Introduction
Primary small bowel adenocarcinoma (SBA) is a rare
cancer; 5,420 patients were diagnosed with SBA, accounting for 2.1%
of all malignant gastrointestinal tumors, in the United States of
America in 2005 (1,2). Early diagnosis of SBA is challenging due
to the site of origin; for example, of 217 patients diagnosed with
SBA at the University of Texas M. D. Anderson Cancer Center between
1978 and 998, 75 patients (35%) were diagnosed at stage IV
(3). However, the use of capsule
endoscopy and double balloon enteroscopy (DBE) has enabled the
observation of the entire small intestine endoscopically (4–6). In
addition, DBE can be used to sample mucosal tissues in order to
diagnose SBA (5,6). The incidence of SBA diagnosis is
increasing, partially due to the development of these novel methods
for diagnosis (7,8). However, delayed diagnosis remains common
despite technical advances in endoscopy.
Resection of the primary tumor and regional lymph
nodes is the standard treatment for localized SBA. However, the
curative resection rate of SBA is 40–60% (9–12).
Chemotherapy is performed for unresectable or recurrent SBA. Raghav
and Overman (13) reported that the
median survival time (MST) of patients with SBA who received
systemic chemotherapy was 13 months, which was longer than the MST
for patients who received best supportive care (BSC) alone (4
months). The 5-year survival rates of SBA are 40–60% for resected
tumors and 15–30% for unresectable tumors (9–12).
Prospective evaluations of the efficacy of chemotherapy are rare
due to the rarity of SBA. SBA is more similar to colorectal cancer
than gastric cancer based on genome-wide DNA copy number
aberrations (14); therefore,
chemotherapy regimens used to treat colorectal cancer are typically
selected for the treatment of SBA. Previous retrospective studies
have demonstrated that chemotherapy, including oxaliplatin with
fluorouracil and folinic acid (FOLFOX) chemotherapy, and
oxaliplatin and capecitabine (CAPOX) chemotherapy, contributes to a
longer survival time in patients with SBA (15–19). The
overall survival (OS) time of patients who received chemotherapy
was 15.1–22.2 months (15–19). Treatment strategies for colorectal
cancer have drastically changed in the last 10 years due to the
development of new molecular targeted therapies. Molecular targeted
therapy has been suggested to be effective for the patients with
SBA due to the similarities between SBA and colorectal cancer
(20). Few studies have evaluated the
efficacy of molecular targeted therapies for SBA (21–23) and
there is an urgent need to explore effective strategies to treat
SBA. In the present study, clinicopathological factors associated
with the effective treatment of SBA, and the efficacy of
chemotherapy, including molecular targeted agents for the treatment
of SBA, were investigated.
Materials and methods
Patients
The treatment and follow-up data of 27 patients with
recurrent, non-curatively resected or unresectable SBA who received
chemotherapy between April 2006 and March 2014 in 10 hospitals
participating in the Osaka Gut Forum (Table I) were retrospectively analyzed. SBA
was defined as histologically diagnosed adenocarcinoma of the
duodenum, jejunum or ileum excluding ampullary carcinoma. The
present study was conducted in accordance with the Declaration of
Helsinki. Ethical approval was obtained from the Institutional
Review Boards of all the institutions where patients were recruited
as listed in Table I; patients were
able to opt out their data from inclusion in the retrospective
study.
 | Table I.Hospitals included in the present
study and the number of patients with small bowel adenocarcinoma
from each. |
Table I.
