Introduction
As populations become increasingly aged and the
magnitude of environmental pollution rises, the incidence of skin
cancer also increases. Skin cancer is one of the most common
malignancies with high invasiveness and metastasis rates. It
usually occurs in the exposed parts of the human body, such as the
neck, face and upper arm. The most common types are basal cell and
squamous cell carcinomas (1). Local
invasion by skin cancer cells can cause damage to the local
tissues, generating deformations that affect the normal appearance
of the patient (2).
Many treatment methods, including traditional
surgery, radiotherapy, chemotherapy, cryotherapy and photodynamic
therapy can be used to treat skin cancers (3). Nevertheless, surgery remains as the
mainstay of treatment. In addition, painless photodynamic therapy
(PDT) with its excellent safety profile, fine cosmetic results and
low recurrence rate has become a standard option for certain cases
even though it is not cost-effective (4,5). Compared
with the traditional photodynamic therapy, 5-aminolevulinic
acid-photodynamic therapy (ALA-PDT), the second-generation
5-aminolevulinic acid (5-ALA), is used as the photosensitizer. In
this case, absorption by the target tissues occurs faster, prompt
excretion from the body ensures minimal photosensitivity adverse
effects, and a shorter time course of lightproof treatment is
necessary. CyPA and CyPB are widely distributed proteins expressed
in various types of human cells, may be involved in the apoptosis
of skin cancer cells, and can be used as markers for progression of
skin cancer.
In the present study, patients with skin cancer were
treated with surgery and local ALA-PDT to evaluate the efficacy and
safety of this method as compared to ALA-PDT alone, and the
findings were reported.
Materials and methods
Clinical data
General information
Seventy-six patients with skin cancer who were
admitted to the Liaocheng People's Hospital were selected from May
2014 to April 2015 and were randomly divided into a control or
observation group (38 cases in each). The patients in the
observation group were first subjected to surgical treatment, and
then treated with ALA-PDT. The patients in the control group
underwent ALA-PDT surgery alone. There were three main inclusion
criteria: ⅰ) patients had the skin cancer diagnosis confirmed by
histopathology; ⅱ) they had not been treated with laser, freezing
or topical treatments before the diagnosis and ⅲ) the patients all
signed informed consent. The exclusion criteria included patients
with severe organ dysfunction, immune system diseases and eczema or
fungal infections in the vicinity of the skin lesion area. No
significant differences following a comparison og the general
information between the two groups (P>0.05, Table I). This study was approved by the
Ethics Committee of Liaocheng People's Hospital. Signed written
informed consents were obtained from all participants or their
families before the study.
 | Table I.General information for patients in
the two groups. |
Table I.
General information for patients in
the two groups.
| Items | Observation group
(n=38) | Control group
(n=38) | t/χ2 | P-value |
|---|
| Age range
(years) | 40–75 | 40–72 |
|
|
| Sex
(male/female) | 20/18 | 22/16 | 0.213 | 0.645 |
| Mean age (years) | 57.56±6.47 | 58.34±6.56 | 0.522 | 0.603 |
| BMI
(kg/m2) | 22.43±3.45 | 22.56±3.38 | 0.166 | 0.869 |
| Mean duration of
disease (months) | 14.56±6.47 | 15.34±6.56 | 0.522 | 0.603 |
| Skin lesion area
(cm2) | 2.43±0.45 | 2.56±0.48 | 1.218 | 0.227 |
| Disease type (n,
%) |
|
|
|
|
| Basal
cell carcinoma | 21 (55.26) | 24 (63.16) | 0.260 | 0.610 |
| Squamous
cell carcinoma | 17 (44.74) | 14 (36.84) | 0.260 | 0.610 |
Treatment
The patients in the control group were only treated
with ALA-PDT. Sodium chloride solution (0.9%) was used to clean the
lesion and the surrounding skin, followed by cleaning with sterile
cotton balls. The lesion was infiltrated with ozone solution for 30
min. 5-ALA was dissolved in 1 ml of a 5% ozone solution to produce
10% solution of 5-aminopentanoic acid. The 10% solution of
5-aminopentanoic acid was then applied to the lesion and the
surrounding skin (approximately 0.5 cm beyond the lesion). The
lesion area was coated with opaque material, which was removed 4 h
later. The lesion area was then irradiated with photodynamic
therapy apparatus (Guilin Xingda Optoelectronic Medical Instruments
Co., Ltd., Guangxi, China) with an output power of 200
mW/cm2, a wavelength of 635 nm and a light dose of 120
J/cm2. The lesion area was treated for 40 min each time
and once per week until the tumor was completely removed.
