plus S-1 in elderly patients, with the combination
regimen yielding a response rate of 27%, a time to progression of
4.2 months and an overall survival of 12.9 months, with minimum
toxicity. Their treatment schedule was the oral administration of
S-1 (30 mg/m2 twice a day) on days 1–14, and intravenous
administration of gemcitabine (1,000 mg/m2) on days 8
and 15. On the other hand, the treatment schedule followed in the
previous study performed by our group was the oral administration
of S-1 (40 mg/m2 twice a day) on days 1–14, and
intravenous administration of gemcitabine (1,000 mg/m2)
on days 1 and 15. The dose of S-1 and the administrative schedule
of gemcitabine differed between the previous study by our group and
the study by Seto et al (17).
In another study, Satouchi et al (18) recommended two treatment schedules that
differed in the administration schedule of gemcitabine: The oral
administration of S-1 (30 mg/m2 twice a day) on days
1–14 and intravenous administration of gemcitabine (1,000
mg/m2) on days 1 and 8 or days 8 and 15 in patients with
chemotherapy-naïve NSCLC. Similarly, Takiguchi et al
(19) also described the oral
administration of S-1 (30 mg/m2 twice a day) on days
1–14 and intravenous administration of gemcitabine (1,000
mg/m2) on days 8 and 15. However, it remains unclear
whether the differences in S-1 dose and gemcitabine administration
affected the response rate and survival time in patients with
advanced NSCLC. Therefore, the present study was conducted to
investigate the efficacy of the regimen comprised of S-1 plus
gemcitabine based on the dosage used in the previous phase I study
performed by our group (16).
Patients and methods
Patients and patient eligibility
A total of 21 patients were enrolled in the present
study between August 2007 and March 2015 at Gunma University
Hospital (Maebashi, Japan) A single patient withdrew because of the
occurrence of vascular disease. Patient characteristics are
detailed in Table I. The inclusion
criteria were histologically and/or cytologically proven
unresectable stage IIIB or IV NSCLC (1,3), no
previous systemic chemotherapy or radiotherapy, an Eastern
Cooperative Oncology Group (ECOG) performance status (PS) of 0 or
1, age ≥70 years, a life expectancy of ≥12 weeks; adequate bone
marrow reserve (leukocyte count ≥4,000 mm−3, neutrophil
count ≥2,000 mm−3, platelet count ≥100,000
mm−3 and hemoglobin ≥10 g/dl), normal liver function
(total serum bilirubin ≤1.5 mg/dl, and aspartate transaminase,
alanine transaminase <2x the upper limits of the normal range),
regardless of epidermal growth factor receptor (EGFR) mutation
status and normal renal function (serum creatinine ≤1.5 mg/dl and
creatinine clearance ≥60 ml/min). Patients with concomitant
malignancies, central nervous system metastases, active infectious
diseases or other serious medical problems were excluded. The
institutional Review Board at Gunma University Hospital approved
the present study, and written informed consent was obtained from
all patients (approval no. UMIN000001750).
 | Table I.Patient demographics. |
Table I.
Patient demographics.
| Clinical
characteristic | Value |
|---|
| Age [years; median
(range)] | 78 (70–86) |
| Sex
(male/female) | 10/10 |
| ECOG PS (0/1) | 16/4 |
| Histology
(AC/SQC/other) | 13/5/2 |
| Clinical stage
(IIIB/IV) | 2/18 |
| Smoking history
(yes/no) | 12/8 |
| Comorbid disease
(yes/no) | 17/3 |
| Recurrence following
operation (yes/no) | 5/15 |
Clinical study design
The present study was a prospective, single-center,
single-arm study investigating the effectiveness of gemcitabine and
S-1 combination therapy for the treatment of elderly patients with
NSCLC. S-1 (80 mg/m2/day) was administered orally twice
daily following a meal for 14 consecutive days, followed by 2 weeks
without treatment. Each S-1 capsule contained 20 or 25 mg tegafur.
Individual doses were rounded down to the nearest pill size less
than the calculated dose, given the available formulation.
