Introduction
Gastric cancer (GC) is the fifth most common type of
malignancy in the world, with 952,000 incident cases estimated to
have occurred in 2012 (1). The
standard treatment for stage II/III GC in Japan is gastrectomy with
extended (D2) lymphadenectomy plus postoperative
tegafur/gimeracil/oteracil (S-1) adjuvant chemotherapy (ACT) for 1
year, according to the Adjuvant Chemotherapy Trial of S-1 for
Gastric Cancer (ACTS-GC) (2,3). However, 30.6% of patients undergo cancer
recurrences, 65.8% continue their S-1 treatments for a full year
and 46.5% of patients are administered reduced doses of their
recommended regimens (2,3). The clinical parameters for continuing
S-1 ACT in these patients has been the focus of several studies
(4–6),
as has the role of S-1 dose intensity in ACT subsequent to curative
gastrectomy for advanced GC (7).
However, the association between relative dose intensity or
continuation of S-1 and patient survival has not been fully
characterized.
The prognostic significance of a number of clinical
parameters has been examined for patients with GC subsequent to
radical surgery (8–15). Of these, the preoperative prognostic
nutritional index (PNI) as a reflection of the immunological and
nutritional condition of the patients has been associated with the
outcomes of patients who undergo gastrectomy for a number of stages
of GC (10,11,16,17).
Although the association between body mass index (BMI) and
postoperative complications has been investigated (18–22), the
association between BMI and long-term survival subsequent to
curative gastrectomy remains unclear (23–28). The
association between BMI and outcome in patients who received ACT
for colon cancer has been demonstrated (29), but not the analogous association for
patients with gastric cancer.
In the present study, the dose-response effects of
S-1 ACT on survival in patients who received gastrectomy for GC
were clarified, and other clinical factors that affected their
survival were analyzed, including BMI and PNI as nutritional
parameters.
Patients and methods
Data collection involved a survey of original
medical records and drug information databases of Nara Hospital,
Kindai University School of Medicine (Ikoma, Japan). Patients with
histologically confirmed primary gastric adenocarcinoma who had
received curative gastrectomy with D2 lymphadenectomy and ACT with
S-1 between January 2007 and December 2014 at Nara Hospital, Kindai
University School of Medicine were enrolled and retrospectively
evaluated. All patients exhibited adenocarcinoma histology, as
demonstrated by endoscopic biopsies, with pathological stage II or
III disease according to the Japanese Classification of Gastric
Carcinoma (13th edition) (30); the
TNM Classification of Malignant Tumors (7th edition) (31) was also used. Gastric adenocarcinomas
can be divided into two major histological types, diffuse and
intestinal type according to the Lauren classification (32). Patients received S-1 beginning between
4 and 8 weeks after surgery, typically at a standard dose of S-1 of
80 mg/m2/day for 4 weeks, followed by 2 weeks of no
chemotherapy. This 6-week cycle was repeated during the first year
following surgery. A 3-week regimen of 80 mg/m2 S-1 for
2 weeks, followed by a 1-week rest, was also permitted. Certain
patients received S-1 dose decreases, according to the criteria
outlined in the ACTS-GC study (3),
but decisions to decrease or cease S-1 ACT were entrusted to the
patients and their physicians. Almost all patients in whom cancer
recurrence was detected during their year of S-1 ACT were
transferred to second-line chemotherapy, which included CPT-11,
cisplatin and taxanes.
Toxicities were graded according to the National
Cancer Institute Common Toxicity Criteria (version 3.0) (33). The present study was approved by the
ethical review committee of Nara Hospital, Kindai University School
of Medicine. Median follow up time was 44.76 months, ranging
between 13.5–92 months. Written informed consent was obtained from
all patients. The concept of the present study is available on the
Nara Hospital website (http://www.kindainara.com/act/goannai.pdf).
Clinical and nutritional
parameters
Clinical and pathological parameters were evaluated
from medical records, and postoperative prognostic factors with S-1
adjuvant therapy were analyzed. The relative performance (RP) value
was evaluated instead of the relative dose intensity. RP value was
calculated as (administrated S-1 dose)/(planned S-1 administration
dose) ×100%. The overall survival (OS) rates were compared between
low- and high-RP groups. Creatinine clearance (CCr), was calculated
using the formula developed by Cockroft and Gault (34). PNI was evaluated and calculated as
[10x serum albumin value (g/dl)] + (0.005x peripheral lymphocyte
counts), using serum albumin (mg/dl) level and peripheral
lymphocyte counts (counts/mm3) assessed between 1 and 2
months after surgery (35), and BMI
(kg/m2) was calculated as weight in kg/(height in
m)2. Body weight (BW), BMI and PNI were evaluated
between 2 and 4 weeks before, and between 1 and 2 months after,
surgery. Postoperative BW and BMI losses were calculated between 1
and 2 months after surgery as (postoperative value-preoperative
value)/preoperative value ×100%). As BW loss and BMI loss were the
same values, we evaluated only BW loss. The threshold values for
postoperative BW loss (BMI loss), PNI, CCr and number of metastatic
lymph nodes were decided by receiver operating characteristic (ROC)
curves for OS. Other clinical and pathological prognostic factors
included age, sex, type of gastrectomy and tumor stage (pStage)
were also evaluated.