The patients in the observation group were treated
with surgery and ALA-PDT. Patients were treated with 2% lidocaine
for local anesthesia. An incision was located 2 mm beyond the edge
of the lesion. The lesion was removed from the subcutaneous fat
layer, fascia surface or deep skin and periosteal surface. Local
bleeding was controlled. A direct suture or a suitable flap was
selected according to the size and depth of the lesion to repair
the wound. ALA-PDT was performed immediately after hemostasis
applying the same method used for the control group.
Detection of indicators
Histopathological examination was performed before
and after treatment, the specimen tissue was embedded with paraffin
and placed at −60°C. The expression of CyPA, CyPB and CD147 was
detected by immunohistochemical staining of specimens. The
necessary reagents were provided by Beijing Zhongshan Jinqiao
Biotechnology Co. Ltd., (Guangdong, China). The tissue samples were
sliced and dewaxed according to standard procedure. After washing,
incubation and color development, the tissues were observed under a
microscope. Pure water was used to wash the samples and terminate
color development. Hematoxylin staining (30 sec) was applied twice
and the samples were then sealed with neutral gum.
Follow-up
After treatment, the patients were followed up for
12 months. The efficacy of the treatment and patient satisfaction
levels were recorded. The appearance of the skin at the lesion site
and detected scar hyperplasias were recorded.
Treatment efficacy and evaluation
criteria
Determination of treatment efficacy
Complete remission (CR) occurred when the
pigmentation was significantly improved, the skin lesions
completely disappeared, and there was no malignancy on pathology
samples after treatment and no recurrences within 6 months. With
partial remission (PR) the hyperpigmentation was lessened and the
lesion area was reduced by ≥50% of the original. A failed treatment
meant no improvement was seen in pigmentation, the skin lesions
decreased by <50% in size and the malignancy recurred within 6
months. The treatment efficiency was calculated by adding the
percentages of CR and PR.
Observation of the surgical treatment outcomes in
the two groups
The average times to wound healing and the number of
treatments needed were recorded. The patients satisfaction with the
postoperative appearance was classified as one of three levels
(very satisfied, generally satisfied and not satisfied). The
percentage of patient satisfaction was calculated by adding the
percentage of very satisfied and generally satisfied patients.
Observation of the incidence of adverse reactions
in the two groups
The cases of burning, redness, swelling, pain and
other adverse reactions were recorded. All the patients were
followed up for 12 months after treatment. Any pathological changes
of the lesion were examined. The recurrence rate was recorded at 6
and 12 months after treatment.
The expression levels of CyPA, CyPB and CD147 before
and after treatment were detected by immunohistochemistry. Eight
visual fields were randomly selected under high magnification
(×400) and 100 cells were selected for each visual field. According
to the number of cells with positive signal, a semi-quantitative
scoring method was used assigning points for positivity and cell
staining degree: 0 points for positive cell percentage of <5%, 1
point for positive cell percentage between 5 and 25%, 2 points for
positive cell percentage between 26 and 50%, 3 points for positive
cell percentage between 51 and 75% or 4 points for positive cell
percentage >75%. Furthermore, scoring for staining included 0
points for no color, 1 point for pale yellow cells, 2 points for
coarse granular brown cells, and 3 points for small block dark
brown cells. The total sum of cell percentage plus cell staining
degree points produced the total score. A total score ≤3 indicated
low-level expression, scores from 4 to 5 indicated moderate
expression, and a score ≥6 indicated a high expression.
Statistical analysis
Data were processed using SPSS 19.0 software (SPSS,
Inc., Chicago, IL, USA). Measurement data were expressed as mean ±
standard deviation. The t-test was used for comparisons between the
groups and within a group, and countable data were expressed as
rates and processed using χ2 test, the treatment
efficiency was evaluated by the rank-sum test. P<0.05 was
considered to be statistically significant.