Gemcitabine (1,000 mg/m2/day) was administered as a
30-min intravenous infusion on days 1 and 15 of each cycle. The
cycle was repeated every 4 weeks. Although the prophylactic
administration of granulocyte-colony stimulating factor (G-CSF) was
not permitted, the administration of G-CSF was permitted in
patients with grade 4 neutropenia and/or grade 3 febrile
neutropenia. Subsequent cycles of chemotherapy were initiated when
the leukocyte counts were ≥4,000 m−3, and the platelet
counts were ≥100,000 m−3 following day 29. If the
leukocyte or platelet counts had not returned to these levels by
day 1 of the next cycle of chemotherapy, the drugs were withheld
until full recovery. Chemotherapeutic treatment was performed for
at least two cycles, unless unacceptable toxicity or disease
progression occurred.
Treatment assessment
Patients were evaluated prior to treatment with
complete blood cell count evaluation, differential count
evaluation, routine chemistry measurements, chest radiography,
chest computed tomography (CT), abdominal CT, whole-brain magnetic
resonance imaging or CT, and isotope bone scintigraphy. Evaluations
performed weekly were complete blood cell count, differential
count, routine chemistry measurements, physical examination, and
toxicity assessment. Response Evaluation Criteria in Solid Tumors
version 1.1 was used to assess the response to S-1 plus gemcitabine
(20). To evaluate the response, CT
scans were performed every 6 weeks until progressive disease
developed. The overall response was defined as the best response.
Second-line chemotherapy or other treatments following the present
study were not prohibited by the protocol. Adverse events were
assessed according to the Common Terminology Criteria for Adverse
Events version 3.0 (21).
Statistical analysis
The primary endpoint of the present study was to
evaluate the overall response rate (ORR), and the secondary
endpoints were to examine the adverse events and survival data.
Progression-free survival (PFS) was defined as the time from
treatment initiation to disease progression or mortality. Overall
survival (OS) was determined as the time from the start of the
treatment to mortality from any cause. Survival estimation was
performed using the Kaplan-Meier method and the log-rank test.
P<0.05 was considered to indicate a statistically significant
difference. The estimated required accrual was 19 patients,
assuming an ORR of 35% in eligible patients would indicate
potential usefulness, whereas an ORR of 13% would constitute the
lower limit of interest, with a power of 80% and α level set at 5%
(one-sided test). The estimates were based on the 32.7–47.0%
response rates reported in previous trials for platinum-based
combination regimens with S-1 (6,7) and
12.5–22.7% response rates reported by a study conducted using S-1
monotherapy (8.9). All statistical analyses were performed using
GraphPad Prism 4 software (GraphPad Software, La Jolla, CA, USA)
for Microsoft Windows.
Results
Patient demographics
Patient characteristics are detailed in Table I. The median age of patients was 78
years (range, 70–86 years); 10 (50%) patients were men, and 10
(50%) were women. Histology indicated 13 (65%) adenocarcinomas, 5
(25%) squamous cell carcinoma and 2 (10%) other. Two patients (10%)
had stage IIIB disease, and 18 (90%) had stage IV disease. Other
demographics included an ECOG performance status score of 0 (80%)
and a history of smoking (60%). The distribution of comorbid
diseases was as follows: 4 patients with chronic obstructive
pulmonary disease; 5 patients with hypertension (medically
treated); 4 patients with diabetes mellitus; 2 patients with
arrhythmia; and 2 patients with angina pectoris (medically
treated). The status of EGFR mutation was assessed in 13 patients
with adenocarcinoma. Of these 13 patients, there were 4 patients
with EGFR mutation, 7 patients with EGFR wild type and the other 2
patients had unknown status.
Treatment delivery
Chemotherapy was administered to 20 patients, and
the median number of cycles was 2 (range, 1–27). Four or more
cycles were administered to ~35% of all patients. Among the total
74 cycles administered, gemcitabine was not skipped in any of the
patients. S-1 was administered at >98% of the scheduled dosage
in all cycles. Following disease progression, 9 patients received
second- or third-line chemotherapy including gefitinib or
erlotinib. The 4 patients harboring EGFR mutations were treated
with gefitinib or erlotinib, and the 5 patients with EGFR wild type
received erlotinib.