Statistical analysis
OS was defined as time between surgery and patient
mortality or the last available information pertaining to vital
status. Differences between cumulative survival rates of the
patient groups were calculated using a log-rank test for comparison
using Kaplan-Meier survival curves. P<0.05 was considered to
indicate a statistically significant difference. PNI, BMI, BW were
expressed as the mean ± standard deviation. The Wilcoxon signed
rank test was used for comparison between preoperative and
postoperative PNI, BMI and BW. Time-dependent survival ROC curves
were used to evaluate the sensitivity and specificity of
postoperative BW loss, CCr, PNI and number of metastatic lymph
nodes for predicting the 3-year OS rate (36). The Youden index was used to determine
optimal threshold values for postoperative BW loss, BMI loss, CCr,
number of metastatic lymph nodes and PNI. Patients were divided
into two subgroups, above and below the threshold values, for each
factor. Factors were also subjected to univariate and multivariate
analyses, using Cox's proportional hazard model, against OS.
Statistical analyses used JMP (version 11; SAS, Tokyo, Japan).
Survival ROC was analyzed using R software (version 3.1.1; R
Project for Statistical Computing, Vienna, Austria).
Results
A total of 62 patients were enrolled in the present
study (Table I), all of whom received
S-1 ACT as outpatients, at 60–120 mg/day.
 | Table I.Clinicopathological characteristics
of patients in the present study (n=62). |
Table I.
Clinicopathological characteristics
of patients in the present study (n=62).
| Factor | n |
|---|
| Sex |
|
|
Male | 41 |
|
Female | 21 |
| Mean age,
years | 64.9 |
| Tumor location in
stomach |
|
|
Upper | 17 |
|
Middle | 21 |
|
Lower | 24 |
| Pathological
typea |
|
|
Intestinal | 22 |
|
Diffuse | 40 |
| Depth of tumor
invasion (pT)b |
| T1 | 2 |
| T2 | 11 |
| T3 | 18 |
|
T4a | 28 |
|
T4b | 3 |
| Pathological nodal
statusc |
|
| N0 | 7 |
| N1 | 23 |
| N2 | 32 |
| No. of lymph node
metastasis (pN)c |
|
| 0
(N0) | 7 |
| 1–2
(N1) | 15 |
| 3–6
(N2) | 12 |
| ≥7
(N3) | 27 |
| Final pathological
stagec |
|
| II | 21 |
|