Results
Comparison of the efficacy between two
groups
The CR and PR of the observation group were 65.79
and 31.58%, respectively. The CR and PR of the control group were
34.21 and 42.11%, respectively. The total treatment efficacy of the
observation group was significantly higher than that of the control
group (P<0.05, Table II).
 | Table II.Comparison of the treatment efficiency
between the two groups (n, %). |
Table II.
Comparison of the treatment efficiency
between the two groups (n, %).
| Groups | N (cases) | CR | PR | Invalid | Treatment
efficiency |
|---|
| Observation | 38 | 25 (65.79) | 12 (31.58) | 1 (2.64) | 37 (97.37) |
| Control | 38 | 13 (34.21) | 16 (42.11) | 9 (23.69) | 29 (76.32) |
Comparison of the treatment outcomes
between two groups
The average healing time in the observation group
was shorter than that in the control group. The number of
treatments needed for those in the observation group was
significantly smaller than the number of treatments needed for
those in the control group. In addition, the apparent treatment
satisfaction in the observation group was significantly higher than
that in the control group (P<0.05, Table III).
 | Table III.Comparison of the treatment outcomes
between the two groups. |
Table III.
Comparison of the treatment outcomes
between the two groups.
| Groups | N (cases) | Average healing time
(days) | Number of treatments
needed | Patient satisfaction
(n, %) |
|---|
| Observation | 38 | 13.32±3.23 | 2.43±1.24 | 36 (94.73) |
| Control | 38 | 15.34±3.24 | 3.45±1.38 | 28 (73.64) |
| t/χ2 |
| 2.722 | 3.389 | 4.862 |
| P-value |
| 0.008 | 0.001 | 0.027 |
Comparison of the incidence of adverse
reactions between two groups
The incidence of pain, redness, swelling and burning
sensation in the observation group was significantly lower than
that in the control group (P<0.05, Table IV).
 | Table IV.Comparison of the incidence of adverse
reactions between the groups (n, %). |
Table IV.
Comparison of the incidence of adverse
reactions between the groups (n, %).
| Groups | N (cases) | Burning
sensation | Redness and
swelling | Pain | Complication
rate |
|---|
| Observation | 38 | 1 (2.63) | 0 (0.00) | 1 (2.63) | 2 (5.26) |
| Control | 38 | 5 (13.16) | 3 (7.89) | 4 (10.53) | 12 (31.58) |
| t/χ2 |
|
|
|
| 7.095 |
| P-value |
|
|
|
| 0.007 |
Comparison of recurrence rates between
the two groups during the 12 months follow-ups
The recurrence rates in the observation group were
2.63 and 5.26% at 6 and 12 months after treatment, respectively.
The recurrence rates in the control group were 13.15 and 34.21% at
6 and 12 months after treatment, respectively. While no significant
difference in the recurrence rate was found at 6 months after
treatment between the two groups (P>0.05), the recurrence rate
of the observation group at 12 months after treatment was
significantly lower than that of the control group at the same time
(P<0.05, Table V).
 | Table V.Comparison of recurrence rate between
the two groups (n, %). |
Table V.
Comparison of recurrence rate between
the two groups (n, %).