Efficacy and survival data
A total of 17 patients completed >2 cycles of
chemotherapy. Three patients discontinued treatment prematurely
following 1 cycle due to adverse effects and patient choice. None
of the patients achieved complete response (CR), and 8 achieved a
partial response (PR) with an ORR of 40.0% [95% confidence interval
(CI): 18.5–61.5%]. The overall disease control rate (CR + PR +
stable disease) was 65.0% (95% CI: 44.1–85.9%; Table II). According to the histological
type, patients with adenocarcinoma exhibited a response rate of
38.5% (95% CI: 12.0–64.9%; Table II)
and those without adenocarcinoma exhibited a response rate of 42.9%
(95% CI: 6.2–79.5%; Table II). The
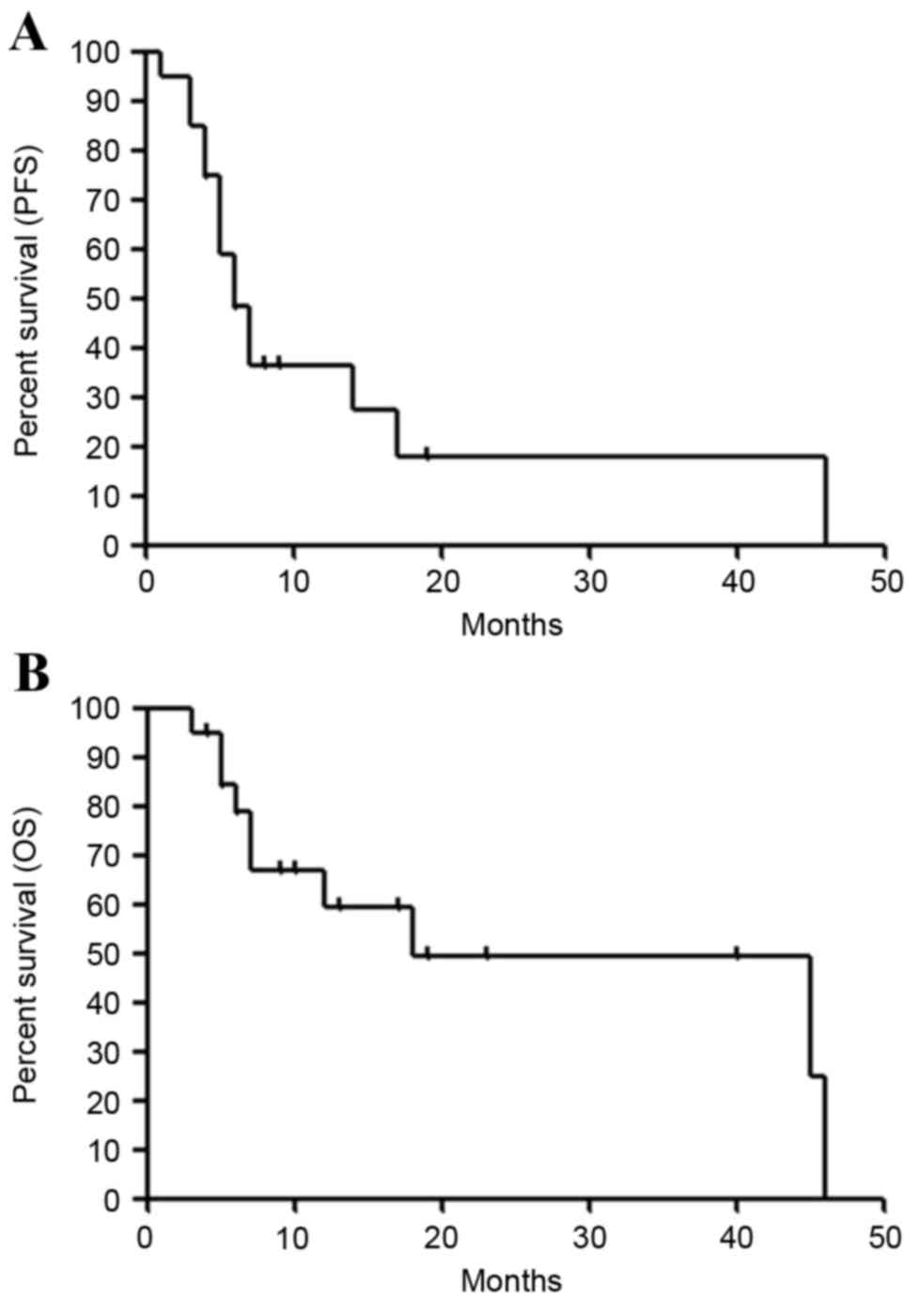
median PFS was 6.4 months (95% CI: 4.0–17.0), and the PFS rates at
3 and 6 months were 85.0 and 48.2%, respectively (Fig. 1A). The median survival time (MST) was
17.8 months (95% CI: 6.0–46.0), and the OS rates at 6 and 12 months
were 78.8 and 59.3%, respectively (Fig.