IIIA | 20 |
|
IIB | 21 |
| Surgery type |
|
| Total
gastrectomy | 21 |
| Distal
gastrectomy | 41 |
Survival
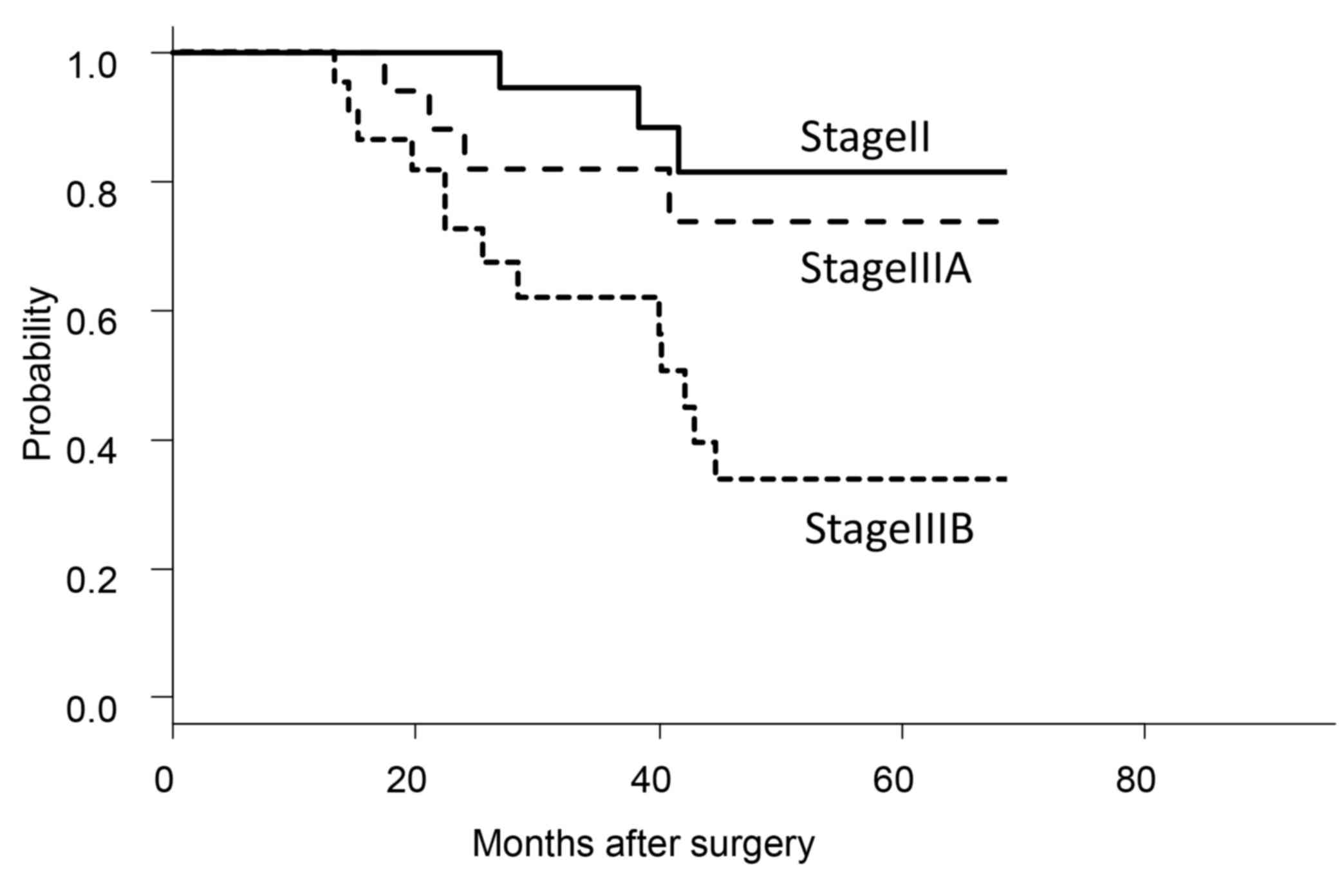
For the cohort of the present study, 3- and 5-year
OS rates were 79.1 and 60.9%, respectively, but varied according to
disease stage (Fig. 1). The stage II
and IIIA groups exhibited significantly improved 5-year OS rates
(stage II, 81.6%; stage IIIA, 73.7%) compared with that of the
stage IIIB group (33.8%; P<0.01).
Recurrence
Of the 62 patients, 25 (40.3%) experienced cancer
recurrences. The sites of recurrence were peritoneal dissemination
in 13 patients, liver in 5 patients, bone in 2 patients, lymph
nodes in 4 patients, and lung in 1 patient.
ROC curves and threshold values
ROC survival curves were used to determine optimal
threshold values for favorable OS in the factors under
investigation. The threshold values were: PNI, 48 [area under curve
(AUC), 0.637; true positive (TP), 0.96; false positive (FP), 0.57];
CCr, 70 ml/min (AUC, 0.541; TP, 0.72; FP, 0.485); BW loss, 10.6%
(AUC, 0.612; TP, 0.669; FP, 0.255) and number of metastatic lymph
nodes, 7 (AUC, 0.733; TP, 0.645; FP, 0.183). The analyses of ROC
curves used a 3-year endpoint and maximum Youden index.
As the ROC curves did not produce an optimal
threshold value for BMI, it was set at 23 kg/m2
according to the WHO Expert Consultation (37); the RP threshold value was set at 70%
according to the ACTS-GC subset report (6). Patients were defined as those above or
below each threshold and the two groups were compared.
RP value and survival
Of the 62 patients, 24 were administered decreased
S-1 doses within the first 3 cycles, at their or their physicians'
decision, for an initial reduction rate of 38.7%. A total of 3
patients elected to end their S-1 ACT regimens following 3 or 4
cycles (patient refusal); these patients did not exhibit cancer
recurrences. The 1-year treatment continuation rate was 79.0%,
including patients who exhibited recurrences during S-1 ACT and
were referred to second-line chemotherapies. Among the patients who
did not exhibit recurrences, the 1-year treatment continuation rate
was 94.2%.
