Introduction
Glioma derived from the neural epithelium is the
most common type of primary brain tumor, accounting for 40–50% of
all central nervous system neoplasms (1). Gliomas include astrocytomas,
oligodendrogliomas, glioblastomas, ependymomas, medulloblastomas
and glioblastoma multiforme (2).
Glioma is genetically complex and is invasive to surrounding
tissues, thus surgical resection is difficult (3). The recurrence of glioma following
treatment is mostly due to the recurrence of the primary lesion
(4). Although postoperative
radiotherapy and chemotherapy may prolong survival times, the
majority of patients with glioma live only 1–2 years after
diagnosis (5).
Tumor occurrence, development, invasion and
metastasis are complex processes involving multiple genes (6). Certain studies have indicated that
non-steroidal anti-inflammatory drugs reduce the risk of various
tumor types, for example, polyps of the large bowel in colorectal
cancer tissues, and that cyclooxygenase (COX) may be associated
with tumorigenesis (7). COX, also
known as prostaglandin endoperoxidase H synthase, has two isoforms:
COX-1, located in the cytoplasm and constitutively and stably
expressed in various tissues, and COX-2, which is expressed at low
levels or is not detectable in the majority of normal tissues, but
can be induced in response to cell activation by growth factors,
cytokines, inflammatory mediators and tumor promoters (8).
Survivin is a small but important member of the
inhibitor-of-apoptosis protein family, with 142 amino acid residues
and one baculovirus inhibitor of apoptosis protein repeat domain,
which is a multifunctional protein that contributes to cellular
homeostasis (9). Survivin is
implicated in the inhibition of apoptosis and mitotic regulation,
and promotes tumor development (10).
Overexpression of survivin has been reported in cancer and is
associated with a poor prognosis for a number of human
malignancies, including non-small cell lung cancer (11). Thus, the present study determined the
COX-2 and survivin expression levels in glioma tissues using
immunohistochemistry methods, it examined the association between
COX-2 and survivin, and it correlated the data with the
clinicopathological characteristics of gliomas, including patient
age, gender, tumor location, histopathological grade and patient
survival.
Materials and methods
Specimen collection
Patients (n=70) with glioma were selected from The
First Affiliated Hospital of Anhui Medical University (Hefei,
Anhui, China) between October 2012 and December 2013; the patient's
detailed clinicopathological characteristics were obtained from
their medical files. The patients selected included 42 males and 28
females, with an age range of 7–70 years (median age 50 years).
Patient characteristics are presented in Table I. The survival time was defined as the
time between the day of the initial diagnosis and the day of
patient mortality due to the glioma. Selected patients had no prior
history of glioma. No patient received radiotherapy or chemotherapy
prior to surgery and all patients were treated with surgery. The
type of surgery patients' underwent was mainly according to the
location and size of the tumor (for example total resection or
subtotal resection). The tissue samples were obtained during the
surgery. Surgically resected tissues were diagnosed by two
pathologists according to World Health Organization (WHO)
histological criteria (12). Tumors
of grades I–II were of low malignancy and tumors graded III–IV were
highly malignant. In addition, 7 normal brain tissues were derived
from patients with arteriovenous malformation by arteriovenous
malformation removal surgery. Written informed consent was obtained
in all patients prior to enrollment in the present study. The study
was approved by the Ethics Committee of The First Affiliated
Hospital of Anhui Medical University.
 | Table I.COX-2 and survivin expression levels
and clinicopathological factors. |
Table I.
COX-2 and survivin expression levels
and clinicopathological factors.
| Clinicopathological
factors | Cases, n | COX-2, n (%) | χ2 | P-value | Survivin, n (%) | χ2 | P-value |
|---|
| Gender |
|
|
|
|
|
|
|
| Male | 42 | 28 (66.7) | 0.653 | >0.05 | 25 (59.5) | 0.618 | >0.05 |
|
Female | 28 | 16 (57.1) |
|
| 14 (50.0) |
|
|
| Age, years |
|
|
|
|
|
|
|
| ≥50 | 46 | 26 (56.5) | 2.307 | >0.05 | 23 (50.0) | 1.776 | >0.05 |
|
<50 | 24 | 18 (52.9) |
|
| 16 (66.7) |
|
|
| Pathological WHO
grade |
|
|
|
|
|
|
|
|
I–II | 28 | 13 (46.4) | 5.395 | <0.05 | 11 (39.3) | 5.105 | <0.05 |
|
III–IV | 42 | 31 (73.8) |
|
| 28 (66.7) |
|
|
| Tumor size, cm |
|
|
|
|
|
|
|
| ≥5 | 43 | 28 (65.1) | 0.244 | >0.05 | 24 (55.8) | 0.000 | >0.05 |
|
<5 | 27 | 16 (59.3) |
|
| 15 (55.6) |
|
|
| Tumor position |
|
|
|
|
|
|
|
| Frontal
lobe | 28 | 19 (67.9) | 3.586 | >0.05 | 17 (60.7) | 2.530 | >0.05 |
|
Temporal lobe | 19 | 14 (73.7) |
|
| 12 (63.2) |
|
|
|
Parietal lobe | 10 | 5
(50.0) |
|
| 5
(50.0) |
|
|
|
Occipital lobe | 7 | 3
(42.9) |
|
| 3
(42.9) |
|
|
|
Epencephala | 6 | 3
(50.0) |
|
| 2
(33.3) |
|
|
Immunohistochemistry
Sections from formalin-fixed, paraffin-embedded
tumor tissues (4-µm thick) were immunostained and assayed using a
PV6000 polymer system (ZSGB-BIO, Beijing, China). All paraffin
sections were heated to 90°C for 20 min. Following routine
deparaffinization by serial baths in xylene 3 times and dehydration
in a graded series of ethanol (100, 95 and 80%) and distilled
water, each section was treated with 0.3% hydrogen peroxide to
block endogenous peroxidase activity. Sections were subsequently
washed in 0.01 M phosphate-buffered saline (PBS; pH 7.4),
microwaved at 100°C for 20 min for antigen retrieval and then
incubated overnight at 4°C with optimal dilutions of primary
antibodies. Antibodies used were anti-COX-2 rabbit polyclonal
antibody (dilution, 1:400; ab15191; Abcam, Cambridge, UK) and
anti-survivin rabbit polyclonal antibody (dilution, 1:1,200;
ab24479; Abcam). Following washing in PBS, the tissue sections were
treated with secondary antibody, IgG-horseradish peroxidase polymer
multimer (PV-6000; ZSGB-BIO, Beijing, China), at 37°C for 20 min.