Hospitals included in the present
study and the number of patients with small bowel adenocarcinoma
from each.
| Hospital | Number of
patients |
|---|
| Osaka
Universitya | 7 |
| Osaka Police
Hospitalb | 5 |
| Osaka Rosai
Hospitalc | 3 |
| Sumitomo
Hospitalb | 3 |
| JCHO Osaka
Hospitalb | 3 |
| Saiseikai Senri
Hospitala | 2 |
| Kansai Rosai
Hospitald | 1 |
| Osaka Medical
Center for Cancer | 1 |
| and Cardiovascular
Diseasesb |
| Hyogo Prefectural
Nishinomiya Hospitale | 1 |
| Higashiosaka City
General Hospitalf | 1 |
Data collection
The following data were collected from medical
records: Patient demographics [age, sex, Eastern Cooperative
Oncology Group (ECOG) performance status (PS) (24)], primary tumor locations (duodenum,
jejunum, ileum), indications for chemotherapy (post-operative
recurrence, non-curative resection, unresectable), adjuvant
chemotherapy, histological type (differentiated, undifferentiated),
tumor biomarker expression [serum carcinoembryonic antigen (CEA)
and carbohydrate antigen (CA) 19–9], metastatic sites (liver and
lung), number of metastatic organs including lymph nodes, resection
of the primary tumor, chemotherapy agent and radiation therapy. The
clinical course of the disease was also investigated as follows:
Best response under chemotherapy, time for disease progression,
subsequent therapies and survival status. Best responses to
chemotherapy were evaluated according to the Response Evaluation
Criteria in Solid Tumors (version 1.1) (25). The National Cancer Institute Common
Terminology Criteria (version 4.0) (26) were used to evaluate the toxicity of
therapeutics. Progression free survival (PFS) was defined as the
time from the initiation of chemotherapy until the confirmation of
disease progression or mortality from any cause. OS was defined as
the time from the initiation of chemotherapy until mortality.
Surviving patients were censored on their last follow-up dates. The
clinicopathological characteristics of the patients included in the
present study are illustrated in Table
II.
 | Table II.Clinicopathological characteristics
of patients with small bowel adenocarcinoma. |
Table II.
Clinicopathological characteristics
of patients with small bowel adenocarcinoma.
| Clinicopathological
characteristic | Number of patients
(%) |
|---|
| Age
(<60/≥60) | 10 (37.0)/17
(63.0) |
| Sex (M/F) | 17 (63.0)/10
(37.0) |
| Location of primary
tumor (duodenum/jejunum/ileum) | 8
(29.6)/11 (40.7)/8 (29.6) |
| Reasons for
chemotherapy (non-curative resection/unresectable/post-operative
recurrence) | 9
(33.3)/13 (48.2)/5 (18.5) |
| Adjuvant
chemotherapy treatment (yes/no) | 4
(14.8)/23 (85.2) |
| Histological type
(differentiated/undifferentiated) | 18 (66.7)/9
(33.3) |
| Performance status
(0/1-2) | 19 (70.4)/8
(29.6) |
| CEA (ng/ml),
(≤5/>5) | 13 (50.0)/13
(50.0) |
| CA19-9 (U/ml),
(≤40/>40) | 15 (60)/10
(40) |
| Liver metastasis
(present/absent) | 8
(29.6)/19 (70.4) |
| Lung metastasis
(present/absent) | 2
(7.4)/25 (92.6) |
| Number of
metastatic organs (1/2/3) | 16 (59.3)/9
(33.3)/2 (7.4) |
| Resection of
primary tumor (yes/no), | 15 (55.6)/12
(44.4) |
Chemotherapy regimens
The modified FOLFOX6 (mFOLFOX6) regimen consisted of
the following: L-leucovorin (LV; 200 mg/m2), oxaliplatin
(85 mg/m2) and bolus 5-fluorouracil (5-FU; 400
mg/m2), followed by infusion of 5-FU (2,400
mg/m2) for 46 h, every 2 weeks. The mFOLFOX6 with
bevacizumab regimen included mFOLOFOX6, as described, with 5 mg/kg
bevacizumab, every 2 weeks. mFOLFOX6 with cetuximab included an
initial dose of 400 mg/m2 cetuximab and 250
mg/m2/week thereafter. The CAPOX regimen was as follows:
Oxaliplatin (130 mg/m2) intravenously on day 1 and
capecitabine (2,000 mg/m2/day) orally on days 1–14 every
3 weeks. The titanium silicate (TS)-1 ± cisplatin (CDDP) regimen
was a follows: TS-1 (80 mg/m2/day) orally on days 1–21
plus CDDP (60 mg/m2) on day 8, every 5 weeks or TS-1 (80
mg/m2/day) orally on days 1–28 every 6 weeks. The 5-FU +
LV regimen was as follows: Bolus 5-FU (600 mg/m2) plus
LV (250 mg/m2) once a week for 6 weeks, every 8 weeks.