1B).
 | Table II.Response rate according to
histological type. |
Table II.
Response rate according to
histological type.
|
|
| Response |
|---|
|
|
|
|
|---|
| Histology | No. of patients | CR | PR | SD | PD |
|---|
| AC | 13 | 0 | 5 | 4 | 4 |
| Non-AC | 7 | 0 | 3 | 1 | 3 |
| Total patients | 20 | 0 | 8 | 5 | 7 |
| Response rate of
total patients |
|
| 40.0% |
|
|
|
| (95% CI;
18.5–61.5%) |
| Disease control rate
of total patients |
|
| 65.0% |
|
|
|
| (95% CI;
44.1–85.9%) |
Toxicity
Adverse events were assessed in all the treated
patients. Hematological and non-hematological adverse events are
listed in Table III. Grade 3 or 4
hematological toxicities (21) were
leukopenia (30%), neutropenia (25%), anemia (0%), and
thrombocytopenia (0%). Febrile neutropenia was not observed in any
patients. The only non-hematological adverse event observed was
grade 3 skin rash (10%). Pulmonary injuries, including interstitial
pneumonia, and treatment-associated mortality were not observed in
the present study.
 | Table III.Hematological and non-hematological
adverse events. |
Table III.
Hematological and non-hematological
adverse events.
|
| Grade |
|---|
|
|
|
|---|
| Adverse event | 1 | 2 | 3 | 4 | 3 or 4 (%) |
|---|
| Leukopenia | 2 | 3 | 5 | 1 | 29 |
| Neutropenia | 2 | 2 | 4 | 1 | 24 |
| Febrile
neutropenia | 0 | 0 | 0 | 0 | 0 |
| Anemia | 5 | 4 | 0 | 0 | 0 |
|
Thrombocytopenia | 4 | 2 | 0 | 0 | 0 |
|
Nausea/vomiting | 2 | 1 | 0 | 0 | 0 |
| Anorexia | 5 | 1 | 0 | 0 | 0 |
| Diarrhea | 1 | 0 | 0 | 0 | 0 |
| Liver
dysfunction | 1 | 0 | 0 | 0 | 0 |
| Infection | 1 | 0 | 0 | 0 | 0 |
| Skin rash | 5 | 1 | 2 | 0 | 10 |
| Constipation | 0 | 1 | 0 | 0 | 0 |
| Fever | 5 | 0 | 0 | 0 | 0 |
|
Neuropathy-sensory | 0 | 0 | 0 | 0 | 0 |
| Pneumonitis | 3 | 2 | 0 | 0 | 0 |
| Vertigo | 0 | 0 | 0 | 0 | 0 |
| Alopecia | 0 | 0 | 0 | 0 | 0 |
| Fatigue | 3 | 1 | 0 | 0 | 0 |
Discussion
In the present study, a combination of gemcitabine
and S-1 was demonstrated to be feasible and effective as a
first-line treatment in elderly patients with advanced NSCLC. A
previous phase I study of this regimen reported mild toxicities and
a response rate of 42.9% (16). The
response rate in the present study almost coincided with that of
this previous phase I study. To date, only three studies have
reported the combination of gemcitabine and S-1 in the treatment of
patients with advanced NSCLC (Table
IV) (17–19). In a previous prospective study
conducted in a first-line setting, two regimens were investigated,
and the efficacy was 22.0 and 28.9%, the PFS was 3.6 and 4.1
months, and the OS was 15.5 and 18.8 months, with mild toxicities
(18). A study reporting a
platinum-refractory case yielded a response rate of 23.5%, a PFS of
6.6 months and an OS of 19.9 months (19). A phase II trial in elderly patients
with NSCLC indicated a response rate of 27%, a time to progression
of 4.2 months, and an OS of 12.9 months (17). The present study suggested a higher
response rate associated with the regimen designed by our group
compared with the other studies (17–19),
although the sample size of the present study was very small.