Finally, the tissue sections were incubated with diaminobenzidine
and counterstained with hematoxylin, cleared and mounted on
neutralbalsam. In order to confirm COX-2 and survivin
immunospecificity, 0.01 M PBS was supplied instead of primary
antibody, as the negative control.
Evaluation
Stained tissue sections were observed under a light
microscope. COX-2 and survivin expression levels were determined in
1,000 cells in 5 high-power fields for each tissue section. COX-2
staining in tumors was categorized as positive or negative and
positive protein expression level was evaluated by two independent
observers using a semi-quantitative immunoreactive score (IRS).
Briefly, IRS was calculated by multiplication of staining intensity
(graded as 0, none; 1, weak; 2, moderate; and 3, strong staining)
and the percentage of positively-stained cells (0, no stained
cells; 1, 1–10% stained cells; 2, 11–50% stained cells; 3, 51–80%
stained cells and 4, 81–100% stained cells). An IRS of >3 was
considered to indicate a positive reaction (13).
Statistical analysis
SPSS version 19.0 was used to analyze data (IBM
SPSS, Armonk, NY, USA). The χ2 test was performed to
evaluate the COX-2 and survivin expression levels in patients with
various clinicopathological characteristics. Spearman's correlation
coefficient method was applied to evaluate the association between
expressions levels of COX-2 and survivin. Survival analysis was
estimated according to the Kaplan-Meier method from the date of
diagnosis to the date of patient mortality due to the glioma. The
difference in survival curves was examined by means of the log-rank
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Immunohistochemistry results
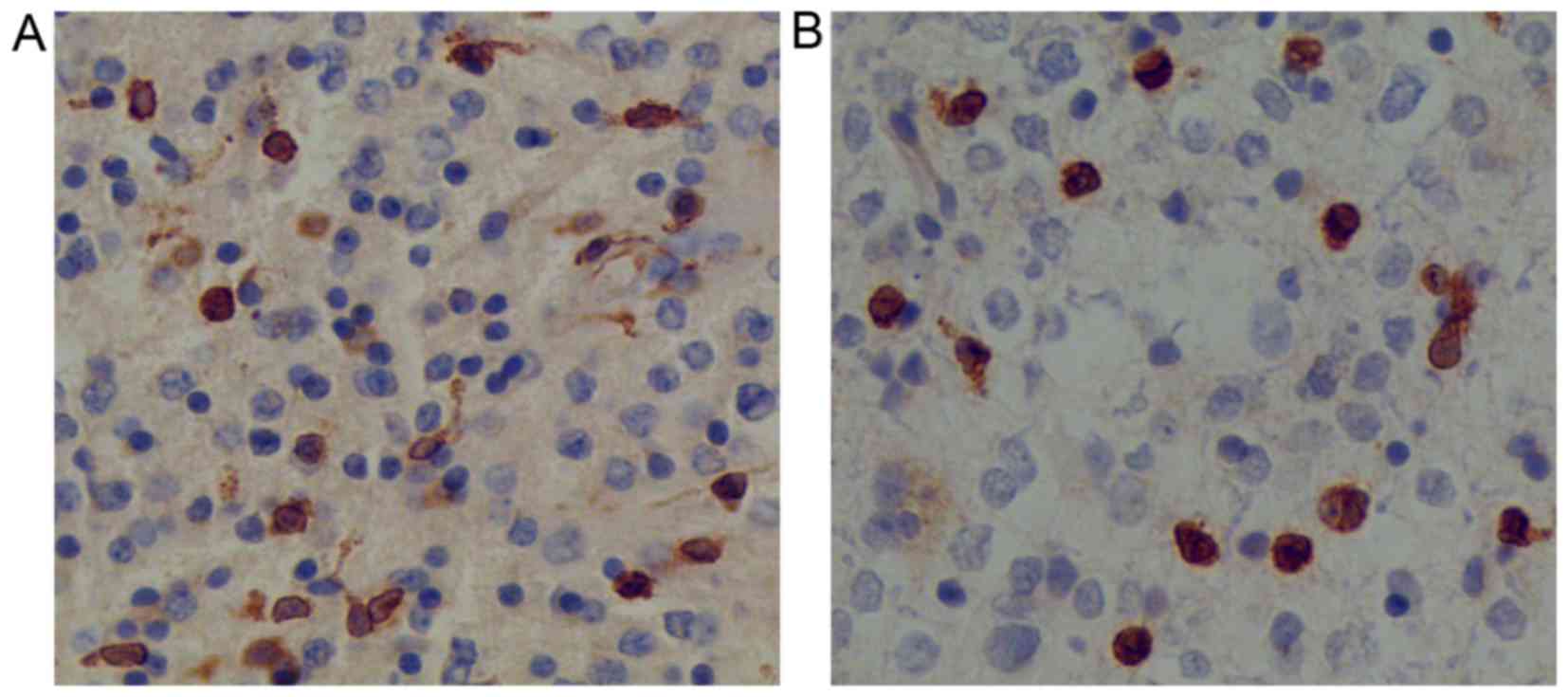
In normal brain tissues, the COX-2 and survivin
protein expression levels were negative. In tumor tissues, COX-2
expression was observed to be localized to the cytoplasm and nuclei
of the tumor cells, whereas survivin expression was confined mostly
to the nuclei of the tumor cells (Fig.
1). Positive COX-2 expression was identified in 44/70 glioma