| Groups | N (cases) | Recurrence rate at 6
months after treatment | Recurrence rate at 12
months after treatment |
|---|
| Observation | 38 | 1 (2.63) | 2 (5.26) |
| Control | 38 | 5 (13.15) | 13 (34.21) |
| t/χ2 |
| 1.627 | 8.308 |
| P-value |
| 0.202 | 0.003 |
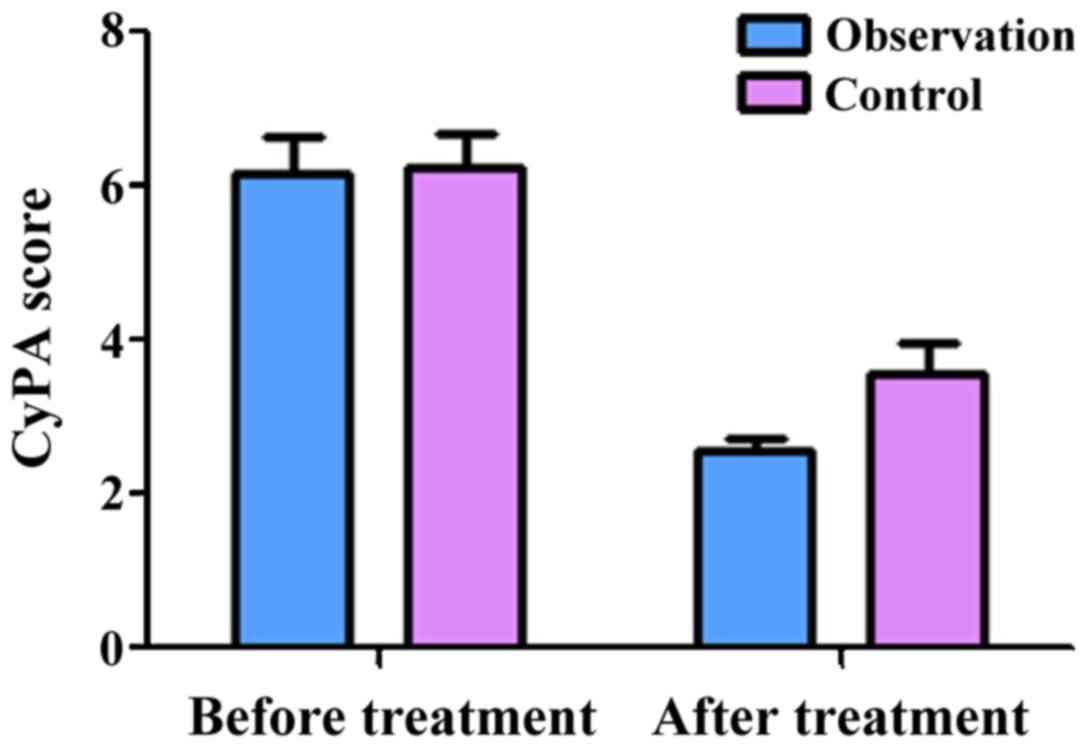
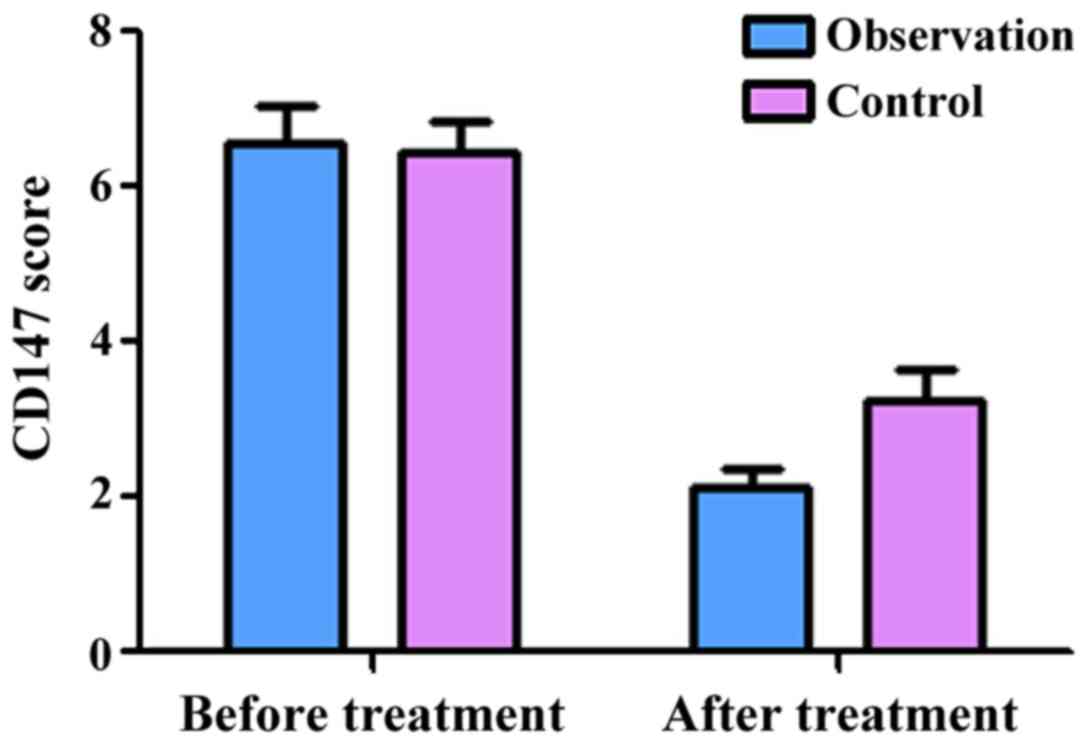
Comparison of CyPA, CyPB and CD147
expression levels before and after treatment between two
groups
The semi-quantitative scores of CyPA in the
observation group before and after treatment were 6.16±0.62 and
2.58±0.23, respectively. The scores of CyPB before and after
treatment were 6.06±0.43 and 2.96±0.54, respectively. The scores of
CD147 before treatment and after treatment were 6.58±0.36 and
2.18±0.15, respectively. These results showed that treatment
significantly decreased the expression of CyPA, CyPB and CD147
(P<0.05) in the observation group. At the same time, the
semi-quantitative scores of CyPA in the control group before and
after treatment were 6.26±0.67 and 3.98±0.43, respectively. The
scores of CyPB before and after treatment were 6.28±0.47 and
3.96±0.57, respectively. The scores of CD147 before and after
treatment were 6.44±0.37 and 3.18±0.25, respectively, indicating
that treatment significantly decreased the expression of CyPA, CyPB
and CD147 in the control group as well (P<0.05). Of note, there
was no significant difference in the expression of CyPA, CyPB and
CD147 between the two groups before treatment (P>0.05). However,
after treatment, the expression of CyPA, CyPB and CD147 in the
observation group was significantly lower than those in the control
group (P<0.05, Figs. 1–3).
Discussion
Skin cancer is a common clinical malignant tumor,
with basal cell carcinoma and squamous cell carcinoma being the two
most common types that account for approximately 90% of all skin
cancers (6). Skin cancer is usually
aggressive and generates metastatic growth. Basal cell carcinoma,
which accounts for the largest proportion of skin cancers, can
initially grow within the epidermal basal cells with less
metastasis, but with time the tumor nest can be extended to the
dermal papilla (7). Squamous cell
carcinoma, which accounts for the second largest proportion of skin
cancers, originates from the epidermis or keratinocytes. Squamous
cell carcinoma usually leads to different degrees of keratosis and
lymphatic metastases (8). The
possible mechanism of invasion and metastasis of skin cancer is
explained by the ability of tumor cells to produce enzymes that can
degrade extracellular matrix components facilitating the migration