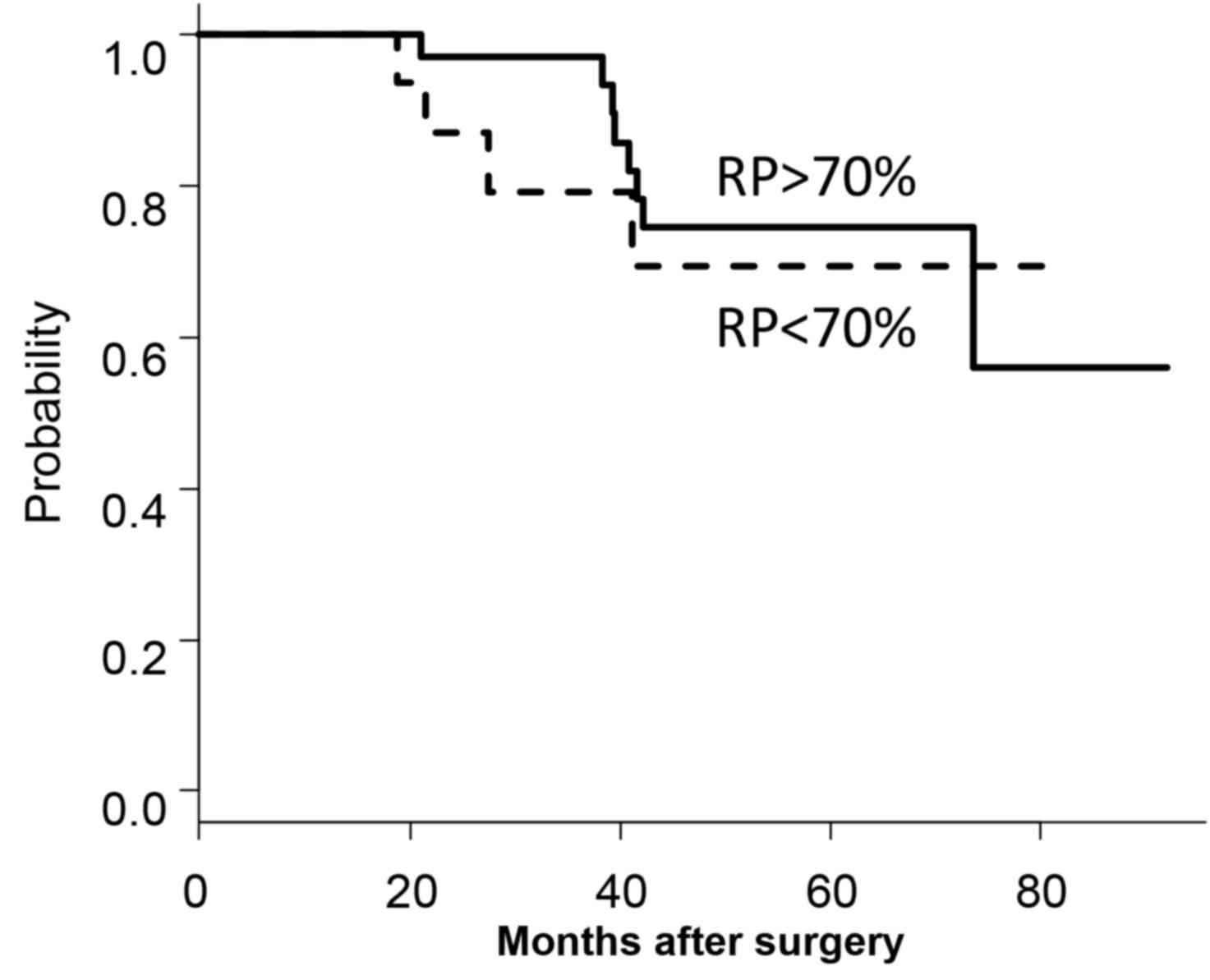
Of the 62 patients, 36 exhibited RP values of
>70%, including 12 patients who possessed 100% RPs, and 26
patients exhibited RP values of <70%. A total of 16 patients in
the <70% RP group were lost to follow-up due to toxicity, and 10
for cancer recurrence leading to second-line chemotherapy between 6
and 12 months after surgery. The 5-year OS rate of the >70% RP
group (74.6%) was significantly increased compared with the <70%
RP group (41.2%; P<0.01). However, as the 10 patients who had
stopped their S-1 ACT regimens due to recurrence between 6 and 12
months after surgery and were on second-line therapies were
excluded, no significant difference between the 5-year OS rates for
the >70% RP group (74.7%) and the <70% RP group was
identified (69.3%; P=0.642; Fig.
2).
Nutritional parameters prior to and
subsequent to surgery
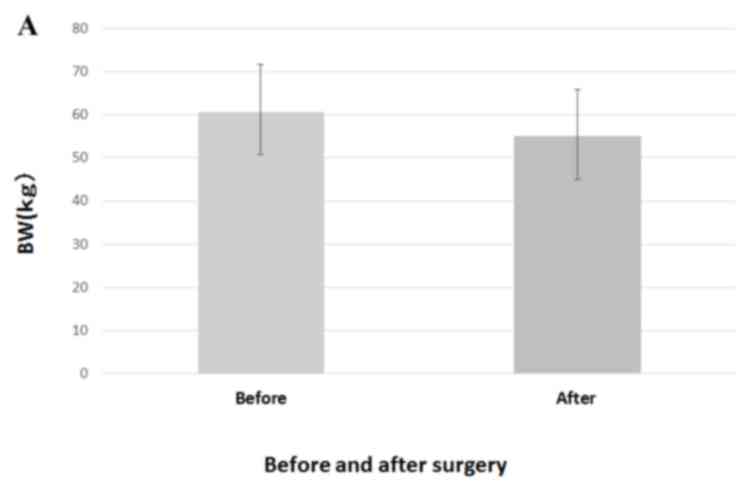
Preoperative values were significantly decreased
subsequent to surgery for BMI (20.69±2.811 vs. 22.79±2.97
kg/m2; P<0.001), BW (55.01±9.94 vs. 60.68±10.94 kg;
P<0.001) and PNI (48.36±4.26 vs. 50.36±4.12; P<0.001;
Fig. 3).