tissues, whereas positive survivin expression was observed in 39/70
glioma tissues.
Correlation with clinicopathological
characteristics
COX-2 and survivin expression levels were correlated
with pathological tumor grade. COX-2 expression occurred in 46.4%
of low-grade malignancies and in 73.8% of high-grade tumors
(P<0.05). Survivin was expressed in 39.3% of low-grade
malignancies and 66.7% of high-grade tumors (P<0.05). COX-2 and
survivin expression levels demonstrated a stepwise increase from
weakly malignant (stage I–II) to highly malignant (stage III–IV)
glioma; this was statistically significant, but expression was not
correlated with gender, age, tumor size or location (P>0.05;
Table I), indicating that these
variables may not affect the expression levels of COX-2 and
survivin.
Correlation between COX-2 and
survivin
A total of 33 cases were identified as being COX-2-
and survivin-positive, 20 were COX-2- and survivin-negative, 11
were COX-2-positive and survivin-negative, and 6 were
COX-2-negative and survivin-positive. There was a significant
positive correlation between the expression level of COX-2 and
survivin in the glioma tissues (r=0.50; P<0.01).
Significant prognostic value of COX-2
and survivin
The follow-up of the patients ended in June 2015,
and at this time, complete follow-up information was available for
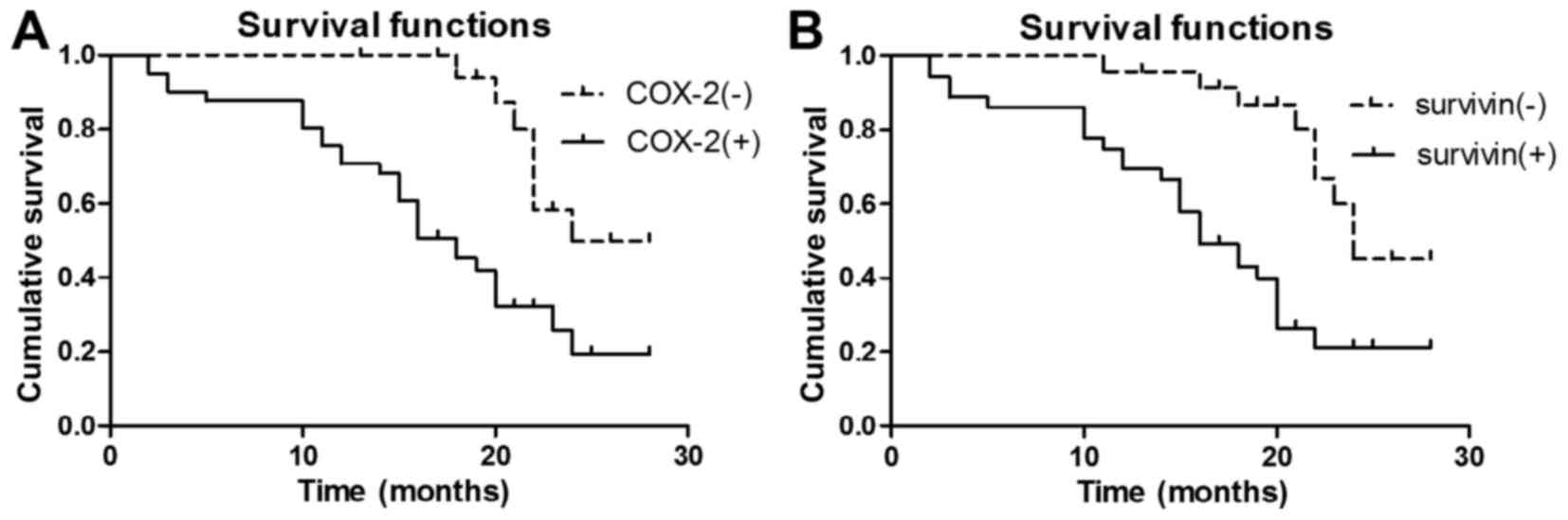
60 patients. Kaplan-Meier survival analysis revealed that the
median survival time for patients who were negative for COX-2
expression was 24 months, while those positive for COX-2 expression
had a median survival time of 18 months. A log-rank test indicated
that survival time for those positive for COX-2 was significantly
lower compared with those negative for COX-2 (χ2=10.113;
P<0.01; Fig. 2A). The median
survival time the for patients who were negative for survivin
expression was 24 months and that for patients positive for
survivin expression was 16 months. A log-rank test confirmed that
survivin expression was associated with a shorter survival time
(χ2=11.847; P<0.01; Fig.