The irinotecan (CPT-11) + CDDP regimen consisted of the following:
CPT-11 (30 mg/m2) plus CDDP (60 mg/m2)
intravenously on days 1 and 15 every 4 weeks. The FOLFILI regimen
was as follows: LV (200 mg/m2), CPT-11 (180
mg/m2) and bolus 5-fluorouracil (5-FU; 400
mg/m2), followed by infusion of 5-FU (2,400
mg/m2) for 46 h, every 2 weeks.
Statistical analysis
The median and interquartile ranges are reported for
continuous variable, and categorical variables are summarized as
frequencies. Differences in the distribution of variables were
evaluated using the Kruskal-Wallis test (reason for chemotherapy)
or χ2 test (other data). PFS and OS were estimated using
the Kaplan-Meier estimator method and compared using the log-rank
test. Hazard ratios (HRs) and the corresponding 95% confidence
intervals (CIs) for PFS and OS were estimated using multivariate
Cox proportional hazards models following stepwise selection of the
covariates. Other than the treatment groups, these covariates
included the following: Age (<60/≥60 years old), sex
(male/female), ECOG PS (continuous variable), primary tumor
locations (duodenum/jejunum/ileum), indications for chemotherapy
(post-operative recurrence/non-curative resection/unresectable),
serum CEA level (≤5/>5 ng/ml), serum CA19-9 level (≤40/>40
ng/ml), liver metastasis (present/absent), lung metastasis
(present/absent), number of metastatic organs (continuous
variable), resection of primary tumor (present/absent),
platinum-containing chemotherapy (yes/no), molecular targeted
agents containing chemotherapy (yes/no), and combined-radiation
therapy (yes/no). All reported P-values were two sided, and
P<0.05 was considered to indicate a statistically significant
difference. Statistical analyses were performed using JMP
statistical software (version 11.1.1; SAS Institute, Inc., Cary,
NC, USA).
Results
Patient clinicopathological
characteristics
A total of 27 patients were included in the present
study. Patient clinicopathological characteristics are illustrated
in Table II. The median age was 63.4
years old (range, 36–83 years old). A total of 17 patients (63.0%)
were ≥60 years old and 17 patients (63.0%) were male. The location
of the primary tumor was in the duodenum in 8 patients (29.6%), in
the jejunum in 11 patients (40.8%) and in the ileum in 8 patients
(29.6%). Primary tumors were surgically resected in 15 patients
(55.6%). Chemotherapy was introduced due to unresectable tumors in
13 patients (48.2%), non-curative resections in 9 patients (33.3%)
and post-operative recurrence in 5 patients (18.5%). Among the
patients with non-curative resections, 4 patients (14.9%) received
adjuvant chemotherapy. The histological types of SBA were
differentiated type in 18 patients (66.7%) and undifferentiated
type in 9 patients (33.3%). The PS was 0 in 19 patients (70.4%), 1
in 7 patients (25.9%) and 2 in 1 patient (3.7%). The serum levels
of CEA and CA19-9 were measured in 26 and 25 patients,
respectively. Elevated levels of serum CEA and CA19-9 were observed
in 50% (13/26) and 64% (16/25) of patients, respectively (data not
shown). The number of metastatic organs was 1 in 16 patients
(59.3%), 2 in 9 patients (33.3%) and 3 in 2 patients (7.4%)
(Table II). A total of 8 patients
(29.6%) had liver metastases and 2 (7.4%) had lung metastases
(Table II).