Prognostic factors for OS
In the univariate analysis (Table II), BW loss between 1 and 2 months
after surgery, pStage and number of lymph node metastases were of
significant prognostic value (P<0.05). In multivariate analysis,
BW loss between 1 and 2 months after surgery, and pStage were
identified to be independent prognostic factors (P<0.05,
Table III).
 | Table II.Univariate analysis of prognostic
factors for overall survival in patients with gastric cancer who
underwent neoadjuvant chemotherapy. |
Table II.
Univariate analysis of prognostic
factors for overall survival in patients with gastric cancer who
underwent neoadjuvant chemotherapy.
| Characteristic | n | HR | 95% CI | P-value |
|---|
| Age, years |
|
|
|
|
|
≥70 | 18 | 1.013 | 0.392–2.616 | 0.979 |
|
<70 | 44 |
|
|
|
| Sex |
|
|
|
|
|
Male | 42 | 1.204 | 0.477–3.041 | 0.696 |
|
Female | 20 |
|
|
|
| Type of
gastrectomy |
|
|
|
|
|
Distal | 41 | 0.596 | 0.242–1.472 | 0.263 |
|
Total | 21 |
|
|
|
| pStage |
|
|
|
|
| II | 21 | 4.64 | 1.318–16.381 | 0.017 |
|
III | 41 |
|
|
|
| Lymph node
metastasis |
|
|
|
|
| ≥7 | 27 | 2.97 | 1.219–7.236 | 0.017 |
|
<7 | 35 |
|
|
|
| Creatinine
clearance |
|
|
|
|
|
≥70 | 36 | 0.441 | 0.168–1.162 | 0.098 |
|
<70 | 26 |
|
|
|
| Postoperative
BMI |
|
|
|
|
|
≥23 | 14 | 0.582 | 0.171–1.980 | 0.386 |
|
<23 | 38 |
|
|
|
| Postoperative
PNI |
|
|
|
|
|
≥48 | 37 | 0.522 | 0.215–1.265 | 0.15 |
|
<48 | 21 |
|
|
|
| Preoperative
BMI |
|
|
|
|
|
≥23 | 27 | 1.179 | 0.496–2.802 | 0.709 |
|
<23 | 35 |
|
|
|
| Preoperative
PNI |
|
|
|
|
|
≥48 | 44 | 0.716 | 0.262–1.956 | 0.514 |
|
<48 | 18 |
|
|
|
| Body weight loss,
% |
|
|
|
|
|
≥10.6 | 23 | 2.744 | 1.157–6.505 | 0.022 |
| (BMI loss) |
|
|
|
|
|
<10.6 | 39 |
|
|
|
| RP, % |
|
|
|
|
|
≥70 | 36 | 0.752 | 0.226–2.506 | 0.643 |
|
<70 | 16 |
|
|
|
 | Table III.Multivariate analysis of
clinicopathological factors compared with overall survival in
patients with gastric cancer who underwent neoadjuvant
chemotherapy. |
Table III.
Multivariate analysis of
clinicopathological factors compared with overall survival in
patients with gastric cancer who underwent neoadjuvant
chemotherapy.
| Factor | HR | 95% CI | P-value |
|---|
| Age | 0.980 | 0.927–1.036 | 0.980 |
| pStage | 5.236 | 1.447–18.95 | 0.012 |
| BW loss subsequent
to surgery | 2.821 | 1.117–7.126 | 0.028 |
Discussion
The present retrospective study was designed to
evaluate the clinical prognostic factors of OS, including the
nutritional parameters in gastrectomy patients who receive ACT with
S-1 for GC in Nara Hospital. As described in the ASTS-GC reports, a
1-year regimen of S-1 ACT was effective for stage II and stage IIIA
GC, but not stage IIIB disease, according to subset analysis
(2,3).
In the present study, 94.2% of patients continued S-1 when the 10
patients who experienced cancer recurrence within the 1-year ACT
period were excluded. However, the 38.7% of patients who decreased
their S-1 doses within the first 3 cycles were included. The OS
rate of the present study was similar to that of the ACTS-GC
report, except for stage IIIB disease (2,3).
It was revealed that, even if the S-1 dose was
reduced, patients who continued to receive it for the recommended
year exhibited a significant survival benefit. The lowest dose of
S-1 administered in the present study was 60 mg/day, for which a
sufficient clinical effect has not been established; however, it
should be established in the future. It is expected that more
effective types of adjuvant therapy may be established in the near
future for stage III disease. According to the ACTS-GC results
[unpublished data, noted in (6)],
improved survival was noted with 1-year continuation of S-1 ACT and
RP values >70%. In the present study, the high and low RP groups
did not significantly differ in OS when the patients with between 6
and 12-month postsurgical recurrences, i.e., those on second-line
regimens, were excluded. Physicians in hospital settings may have
decreased their patients' S-1 doses out of concern for toxic
effects, which may have affected these numbers. Additionally, the
present study involved relatively small numbers of patients, which
may also have affected the results. However, no association between
RP values and survival was observed in the present study.