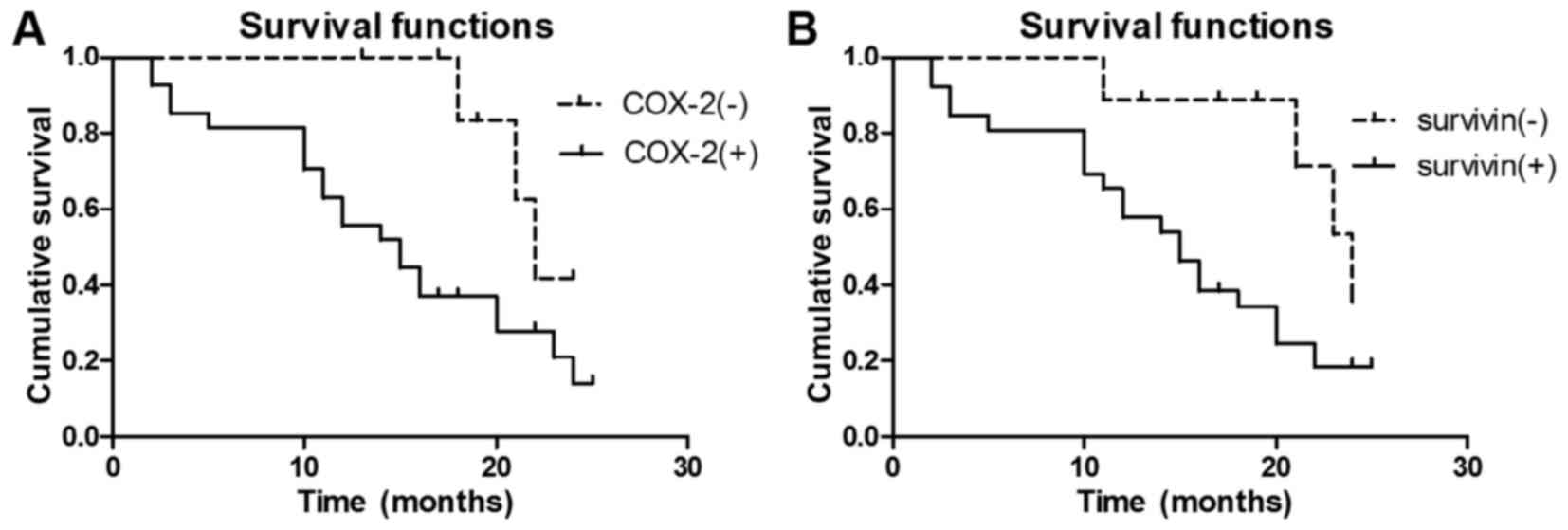
2B). Kaplan-Meier survival curves demonstrated that COX-2
(III–IV; χ2=4.252; P<0.05; Fig. 3A) and Survivin (III–IV;
χ2=4.571; P<0.05; Fig.