Regarding adverse events, the previous three studies demonstrated
hematological toxicities in 45.9–61.0% with grade 3/4 neutropenia,
21.0–28.9% with grade 3/4 leukopenia and 4.9–13.5% with
thrombocytopenia, and non-hematological toxicities in 0–6.0% with
grade 3/4 skin rash (17–19). Furthermore, febrile neutropenia was
observed in 3.0–7.3% of patients, and grade 3/4 pneumonia was
observed in 4.9–9.0%. On the other hand, the toxicities observed in
the present study appeared to be mild compared with those observed
in other studies with gemcitabine and S-1. Bi-weekly administration
of gemcitabine plus S-1 may contribute to increased tolerability
and efficacy, although the mechanisms underlying the effects of the
regimen remain to be delineated. In addition, the survival data in
the present study, although biased due to the small sample size,
suggested that it was possible to compare the effectiveness of this
regimen with that of the previous studies (17–19).
Therefore, further studies conducted with a larger sample size to
confirm the findings of the present study are warranted.
 | Table IV.Summary of previous studies in
combination therapy of gemcitabine and S-1. |
Table IV.
Summary of previous studies in
combination therapy of gemcitabine and S-1.
|
| Treatment
schedule |
|
|
| Grade 3/4 toxicity
(%) |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Subject | Gemcitabine | S-1 | Interval | n | ORR (%) | PFS/TTP
(month) | OS (month) | Leukopenia | Neutropenia |
Thrombocytopenia | Anemia | (Refs.) |
|---|
| 1st-line, elderly
(≥70) | 1,000
mg/m2, days 8 and 15 | 30 mg/m2
b.i.d., days 1–14 | q4w | 37 | 27.0 (95% CI:
15–42) | 4.2 (95% CI:
3.2–5.7) | 12.9 (95%CI:
10.4–14.7) | 27.0 | 45.9 | 13.5 | 13.5 | (17) |
| 1st-line | 1,000
mg/m2, days 1 and 8 | 30 mg/m2
b.i.d., days 1–14 | q3w | 41 | 22.0 (95% CI:
10.6–37.6) | 4.1 (95% CI:
2.8–5.6) | 15.5 (95% CI:
8.0–23.6) | 26.8 | 61.0 |
4.9 |
2.4 | (18) |
| 1st-line | 1,000
mg/m2, days 8 and 15 | 30 mg/m2
b.i.d., days 1–14 | q3w | 38 | 28.9 (95% CI:
15.4–45.9) | 5.5 (95% CI:
3.8–6.3) | 18.8 (95% CI:
11.7–23.9) | 28.9 | 50.0 | 18.4 |
5.3 |
| 2nd-/3rd-line | 1,000
mg/m2, days 8 and 15 | 30 mg/m2
b.i.d., days 1–14 | q3w | 34 | 23.5 (95% CI:
9.1–38.0) | 6.6 (95% CI:
4.2–7.8) | 19.9 (95% CI:
9.3–22.2) | 21.0 | 50.0 | 12.0 | 18.0 | (19) |
| 1st-line, elderly
(≥70) | 1,000
mg/m2, days 1 and 15 | 40 mg/m2
b.i.d., days 1–14 | q4w | 20 | 40.0 (95% CI:
18.5–61.5) | 6.4 (95% CI:
4.0–17.0) | 17.8 (95% CI:
7.0–46.0) | 29.0 | 24.0 |
0.0 |
0.0 | The present
study |
Single agents, including docetaxel or vinorelbine,
have been recommended for the treatment of elderly patients with
advanced NSCLC. In Japan, the results of phase III trials reported
by Kudoh et al (22) and Abe
et al (5) indicated that
docetaxel was suitable for such patients. Kudoh et al
(22) reported the OS, PFS and ORR of
docetaxel were 14.3 months, 5.5 months and 22.7%, respectively. In
the phase III trial conducted by Abe et al (5) the ORR, MST and PFS were reported to be
24.6%, 14.3 months and 4.4 months, respectively, which was superior
to the results of survival data from platinum combination therapies
including docetaxel. These studies had similar profiles of adverse