Additional examination may be required.
Pre- and postsurgical nutritional parameters were
also evaluated. The deterioration in nutritional status subsequent
to gastrectomy is induced by decreased food intake due to decreased
capacity (38–40). Several nutritional parameters were
markedly decreased subsequent to surgery in the present study. Body
weight loss between 1 and 2 months after surgery was a significant
prognostic factor in the multivariate analysis for patients who
received S-1 ACT. The prognosis of patients with BW loss >10.6%
between 1 and 2 months after surgery was poorer in the present
study. Aoyama et al (5)
demonstrated that BW loss of >15% at 1 month after surgery was
the most important risk factor for compliance of S-1 ACT.
Additionally, BW loss at presentation is associated with poor
chemotherapy compliance and poor prognosis in gastrointestinal
malignancies (41). A previous study
from Korea revealed that weight loss at the first month of
palliative chemotherapy predicted unfavorable survival outcomes in
patients with advanced GC (42).
However, it was not possible to determine the potential association
between S-1 continuation and body weight loss between 1 and 2
months after surgery owing to the high S-1 continuation in the
present study; however, BW loss between 1 and 2 months after
surgery is hypothesized to affect the prognosis of patients who
receive S-1 ACT subsequent to gastrectomy. To the best of our
knowledge, the present study has provided the first evidence that
BW loss between 1 and 2 months after surgery is associated with
survival rate in patients with GC who received ACT. However,
whether BW loss affects prognoses directly or indirectly, for
example by decreasing the effectiveness of S-1, remains unclear. In
any case, diminished nutritional status may worsen the prognosis of
any patient with a serious illness, including gastric cancer.
The association between BMI and long-term outcome
has been investigated with regard to certain malignancies (43,44),
including a number of controversial studies evaluating BMI and GC
prognosis (23–28). In the present study, preoperative and
postoperative BMI of patients with stage II/III GC were evaluated,
although no association between BMI and survival rates was
identified.
Preoperative PNI is hypothesized to be a prognostic
marker for a number of malignancies, including long-term survival
for patients with GC (10,11,17,45).
However, no association between either preoperative or
postoperative PNI and survival in patients with GC who received S-1
ACT was demonstrated. As it was revealed that post-surgical BMI and
BW loss were more reliable predictors of survival than PNI, the
present study suggests that nutritional support subsequent to
surgery improves survival rates in patients with stage II and III
GC who expect to receive S-1 ACT.
In conclusion, 1-year continuation of S-1 ACT had an
increased effect on survival compared with relative dose intensity,
as demonstrated by RP value, for patients with stage II/III GC.
Additionally, postoperative nutritional intervention may improve
survival rates of these patients. A novel treatment strategy for
stage III gastric cancer may be warranted.
Acknowledgements
The authors wish to thank the department's
assistant, Mrs Akiko Kaku, and the pharmacologist, Mrs. Saori
Nishiura, for their help with data collection from the
patients.
Glossary
Abbreviations
Abbreviations:
|
ACT
|
adjuvant chemotherapy
|
|
BMI
|
body mass index
|
|
CCr
|
creatinine clearance
|
|
PNI
|
prognostic nutritional index
|
|
RP
|
relative performance
|
|
BW
|
body weight
|
References
|
1
|
WHO, . GLOBOSCAN 2012: Estimated cancer
incidence, mortality and prevalence worldwide in 2012 from.
http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspxFebruary
6–2014
|
|
2
|
Sasako M, Sakuramoto S, Katai H, Kinoshita
T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T and
Ohashi Y: Five-year outcomes of a randomized phase III trial
comparing adjuvant chemotherapy with S-1 versus surgery alone in
stage II or III gastric cancer. J Clin Oncol. 29:4387–4393. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sakuramoto S, Sasako M, Yamaguchi T,
Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi
Y, Imamura H, et al: Adjuvant chemotherapy for gastric cancer with
S-1, an oral fluoropyrimidine. N Engl J Med. 357:1810–1820. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aoyama T, Yoshikawa T, Hayashi T, Kuwabara
H, Mikayama Y, Ogata T, Cho H and Tsuburaya A: Risk factors for
6-month continuation of S-1 adjuvant chemotherapy for gastric
cancer. Gastric Cancer. 16:133–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aoyama T, Yoshikawa T, Shirai J, Hayashi
T, Yamada T, Tsuchida K, Hasegawa S, Cho H, Yukawa N, Oshima T, et
al: Body weight loss after surgery is an independent risk factor
for continuation of S-1 adjuvant chemotherapy for gastric cancer.