3B) expression levels were significant prognostic predictors
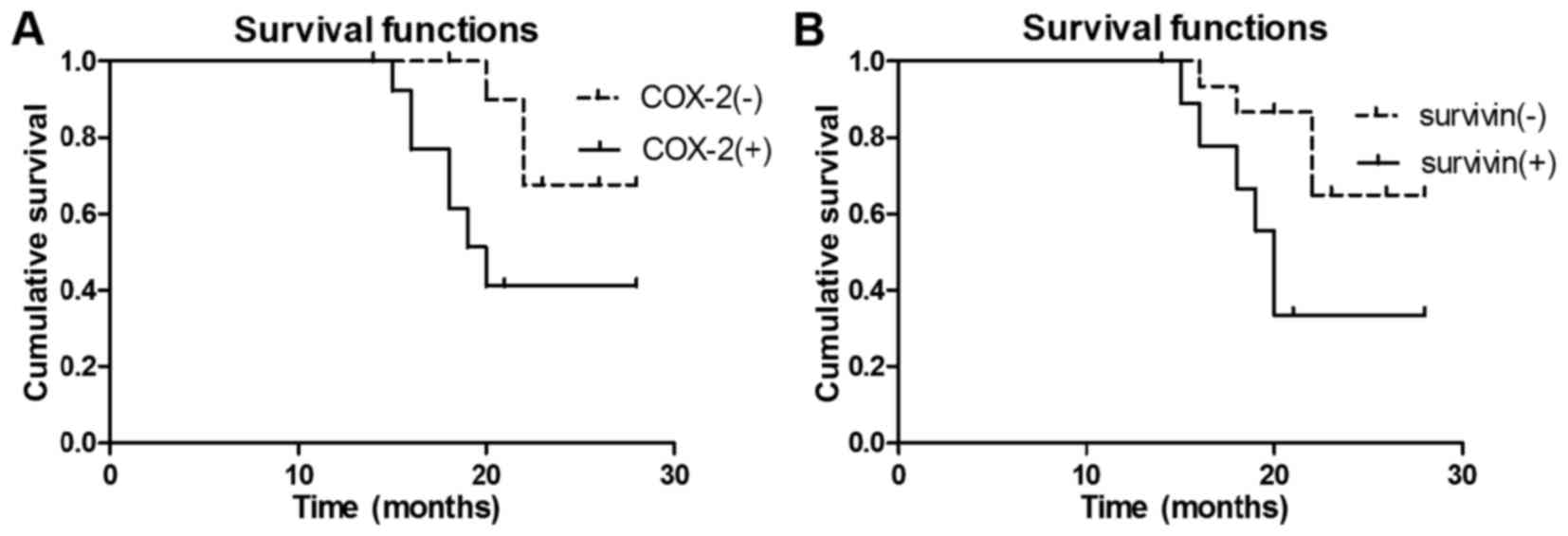
for high-grade malignant glioma. Additionally, COX-2 (I–II;
χ2=5.069; P<0.05; Fig.
4A) and Survivin (I–II; χ2=4.709; P<0.05;
Fig. 4B) expression levels were
significant prognostic predictors for low-grade malignant glioma.
COX-2 and survivin expression levels were significantly associated
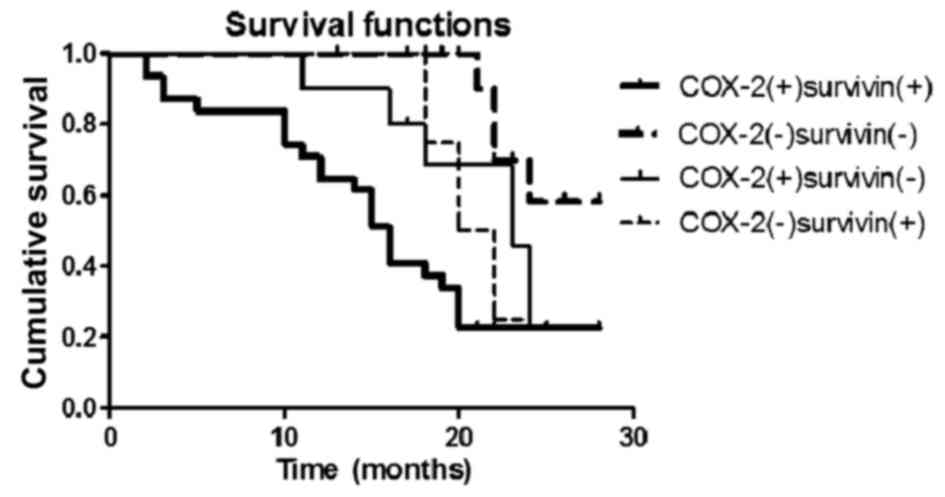
with poor survival. The 2-year survival rate for patients who were
negative for COX-2 and survivin expression was significantly higher
compared with that for patients who were positive for COX-2 and
survivin expression (58.3 vs. 22.5%; χ2=12.156;
P<0.05; Fig. 5). Patients with
grade IV glioma who were negative for COX-2 and survivin expression
experienced a median survival time of 24 months, and this was
significantly longer compared with the survival time for patients
who were positive for COX-2 and survivin expression (14 months;
χ2=3.933; P<0.05).
Discussion
Abnormal expression levels of apoptotic genes
perturbs apoptosis, allowing cells to escape normal control
mechanisms, and inducing cell cycle disorders and tumorigenesis
(14). COX-2 and survivin have been
suggested to be involved in tumorigenesis (15,16), thus
they are of interest as potential therapeutic targets. Currently,
pathological diagnosis, in particular WHO tumor grade guidelines,
are important for predicting prognosis in those patients with
gliomas. Therefore, the present study determined COX-2 and survivin
expression levels, and evaluated their utility as prognostic
markers for glioma.
COX-2 is an inducible enzyme involved in the
conversion of arachidonic acid to prostaglandin H2, which is
subsequently converted by specific prostaglandin synthases to
prostaglandin E2 (17). Increasing
levels of prostaglandin E2 (main product of COX-2) promotes cell
proliferation, invasion and angiogenesis, and inhibits apoptosis, a
feature closely associated with tumorigenesis (18). Previous studies have suggested that
positive staining for COX-2 is associated with cancer, thus it is
used as a diagnostic and therapeutic target for breast, colon,
gastric and esophageal cancer (19–21).