Chemotherapy regimens and clinical
efficacy of first-line chemotherapy
The following first-line chemotherapy regimens were
used for patients in the present study: mFOLFOX6 in 14 patients,
CAPOX in 4 patients, TS-1 ± CDDP in 7 patients, 5-FU + LV in 1
patient, CPT-11 + CDDP in 1 patient (Table III). A total of 4 patients received
mFOLFOX6 with bevacizumab, 1 patient received CAPOX with
bevacizumab, 1 patient received mFOLFOX6 with cetuximab and 8
patients received mFOLFOX6 without molecular targeted agents. The
clinical efficacy of the first-line chemotherapy was evaluated; 2
patients exhibited a complete response (CR), 8 exhibited a partial
response (PR), 12 exhibited stable disease (SD) and 5 exhibited
progressive disease (PD). The response rate (RR) and disease
control rate (DR) were 37.0 and 81.5%, respectively (Table III).
 | Table III.Chemotherapy regimens and efficacy of
the first-line chemotherapy treatment for small bowel
adenocarcinoma. |
Table III.
Chemotherapy regimens and efficacy of
the first-line chemotherapy treatment for small bowel
adenocarcinoma.
| Regimen | Number of patients
(%) |
|---|
| First-line
chemotherapy |
|
|
mFOLFOX6 | 14 (51.9) |
|
CAPOX | 4
(14.8) |
| TS-1 ±
CDDP | 7
(25.9) |
| 5-FU +
LV | 1 (3.7) |
| CPT-11
+ CDDP | 1 (3.7) |
| Platinum-containing
1st line chemotherapy treatment (yes/no) | 24 (88.9)/3
(11.1) |
| Molecular-targeted
agent-containing 1st line chemotherapy treatment (yes/no) | 6
(22.2)/21 (77.8) |
| Combined-radiation
therapy treatment (yes/no) | 2
(7.4)/25 (92.6) |
| Efficacy of 1st