Ann Surg Oncol. 20:2000–2006. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kawabata R, Imamura H, Kishimoto T,
Hachino Y, Yasui Y, Fujino M, Fujii C, Fukunaga M, Ohzato H and
Furukawa H: Examination of factors influencing continuity of S-1
adjuvant chemotherapy for gastric cancer patients. Gan To Kagaku
Ryoho. 39:1205–1208. 2012.PubMed/NCBI
|
|
7
|
Kim SJ, Kim YJ, Kim JH, Park DJ, Kim HH,
Lee JS and Lee KW: Safety, compliance, and predictive parameters
for dosage modification in adjuvant S-1 chemotherapy for gastric
cancer. Cancer Sci. 104:116–123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lien YC, Hsieh CC, Wu YC, Hsu HS, Hsu WH,
Wang LS, Huang MH and Huang BS: Preoperative serum albumin level is
a prognostic indicator for adenocarcinoma of the gastric cardia. J
Gastrointest Surg. 8:1041–1048. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu H, Deng J, Zhang R, Hao X, Jiao X and
Liang H: The RML of lymph node metastasis was superior to the LODDS
for evaluating the prognosis of gastric cancer. Int J Surg.
11:419–424. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Migita K, Takayama T, Saeki K, Matsumoto
S, Wakatsuki K, Enomoto K, Tanaka T, Ito M, Kurumatani N and
Nakajima Y: The prognostic nutritional index predicts long-term
outcomes of gastric cancer patients independent of tumor stage. Ann
Surg Oncol. 20:2647–2654. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sakurai K, Ohira M, Tamura T, Toyokawa T,
Amano R, Kubo N, Tanaka H, Muguruma K, Yashiro M, Maeda K and
Hirakawa K: Predictive potential of preoperative nutritional status
in long-term outcome projections for patients with gastric cancer.
Ann Surg Oncol. 23:525–533. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ema A, Yamashita K, Sakuramoto S, Wang G,
Mieno H, Nemoto M, Shibata T, Katada N, Kikuchi S and Watanabe M:
Lymph node ratio is a critical prognostic predictor in gastric
cancer treated with S-1 chemotherapy. Gastric Cancer. 17:67–75.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pattison S, Mann GB, Crosthwaite G, Lade
S, Mitchell C, Leong T, Busuttil RA and Boussioutas A: Predictors
of outcome after surgery for gastric cancer in a Western cohort.
ANZ J Surg. 86:469–474. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maruyama K: The most important prognostic
factors for gastric cancer patients: A study using univariate and
multivariate analysis. Scandinavian J Gastroenterol. 22:63–68.
1987. View Article : Google Scholar
|
|
15
|
Kim JP, Lee JH, Kim SJ, Yu HJ and Yang HK:
Clinicopathological characteristics and prognostic factors in 10
783 patients with gastric cancer. Gastric Cancer. 1:125–133. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nozoe T, Ninomiya M, Maeda T, Matsukuma A,
Nakashima H and Ezaki T: Prognostic nutritional index: A tool to
predict the biological aggressiveness of gastric carcinoma. Surg
Today. 40:440–443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Watanabe M, Iwatsuki M, Iwagami S,
Ishimoto T, Baba Y and Baba H: Prognostic nutritional index
predicts outcomes of gastrectomy in the elderly. World J Surg.
36:1632–1639. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kodera Y, Sasako M, Yamamoto S, Sano T,
Nashimoto A and Kurita A; Gastric Cancer Surgery Study Group of
Japan Clinical Oncology Group, : Identification of risk factors for
the development of complications following extended and
superextended lymphadenectomies for gastric cancer. Br J Surg.
92:1103–1109. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gretschel S, Christoph F, Bembenek A,
Estevez-Schwarz L, Schneider U and Schlag PM: Body mass index does
not affect systematic D2 lymph node dissection and postoperative
morbidity in gastric cancer patients. Ann Surg Oncol. 10:363–368.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barry JD, Blackshaw GR, Edwards P, Lewis
WG, Murphy P, Hodzovic I, Thompson IW and Allison MC: Western body
mass indices need not compromise outcomes after modified D2
gastrectomy for carcinoma. Gastric Cancer. 6:80–85. 2003.PubMed/NCBI
|
|
21
|
Inagawa S, Adachi S, Oda T, Kawamoto T,
Koike N and Fukao K: Effect of fat volume on postoperative
complications and survival rate after D2 dissection for gastric
cancer. Gastric Cancer. 3:141–144. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee JH, Paik YH, Lee JS, Ryu KW, Kim CG,
Park SR, Kim YW, Kook MC, Nam BH and Bae JM: Abdominal shape of
gastric cancer patients influences short-term surgical outcomes.
Ann Surg Oncol. 14:1288–1294. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kodera Y, Ito S, Yamamura Y, Mochizuki Y,
Fujiwara M, Hibi K, Ito K, Akiyama S and Nakao A: Obesity and
outcome of distal gastrectomy with D2 lymphadenectomy for
carcinoma. Hepatogastroenterology. 51:1225–1228. 2004.PubMed/NCBI
|
|
24
|
Dhar DK, Kubota H, Tachibana M, Kotoh T,
Tabara H, Masunaga R, Kohno H and Nagasue N: Body mass index
determines the success of lymph node dissection and predicts the
outcome of gastric carcinoma patients. Oncology. 59:18–23. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Murphy PM, Blackshaw GR, Paris HJ, Edwards
P, Barry JD and Lewis WG: Prospective evaluation of nutritional
status related to body mass indices and outcomes after modified D2
gastrectomy for carcinoma. Clin Nutr. 23:477–483. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tokunaga M, Hiki N, Fukunaga T, Ohyama S,
Yamaguchi T and Nakajima T: Better 5-year survival rate following
curative gastrectomy in overweight patients. Ann Surg Oncol.