events and grade 3/4 leukopenia was observed in 58.0–62.7%, grade
3/4 neutropenia in 82.9–88.8% and febrile neutropenia in 12.5–15.2%
of the patients. The toxicities following docetaxel administration
were markedly severe compared with those following gemcitabine plus
S-1. However, the efficacy did not differ between docetaxel alone
and gemcitabine plus S-1. In the present study, the ORR of
gemcitabine plus S-1 may be superior to that of docetaxel alone
irrespective of mild toxicities, comparable with that of the
combination of platinum doublet. As anticancer therapies would be
preferable in the outpatient rather than the inpatient treatment
setting, the regimen examined in the present study may be
appropriate for the treatment of advanced NSCLC, in particular in
elderly patients with short life expectancies. Therefore, in terms
of efficacy and tolerability, the administration of this regimen
may be more effectively compared with previous treatment settings
involving gemcitabine plus S-1 (17–19).
There are several limitations to the present study.
Firstly, the selection procedure of patients eligible to be
included in the present study took a long time (from 2007 to 2015
for only 20 patients). Therefore, a number of novel strategies have
become available for the treatment of patients with advanced NSCLC,
which may have rendered the survival time in the present study
biased. Secondly, the treatment of all the patients was not judged
according to the EGFR mutation status. It may be difficult
for the results of the present study to indicate suitability for
daily practice. A total of 4 patients harboring EGFR
mutations received gefitinib and erlotinib, thus, the efficacy of
EGFR-tyrosine kinase inhibitors may affect the survival results. It
is necessary to perform further studies on patients without
EGFR mutations. Thirdly, the relationship between the
efficacy of gemcitabine plus S-1 and any biomarkers, including
thymidylate synthase or ribonucleotide reductase catalytic subunit
M1, was not investigated. The discovery of any predictive
biomarkers would improve the outcome following therapy. It is
important to determine whether the efficacy of the regimen used in
the present study would improve depending on the expression of any
predictive markers. Finally, the sample size is limited and this
may bias the results of the present study. Furthermore, the
potential for effective treatment of elderly patients depends on
physical function, mobility, nutrition, social support and the
condition of comorbid diseases, thus, evaluating the individual
patient is comprehensive and possible to achieve with a geriatric
assessment in cooperation with PS (23). A previous report focused on the
prognostic significance of a baseline assessment of functional
status, comorbidity and quality of life and demonstrated that
improved baseline quality of life and greater facility with
activities of daily living were associated with a favorable outcome
(24). In the present study,
therefore, the absence of geriatric assessment may disturb the
appropriate evaluation of the therapeutic efficacy against elderly
patients. Further investigation and further studies using geriatric
assessment are therefore warranted.