of tumor cells. At the same time, stromal or tumor cells are
induced to produce adhesion molecules and cytokines, such as
vascular endothelial growth factor to facilitate migration and
neovascularization (9,10). The etiology of skin cancer is not yet
fully understood. It may be related to chemical stimulants,
excessive sunlight exposure, long-term radiation exposure, or
trauma (11).
Many treatment methods, including surgery, laser,
cryotherapy, electrocautery and radiotherapy, can be used in the
treatment of skin cancer, and surgical treatment is the gold
standard (1,4). The 5-year cure rate of surgical
treatment can reach 90% or even higher, but these treatment methods
are usually followed by a persistent recurrence rate, that even if
treated successfully, are likely to affect the patient's appearance
(12,13). Therefore, prevention of local
recurrence and improvement of appearance have become hot research
goals.
ALA-PDT, which combines a photosensitizer with a
source light and oxygen can specifically destroy the lesion,
thereby killing only tumor cells (14). As a precursor of hemoglobin, 5-ALA is
a recently developed second-generation photosensitizer. 5-ALA can
be absorbed by the rapidly proliferating cells and selectively
accumulates in the cells, increasing the effectiveness of treatment
(15). The results of our study
showed the treatment of surgery and ALA-PDT was superior to the
treatment with ALA-PDT alone, the efficacy rate was close to 100%,
the average wound healing time was significantly shorter, and the
number of treatments needed significantly less. This is probably
because surgical treatment can effectively open the tumor barrier,
and remove most of the lesion to fully expose any hard to reach
tumor cells, effectively increasing the depth of the tissue being
treated. The surgical treatment facilitates the penetration of
5-ALA and increases the accumulation of 5-ALA in tumor cells, which
in turn increase the production of protoporphyrin IX (PPIX) and
hemoglobin (16). With the
irradiation of light at 635 nm wavelengths, PPIX produces reactive
oxygen species that specifically kill the tumor cells without
bringing damage to the surrounding normal cells (17). Thus, the treatment effect of this
combined method is stronger than that of ALA-PDT alone and the
number of treatments needed is significantly less. In the case of
facial tumors, surgical treatment combined with ALA-PDT is likely
to achieve the best efficacy with the best cosmetic results
ensuring higher appearance satisfaction (18).