Elevated COX-2 expression levels in tumors are associated with the
inhibition of apoptosis, increased cell proliferation and
differentiation, increased angiogenesis, tumor invasion and
metastasis, and the inhibition of cell immune function (22). Temel and Kahveci (23) reported that glial cells did not
express COX-2 in normal brain tissues and that COX-2 protein
expression was observed predominantly in all glioma types; its
immunoreactivity was upregulated in high-grade malignancies,
particularly in glioblastoma multiforme (grade IV). COX-2 has also
been confirmed to be present in the surrounding tissues of necrotic
tumor areas in glioblastoma multiforme (23). The present study demonstrated that
COX-2 expression was detected in 13/28 (46.4%) low-grade malignant
gliomas and in 31/42 (73.8%) high-grade malignant gliomas. COX-2
overexpression in gliomas and a stepwise increase in COX-2
immunoreactivity from weakly to highly malignant gliomas occurred,
and this was statistically significant. El-Sayed and Taha (24) revealed that COX-2 expression was
significantly associated with poor survival (r=0.58; P<0.001).
The follow-up data of the present study demonstrated that patients
with COX-2-positive tumors had a significantly shorter survival
time compared with patients who were negative for COX-2 (18 vs. 24
months). Furthermore, COX-2 may be used to predict prognosis in
high- and low-grade glioma types. Consequently, COX-2 expression
may be a significant negative predictor of prognosis for
glioma.
Survivin is an inhibitor of apoptosis that has other
properties that modulate mitosis, apoptosis and the cellular stress
response (25). Survivin is expressed
during embryonic and fetal development, and is found in cancer
cells (including hepatocellular carcinoma and gastrointestinal
cancer), but not in adult terminal differentiated tissues (26). Survivin enhances tumor cell survival
primarily by suppressing apoptosis via the direct inhibition of
caspase-associated proteins (27).
Survivin is expressed during the G2/M phase of the cell
cycle and is a member of the chromosomal passenger complex, a
regulator of chromosome-microtubule attachment, spindle assembly
checkpoint and cytokinesis at cell division (28). Therefore, survivin functions as a key
regulator of chromosomal segregation and cytokinesis, repressing
cell death (29). A previous study of
patients with glioblastoma indicated that survivin was associated
with patient prognosis (30). While
cytoplasmic survivin did not modify prognosis, nuclear survivin
localization was correlated with a significantly lower survival
rate compared with patients with low nuclear survivin levels. The
present study observed that survivin accumulated in the nuclei of
glioma cells, thus it may be useful for the survival prognosis.
Chakravarti et al (31)
demonstrated a negative association between survivin expression and
the survival of glioma patients. Kogiku et al (32) also observed a significant association
between survivin and age, Karnofsky performance scale (KPS) score
and grade (P=0.0017, P=0.0006 and P=0.0002, respectively). This
indicated that older patients with glioblastoma multiforme (grade
IV) and a lower KPS score tended to have a higher survivin
expression level. In addition, the median survival time of patients
with high survivin expression was significantly shorter compared
with that for patients with low expression (322 vs. 1,084 days)
(32). In the present study, survivin
expression was detected in 11/28 (39.3%) low-grade glioma tissues
and in 28/42 (66.7%) high-grade glioma tissues. Survivin
immunoreactivity was upregulated significantly in high-grade
glioma, but it was not correlated with gender, age, tumor size or
location. In the follow-up data, patients with survivin-positive
tumors experienced a significantly shorter survival time compared
with those who were negative for survivin (16 vs. 24 months), and
this was in agreement with previous studies (31,32).
Furthermore, survivin may be used to predict prognosis for
low-grade and high-grade gliomas.
Therefore, COX-2 and survivin proteins may be
important for changes in malignancy during glioma tumorigenesis. A
study by Mehar et al (33)
indicated that survivin expression could be induced by celecoxib
and that it was dependent on COX-2 expression. A significant
immunoreactive association between COX-2 and survivin protein was
observed in ovarian cancer (34) and
endometrial carcinoma (35). Thus,
COX-2 may act as an upstream regulatory factor, with high
expression of COX-2 upregulating survivin by preventing survivin
ubiquitination and proteasome degradation. To the best of our
knowledge, this interaction has not yet been investigated in
glioma.
The present study demonstrated a significant
positive correlation between the expression of COX-2 and survivin
in glioma, and hypothesized that COX-2 and survivin may facilitate
tumor occurrence and progression via a common signaling pathway.
The two proteins were potent indicators of glioma survival, and
follow-up data revealed that the survival time for patients who
were positive for the two proteins was significantly lower compared
with patients who were negative for the two proteins. This
significant correlation of COX-2 and survivin may further highlight
the advantage of a combinational evaluation with COX-2 and survivin
to determine the prognosis in patients with glioma. Furthermore,
the significantly longer survival time of patients diagnosed with
grade IV glioma without the expression of COX-2 and survivin
suggested an advantage of combining a pathological diagnosis with
the expression of COX-2 and survivin. Ren et al (36) confirmed that inhibition of
proliferation and promotion of apoptosis in U251 glioma cells could
be induced by celecoxib in a dose- and time-dependent manner, and
that this may occur by downregulation of survivin expression.