In conclusion, the results of the present study
demonstrated that the combination of gemcitabine and S-1 was an
effective and well-tolerated regimen in elderly patients with
chemo-naïve advanced NSCLC. The treatment schedule followed in the
present study seemed to be more effective compared with regimens
evaluated in previous studies. Future studies comparing gemcitabine
plus S-1 combination therapy to single-agent regimens including
docetaxel or vinorelbine are warranted.
Acknowledgements
The authors would like to thank Ms. Yuka Matsui for
her technical assistance during manuscript submission and Ms.
Tomoko Okada for data collection and technical assistance.
References
|
1
|
Siegel R, DeSantis C, Virgo K, Stein K,
Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chemotherapy in non-small cell lung
cancer, . A meta-analysis using updated data on individual patients
from 52 randomised clinical trials. Non-small Cell Lung Cancer
Collaborative Group. BMJ. 311:899–909. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Azzoli CG, Temin S and Giaccone G: 2011
Focused Update of 2009 American Society of Clinical Oncology
Clinical Practice Guideline Update on Chemotherapy for Stage IV
Non-Small-Cell. Lung Cancer. J Oncol Pract. 8:63–66. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Quoix E, Zalcman G, Oster JP, Westeel V,
Pichon E, Lavolé A, Dauba J, Debieuvre D, Souquet PJ, Bigay-Game L,
et al: Carboplatin and weekly paclitaxel doublet chemotherapy
compared with monotherapy in elderly patients with advanced
non-small-cell lung cancer: IFCT-0501 randomized, phase 3 trial.
Lancet. 378:1079–1088. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abe T, Takeda K, Ohe Y, Kudoh S, Ichinose
Y, Okamoto H, Yamamoto N, Yoshioka H, Minato K, Sawa T, et al:
Randomized phase III trial comparing weekly docetaxel plus
cisplatin versus docetaxel monotherapy every 3 weeks in elderly
patients with advanced non-small-cell lung cancer: The intergroup
trial JCOG0803/WJOG4307L. J Clin Oncol. 33:575–581. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shirasaka T, Shimamato Y, Ohshimo H,
Yamaguchi M, Kato T, Yonekura K and Fukushima M: Development of a
novel form of an oral 5-fluorouracil derivative (S-1) directed to
the potentiation of the tumor selective cytotoxicity of
5-fluorouracil by two biochemical modulators. Anticancer Drugs.
7:548–557. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kubota K, Sakai H, Yamamoto N, Kunitoh H,
Nakagawa K, Takeda K, Ichinose Y, Saijo N, Ariyoshi Y and Fukuoka
M: A multi-institution phase I/II trial of triweeky regimen with
S-1 plus cisplatin in patients with advanced non-small cell lung
cancer. J Thorac Oncol. 5:702–706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ichinose Y, Yoshimori K, Sakai H, Nakai Y,
Sugiura T, Kawahara M and Niitani H: S-1 plus cisplatin combination
chemotherapy in patients with advanced non-small cell lung cancer:
A multi-institutional phase II trial. Clin Cancer Res.
10:7860–7864. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kawahara M, Furuse K, Segawa Y, Yoshimori
K, Matsui K, Kudoh S, Hasegawa K and Niitani H; S-1 Cooperative
Study Group (Lung Cancer Working Group), : Phase II study of S-1, a
novel oral fluorouracil, in advanced non-small-cell lung cancer. Br
J Cancer. 85:939–943. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Furuse K, Kawahara M, Hasegawa K, Kudoh S,
Takada M, Sugiura T, Ichinose Y, Fukuoka M, Ohashi Y and Niitani H;
S-1 Cooperative Study Group (Lung Cancer Working Group), : Early
phase II study of S-1, a new oral fluoropyrimidine, for advanced
non-small-cell lung cancer. Int J Clin Oncol. 6:236–241. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okamoto I, Yoshioka H, Morita S, Ando M,
Takeda K, Seto T, Yamamoto N, Saka H, Asami K, Hirashima T, et al:
Phase III trial comparing oral S-1 plus carboplatin with paclitaxel
plus carboplatin in chemotherapy-naïve patients with advanced
non-small-cell lung cancer: Results of a west Japan oncology group
study. J Clin Oncol. 28:5240–5246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kubota K, Sakai H, Katakami N, Nishio M,
Inoue A, Okamoto H, Isobe H, Kunitoh H, Takiguchi Y, Kobayashi K,
et al: A randomized phase III trial of oral S-1 plus cisplatin
versus docetaxel plus cisplatin in Japanese patients with advanced
non-small-cell lung cancer: TCOG0701 CATS trial. Ann Oncol.