Our results showed that no significant differences
were found in the recurrence rates at 6 months after treatment
between the two groups (P>0.05), while the recurrence rate of
the observation group was significantly lower than that of the
control group at 12 months after treatment (P<0.05). These data
suggest that ALA-PDT can kill tumor cells by itself. Nevertheless,
surgical treatment combined with ALA-PDT ensures eradication of
tumor cells is more efficient in modulating the recurrence after
longer periods of time.
CyPA and CyPB are the main members of the CyP
family, CyPA is mainly located in the cytoplasm, while CyPB is
mainly located in the endoplasmic reticulum, but can also be
secreted outside of cells. Both CyPA and CyPB play important roles
in cell-cell signaling transduction. CyPA and CyPB can block the
dephosphorylation of T-cell activation factor, inhibit the
transcription of IL-2 and other cytokines, and reduce the release
of these cytokines to achieve their function of immunosuppression.
CyPA and CyPB are highly expressed in pancreatic cancer cells,
non-small cell lung cancer cells, tongue squamous cell carcinoma
cells and many others (19). CD147, a
member of the immunoglobulin superfamily, is widely distributed in
the body. CD147 can stimulate fibroblasts to produce collagenase,
which is involved in tumor infiltration and metastasis. CD147 is
highly expressed in liver and lung cancer cells, lymphoma cells,
skin squamous cell carcinomas and other cancer cells (20). The results of our study showed that
the levels of CyPA, CyPB and CD147 in the two groups were
significantly decreased after treatment and that the decrease in
the observation group was significantly higher than that of the
control group (P<0.05). It is possible that CyPA and CyPB were
highly expressed in the tumor tissue before treatment, enhancing
the expression of CD147 and its transportation to the cell
membrane. CD147, in turn, promoted the secretion of CyPA and CyPB,
a function for which it is known (21). Treatment with ALA-PDT can induce tumor
cells to produce more TNF-α, IL-1β and other pro-inflammatory
cytokines, thereby activating NF-κβ, promoting tumor cell apoptosis
and necrosis, and effectively reducing the secretion of CyPA and
CyPB. In addition, inhibiting the activation and angiogenesis of
vascular endothelial cells, and blocking the interaction between
CD147 and CyPA, and CyPB, will in turn prevent tumor invasion and
metastasis, and reduce recurrence rates (21).
In conclusion, the treatment of surgery combined
with ALA-PDT in skin cancer can fully take advantage of the
strengths of each method. This approach cannot only increase the
survival rate but can also improve the recurrence rate and even
improve appearance. Further studies are required to promote this
treatment method in the clinical practice.
References
|
1
|
LeSueur BW, DiCaudo DJ and Connolly SM:
Axillary basal cell carcinoma. Dermatol Surg. 29:1105–1108. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Climstein M, Furness J, Hing W and Walsh
J: Lifetime prevalence of non-melanoma and melanoma skin cancer in
Australian recreational and competitive surfers. Photodermatol
Photoimmunol Photomed. 32:207–213. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leiter U, Eigentler T and Garbe C:
Epidemiology of skin cancer. Adv Exp Med Biol. 810:120–140.
2014.PubMed/NCBI
|
|
4
|
Lucena SR, Salazar N, Gracia-Cazaña T,
Zamarrón A, González S, Juarranz Á and Gilaberte Y: Combined
treatments with photodynamic therapy for non-melanoma skin cancer.
Int J Mol Sci. 16:25912–25933. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoon HE, Oh SH, Kim SA, Yoon JH and Ahn
SG: Pheophorbide a- mediated photodynamic therapy induces autophagy
and apoptosis via the activation of MAPKs in human skin cancer
cells. Oncol Rep. 31:137–144. 2014.PubMed/NCBI
|
|
6
|
Abbas M and Kalia S: Trends in
non-melanoma skin cancer (basal cell carcinoma and squamous cell
carcinoma) in Canada: A descriptive analysis of available data. J
Cutan Med Surg. 20:166–175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hussain AA, Themstrup L, Nürnberg BM and
Jemec G: Adjunct use of optical coherence tomography increases the
detection of recurrent basal cell carcinoma over clinical and
dermoscopic examination alone. Photodiagn Photodyn Ther.