However, the specific signal transduction pathway by which this may
occur in glioma remains unknown. Thus, the link between COX-2 and
survivin requires further investigation. Furthermore, these
proteins may have potential value for glioma therapy, as COX-2
inhibitors are effective for reducing the risk of malignancies and
inhibiting tumorigenesis (37). Rödel
et al (38) revealed that a
drug to block survivin activity by downregulation may inhibit tumor
proliferation.
There are certain limitations to the present study.
Firstly, the patients underwent surgery using a variety of methods
and surgeons. Also, the tissue specimens were evaluated by
different pathologists, although all tissues were re-examined based
on strict criteria (WHO guidelines). Furthermore, the sample size
was limited, which may have obscured significant findings. A study
with a larger sample size is required. A multi-institutional study
of patient populations in Anhui or all of China would aid in
improving our current understanding of the prognosis of glioma
patients using COX-2 and survivin expression analysis.
Despite the aforementioned factors, the results of
the present study may aid in defining the tumorigenic mechanism
underlying glioma and assist with determining an effective
diagnosis and prognosis. The present study provided convincing
evidence for the specific accumulation of COX-2 and survivin
existing in glioma tissues, suggesting that they serve an important
role in the process of carcinogenesis. The present study suggested
that COX-2 and survivin are associated with the severity of glioma,
as patients who were positive for the expression of the two
proteins demonstrated shorter survival times. This may aid the
establishment of a comprehensive therapeutic evaluation of glioma,
and these proteins may have potential and suitable uses for
targeted therapy. Further studies are required in order to
investigate these findings. Additionally, COX-2 and survivin
proteins may be useful for biomarker studies to predict glioma
severity and patient prognosis.
Acknowledgements
The present study was supported by the Anhui
Provincial Natural Science Foundation (grant no. 1308085MH134).
References
|
1
|
Holland EC: Glioblastoma multiforme: The
terminator. Proc Natl Acad Sci USA. 97:pp. 6242–6244. 2000;
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Louis DN, Pomeroy SL and Cairncross JG:
Focus on central nervous system neoplasia. Cancer Cell. 1:125–128.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakada M, Nakada S, Demuth T, Tran NL,
Hoelzinger DB and Berens ME: Molecular targets of glioma invasion.
Cell Mol Life Sci. 64:458–78. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Omay SB and Vogelbaum MA: Current concepts
and newer developments in the treatment of malignant gliomas.
Indian J Cancer. 46:88–95. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lima FR, Kahn SA, Soletti RC, Biasoli D,
Alves T, da Fonseca AC, Garcia C, Romão L, Brito J, Holanda-Afonso
R, et al: Glioblastoma: Therapeutic challenges, what lies ahead.
Biochim Biophys Acta. 1826:338–349. 2012.PubMed/NCBI
|
|
6
|
Altieri DC: Survivin, cancer networks and
pathway-directed drug discovery. Nat Rev Cancer. 8:61–70. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ke HL, Tu HP, Lin HH, Chai CY, Chang LL,
Li WM, Li CC, Lee YC, Yeh HC, Wu WJ and Bau DT: Cyclooxygenase-2
(COX-2) up-regulation is a prognostic marker for poor clinical
outcome of upper tract urothelial cancer. Anticancer Res.
32:4111–4116. 2012.PubMed/NCBI
|
|
8
|
Cebola I and Peinado MA: Epigenetic
deregulation of the COX pathway in cancer. Prog Lipid Res.
51:301–313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rödel F, Reichert S, Sprenger T, Gaipl US,
Mirsch J, Liersch T, Fulda S and Rödel C: The Role of Survivin for
Radiation Oncology: Moving Beyond Apoptosis Inhibition. Curr Med
Chem. 18:191–199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kanwar JR, Kamalapuram SK and Kanwar RK:
Targeting survivin in cancer: Patent review. Expert Opin Ther Pat.