26:1401–1408. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Guo Zw and Gallo JM: Selective Protection
of 2′,2′-Difluorodeoxycytidine (Gemcitabine). J Org Chem.
64:8319–8322. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ichinose Y, Seto T, Semba H, Itoh K, Inoue
Y, Tanaka F, Araki J, Tamanoi M, Yamamoto H and Iwamoto N: UFT plus
gemcitabine combination chemotherapy in patients with advanced
non-small-cell lung cancer: A multi-institutional phase II trial.
Br J Cancer. 93:770–773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakamura K, Yamaguchi T, Ishihara T, Sudo
K, Kato H and Saisho H: Phase II trial of oral S-1 combined with
gemcitabine in metastatic pancreatic cancer. Br J Cancer.
94:1575–1579. 2006.PubMed/NCBI
|
|
16
|
Kaira K, Sunaga N, Yanagitani N, Aoki H,
Kawata T, Utsugi M, Shimizu Y, Shimizu K, Hisada T, Ishizuka T and
Mori M: Phase I trial of oral S-1 plus gemcitabine in elderly
patients with non-small cell lung cancer. Anticancer Drugs.
19:289–294. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seto T, Yamanaka T, Wasada I, Seki N,
Okamoto H, Ogura T, Shibuya M, Takiguchi Y, Shinkai T, Masuda N, et
al: Phase I/II trial of gemcitabine plus oral TS-1 in elderly
patients with advanced non-small cell lung cancer: Thoracic
oncology research group study 0502. Lung Cancer. 69:213–217. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Satouchi M, Kotani Y, Katakami N, Shimada
T, Urata Y, Yoshimura S, Funada Y, Hata A, Ando M and Negoro S:
Randomized phase II study of two different schedules of gemcitabine
and oral S-1 in chemo-naïve patients with advanced non-small cell
lung cancer. J Thorac Oncol. 5:696–701. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Takiguchi Y, Seto T, Ichinose Y, Nogami N,
Shinkai T, Okamoto H, Minato K, Seki N, Eguchi K, Kishi K, et al:
Long-term administration of second-line chemotherapy with S-1 and
gemcitabine for platinum-resistant non-small cell lung cancer. A
phase II study. J Thorac Oncol. 6:156–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. J Natl Cancer
Inst. 92:205–216. 2000. View Article : Google Scholar
|
|
21
|
Trotti A, Colevas AD, Setser A, Rusch V,
Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN and
Rubin P: CTCAE v3.0: Development of a comprehensive grading system
for the adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kudoh S, Takeda K, Nakagawa K, Takada M,
Katakami N, Matsui K, Shinkai T, Sawa T, Goto I, Semba H, et al:
Phase III study of docetaxel compared with vinorelbine in elderly
patients with advanced non-small-cell lung cancer: Results of the
West Japan Thoracic Oncology Group Trial (WJTOG 9904). J Clin
Oncol. 24:3657–3663. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gajra A and Jatoi A: Non-small-cell lung
cancer in elderly patients: A discussion of treatment options. J
Clin Oncol. 32:2562–2569. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Maione P, Perrone F, Gallo C, Manzione L,
Piantedosi F, Barbera S, Cigolari S, Rosetti F, Piazza E, Robbiati
SF, et al: Pretreatment quality of life and functional status
assessment significantly predict survival of elderly patients with
advanced non-small-cell lung cancer receiving chemotherapy: A
prognostic analysis of the multicenter Italian lung cancer in the
elderly study. J Clin Oncol. 23:6865–6872. 2005. View Article : Google Scholar : PubMed/NCBI
|