14:178–184. 2016. View Article : Google Scholar
|
|
8
|
Kauvar AN, Cronin T Jr, Roenigk R, Hruza G
and Bennett R: Consensus for nonmelanoma skin cancer treatment:
Basal cell carcinoma, including a cost analysis of treatment
methods. Dermatol Surg. 41:550–571. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cho E and Grim JE: A (heat) shocking
development: FBXW7 loss unleashes HSF1 to drive melanoma invasion
and metastasis. Pigment Cell Melanoma Res. 28:643–644. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Campione E, Paternò EJ, Candi E, Falconi
M, Costanza G, Diluvio L, Terrinoni A, Bianchi L and Orlandi A: The
relevance of piroxicam for the prevention and treatment of
nonmelanoma skin cancer and its precursors. Drug Des Devel Ther.
9:5843–5850. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang L, Yi XM, Chen J, Chen FJ, Lou W, Gao
YL, Zhou J, Su LN, Xu X, Lu JQ, et al: Ubiquitin ligase UBE3C
promotes melanoma progression by increasing epithelial-mesenchymal
transition in melanoma cells. Oncotarget. 7:15738–15746.
2016.PubMed/NCBI
|
|
12
|
Campos PM, Bentley MV Lopes Badra and
Torchilin VP: Nanopreparations for skin cancer
therapyNanobiomaterials in Cancer Therapy. Grumezescu AM: 7. 1st.
Elsevier; New York, NY: pp. 1–28. 2016, View Article : Google Scholar
|
|
13
|
Dummer R: Precision medicine and skin
cancer therapy: Dealing with a moving target. Curr Opin Oncol.
26:182–183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dixon AJ, Anderson SJ, Mazzurco JD and
Steinman HK: Novel photodynamic therapy does not prevent new skin
cancers - randomized controlled trial. Dermatol Surg. 40:412–419.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Na JI, Kim SY, Kim JH, Youn SW, Huh CH and
Park KC: Indole-3-acetic acid: A potential new photosensitizer for
photodynamic therapy of acne vulgaris. Lasers Surg Med. 43:200–205.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Valdes PA, Bekelis K, Harris BT, Wilson
BC, Leblond F, Kim A, Simmons NE, Erkmen K, Paulsen KD and Roberts
DW: 5-Aminolevulinic acid-induced protoporphyrin IX fluorescence in
meningioma: Qualitative and quantitative measurements in vivo.
Neurosurgery. 10 Suppl 1:74–83. 2014.PubMed/NCBI
|
|
17
|
Kanick SC, Davis SC, Zhao Y, Hasan T,
Maytin EV, Pogue BW and Chapman MS: Dual-channel red/blue
fluorescence dosimetry with broadband reflectance spectroscopic
correction measures protoporphyrin IX production during
photodynamic therapy of actinic keratosis. J Biomed Opt.
19:750022014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sidoroff A and Thaler P: Taking treatment
decisions in non-melanoma skin cancer - the place for topical
photodynamic therapy (PDT). Photodiagn Photodyn Ther. 7:24–32.
2010. View Article : Google Scholar
|
|
19
|
Huang C, Sun Z, Sun Y, Chen X, Zhu X, Fan
C, Liu B, Zhao Y and Zhang W: Association of increased ligand
cyclophilin A and receptor CD147 with hypoxia, angiogenesis,
metastasis and prognosis of tongue squamous cell carcinoma.
Histopathology. 60:793–803. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fei F, Li X, Xu L, Li D, Zhang Z, Guo X,
Yang H, Chen Z and Xing J: CD147-CD98hc complex contributes to poor
prognosis of non-small cell lung cancer patients through promoting
cell proliferation via the PI3K/Akt signaling pathway. Ann Surg
Oncol. 21:4359–4368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li L, Tang W, Wu X, Karnak D, Meng X,
Thompson R, Hao X, Li Y, Qiao XT, Lin J, et al: HAb18G/CD147
promotes pSTAT3-mediated pancreatic cancer development via CD44s.
Clin Cancer Res. 19:6703–6715. 2013. View Article : Google Scholar : PubMed/NCBI
|