20:1723–1737. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen XQ, Yang S, Li ZY, Lu HS, Kang MQ and
Lin TY: Effects and mechanism of downregulation of survivin
expression by RNA interference on proliferationand apoptosis of
lung cancer cells. Mol Med Rep. 5:917–922. 2012.PubMed/NCBI
|
|
12
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumours of the central nervous system. Acta
Neuropathol. 114:97–1099. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German). PubMed/NCBI
|
|
14
|
Fesik SW: Promoting apoptosis as a
strategy for cancer drug discovery. Nat Rev Cancer. 5:876–885.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Trifan OC and Hla T: Cyclooxygenase-2
modulates cellular growth and promotes tumorigenesis. J Cell Mol
Med. 7:207–222. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Altieri DC: Survivin, versatile modulation
of cell division and apoptosis in cancer. Oncogene. 22:8581–8589.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grösch S, Maier TJ, Schiffmann S and
Geisslinger G: Cyclooxygenase-2 (COX-2)-independent
anticarcinogenic effects of selective COX-2 inhibitors. J Natl
Cancer Inst. 98:736–47. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim YM, Shin YK, Jun HJ, Rha SY and Pyo H:
Systematic analyses of genes associated with radiosensitizing
effect by celecoxib, a specific cyclooxygenase-2 inhibitor. Radiat
Res. 52:752–765. 2011. View Article : Google Scholar
|
|
19
|
Hoellen F, Kelling K, Dittmer C, Diedrich
K, Friedrich M and Thill M: Impact of cyclooxygenase-2 in breast
cancer. Anticancer Res. 31:4359–4367. 2011.PubMed/NCBI
|
|
20
|
Moreira L and Castells A: Cyclooxygenase
as a target for colorectal cancer chemoprevention. Curr Drug
Targets. 12:1888–1894. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zimmermann KC, Sarbia M, Weber AA,
Borchard F, Gabbert HE and Schrör K: Cyclooxygenase-2 expression in
human esophageal carcinoma. Cancer Res. 59:198–204. 1999.PubMed/NCBI
|
|
22
|
Greenhough A, Smartt HJ, Moore AE, Roberts
HR, Williams AC, Paraskeva C and Kaidi A: The COX-2/PGE 2 pathway:
Key roles in the hallmarks of cancer and adaptation to the tumour
microenvironment. Carcinogenesis. 30:377–386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Temel SG and Kahveci Z: Cyclooxygenase-2
expression in astrocytes and microgliain human oligodendroglioma
and astrocytoma. J Mol Histol. 40:369–377. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
El-Sayed M and Taha MM:
Immunohistochemical expression of cycloxygenase-2 in astrocytoma:
Correlation with Angiogenesis, tumor progression and survival. Turk
Neurosurg. 21:27–35. 2011.PubMed/NCBI
|
|
25
|
Altieri DC: Targeting survivin in cancer.
Cancer Lett. 332:225–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu W, Zhu F, Jiang Y, Sun D, Yang B and
Yan H: SiRNA targeting survivin inhibits the growth and enhances
the chemosensitivity of hepatocellularcarcinoma cells. Oncol Rep.
29:1183–1188. 2013.PubMed/NCBI
|
|
27
|
Mobahat M, Narendran A and Riabowol K:
Survivin as a preferential target for cancer therapy. Int J Mol
Sci. 15:2494–2516. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jeyaprakash AA, Klein UR, Lindner D, Ebert
J, Nigg EA and Conti E: Structure of a Survivin-Borealin-INCENP
core complex reveals how chromosomal passengers travel together.
Cell. 131:271–285. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Altieri DC: Survivin-The inconvenient IAP.
Semin Cell Dev Biol. 39:91–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shirai K, Suzuki Y, Oka K, Noda SE, Katoh
H, Suzuki Y, Itoh J, Itoh H, Ishiuchi S, Sakurai H, et al: Nuclear
survivin expression predicts poorer prognosis in glioblastoma. J
Neurooncol. 91:353–358. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chakravarti A, Noll E, Black PM,
Finkelstein DF, Finkelstein DM, Dyson NJ and Loeffler JS:
Quantitatively determined survivin expression levels are of
prognostic value in human gliomas. Oncol. 20:1063–1068. 2002.
|
|
32
|
Kogiku M, Ohsawa I, Matsumoto K, Sugisaki
Y, Takahashi H, Teramoto A and Ohta S: Prognosis of glioma patients
by combined immunostaining for survivin, Ki-67 and epidermal growth
factor receptor. J Clin Neurosci. 15:1198–1203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mehar A, Macanas-Pirard P, Mizokami A,
Takahashi Y, Kass GE and Coley HM: The effects of cyclooxygenase-2
expression in prostate cancer cells: Modulation of response to
cytotoxic agents. J Pharmocol Exp Ther. 324:1181–1187. 2008.
View Article : Google Scholar
|
|
34
|
Athanassiadou P, Grapsa D, Athanassiades
P, Gonidi M, Athanassiadou AM, Tsipis A and Patsouris E: The
prognostic significance of COX-2 and survivin expression in ovarian
cancer. Pathol Res Pract. 204:241–249. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Erkanli S, Bolat F, Kayaselcuk F, Demirhan
B and Kuscu E: COX-2 and survivin are overexpressed and positively
correlated in endometrial carcinoma. Gynecol Oncol. 104:320–325.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ren Z, Zhang LZ and Wang RZ: Effect of
celecoxib on proliferation, apoptosis and Survivin expression in
human glioma cell line U251. Chin J Can. 29:294–299. 2010.
View Article : Google Scholar
|
|
37
|
Schetter AJ, Heegaard NH and Harris CC:
Inflammation and cancer: Interweaving micro RNA, free radical,
cytokine and p53 pathways. Carcinogenesis. 31:37–49. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rödel F, Frey B, Leitmann W, Capalbo G,
Weiss C and Rödel C: Survivin antisense oligonucleotides
effectively radiosensitize colorectal cancer cells in both tissues
culture and murine xenograft models. Int J Radiat oncol Biol Phys.
71:247–255. 2008. View Article : Google Scholar : PubMed/NCBI
|