16:3245–3251. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nozoe T, Kohno M, Iguchi T, Mori E, Maeda
T, Matsukuma A and Ezaki T: Analysis of the impact of the body mass
index in patients with gastric carcinoma. Surg Today. 42:945–949.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moriwaki Y, Kunisaki C, Kobayashi S,
Harada H, Imai S and Kasaoka C: Does body mass index (BMI)
influence morbidity and long-term survival in gastric cancer
patients after gastrectomy? Hepatogastroenterology. 50:284–288.
2003.PubMed/NCBI
|
|
29
|
Dignam JJ, Polite BN, Yothers G, Raich P,
Colangelo L, O'Connell MJ and Wolmark N: Body mass index and
outcomes in patients who receive adjuvant chemotherapy for colon
cancer. J Natl Cancer Inst. 98:1647–1654. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Japanese Gastric Cancer Association, .
Japanese classification of gastric carcinoma. 13th. Kanehara,
Tokyo, Japan: 1999
|
|
31
|
Sobin LH, Gospodarowics MK and Wittekind
CH: TNM classification of malignant tumours. 7th. John Wiley &
Sons; New York: 2009
|
|
32
|
Lauren P: The two histologic main types of
gastric carcinoma: Diffuse and so-called intestinal-type carcinoma.
An attempt at a Histo-Clinical classification. Acta Parhol Microbid
Scan. 64:31–49. 1965. View Article : Google Scholar
|
|
33
|
Common terminology criteria for adverse
events v3.0 (CTCAE). http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdfJournal.
August 9–2006.
|
|
34
|
Cockcroft DW and Gault MH: Prediction of
creatinine clearance from serum creatinine. Nephron. 16:31–41.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Onodera T, Goseki N and Kosaki G:
Prognostic nutritional index in gastrointestinal surgery of
malnourished cancer patients. Nihon Geka Gakkai Zasshi.
85:1001–1005. 1984.(In Japanese). PubMed/NCBI
|
|
36
|
survivalROC: Time-dependent ROC curve
estimation from censored survival data. Journal. May
6–2016https://cran.r-project.org/web/packages/survivalROC/index.html
|
|
37
|
WHO Expert Consultation, . Appropriate
body-mass index for Asian populations and its implications for
policy and intervention strategies. Lancet. 363:157–163. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kiyama T, Mizutani T, Okuda T, Fujita I,
Tokunaga A, Tajiri T and Barbul A: Postoperative changes in body
composition after gastrectomy. J Gastrointest Surg. 9:313–319.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hirao M, Takiguchi S, Imamura H, Yamamoto
K, Kurokawa Y, Fujita J, Kobayashi K, Kimura Y, Mori M and Doki Y;
Osaka University Clinical Research Group for Gastroenterological
Study, : Comparison of Billroth I and Roux-en-Y reconstruction
after distal gastrectomy for gastric cancer: One-year postoperative
effects assessed by a multi-institutional RCT. Ann Surg Oncol.
20:1591–1597. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hiki N: Body weight loss in cancer
patients: Mechanism of body weight loss by treatment. The Japanese
Journal of Clinical Nutrition. 120:848–851. 2012.(In Japanese).
|
|
41
|
Andreyev HJ, Norman AR, Oates J and
Cunningham D: Why do patients with weight loss have a worse outcome
when undergoing chemotherapy for gastrointestinal malignancies? Eur
J Cancer. 34:503–509. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ock CY, Oh DY, Lee J, Kim TY, Lee KH, Han
SW, Im SA, Kim TY and Bang YJ: Weight loss at the first month of
palliative chemotherapy predicts survival outcomes in patients with
advanced gastric cancer. Gastric Cancer. 19:597–606. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sinicrope FA, Foster NR, Sargent DJ,
O'Connell MJ and Rankin C: Obesity is an independent prognostic
variable in colon cancer survivors. Clin Cancer Res. 16:1884–1893.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dawood S, Broglio K, Gonzalez-Angulo AM,
Kau SW, Islam R, Hortobagyi GN and Cristofanilli M: Prognostic
value of body mass index in locally advanced breast cancer. Clin
Cancer Res. 14:1718–1725. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nozoe T, Kohno M, Iguchi T, Mori E, Maeda
T, Matsukuma A and Ezaki T: The prognostic nutritional index can be
a prognostic indicator in colorectal carcinoma. Surg Today.
42:532–535. 2012. View Article : Google Scholar : PubMed/NCBI
|