line chemotherapy (CR/PR/SD/PD) | 2 (7.4)/8 (29.6)/12
(44.5)/5 (18.5) |
|
Response rate (RR)/Disease
control rate (DR) | 10
(37.0%)/22 (81.5%) |
| Molecular-targeted
agent-containing 1st or 2nd line chemotherapy treatment
(yes/no) | 8 (7, bevacizumab;
1, cetuximab,1) |
|
|
(29.6)/19 (70.4) |
Salvage chemotherapy following
first-line therapy
A total of 16 patients (59.2%) received second-line
chemotherapy and 5 patients (18.5%) received third-line
chemotherapy. Molecular targeted agents (bevacizumab or cetuximab)
were administered as the second-line therapy in 5 patients, as the
third-line therapy in 4 patients, as the fourth-line therapy in 4
patients and as the sixth-line therapy in 1 patient (data not
shown).
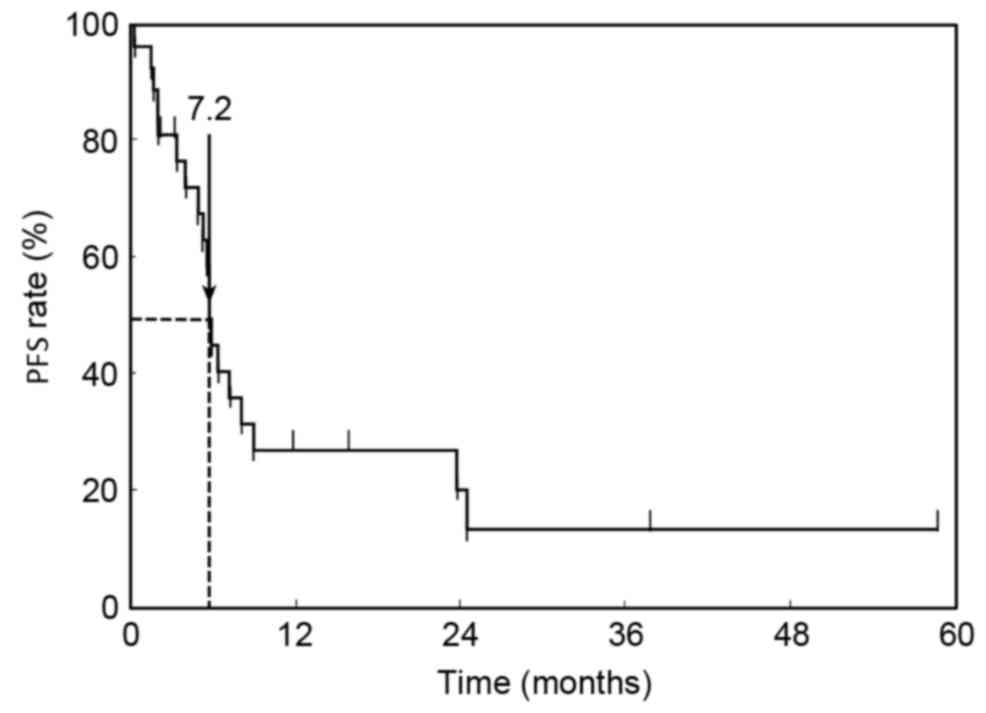
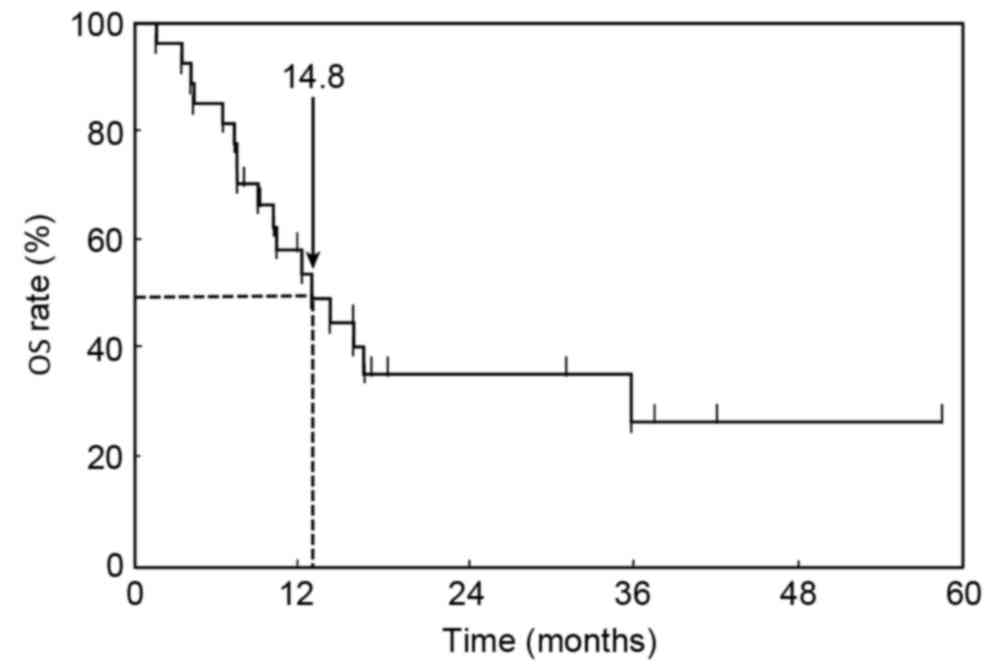
PFS and OS
At the time of analysis, 17 patients (63.0%) had
succumbed to their disease with a median follow-up of 21.3 months
following initiation of first-line chemotherapy. Kaplan-Meier
estimator curves for PFS and OS were calculated. The median PFS of
first-line chemotherapy was 7.2 months (95% CI, 6.2–11.2 months;
Fig. 1). The MST for all patients in
the OS curve was 14.8 months (95% CI, 9.3–45.1 months; Fig. 2).
Survival analysis and probability of
receiving salvage chemotherapy
Univariate and multivariate analyses of the baseline
clinicopathological characteristics associated with prognostic
factors for survival were performed. The univariate analysis
revealed that a PS of 0 and platinum-containing chemotherapy were
significant prognostic factors for improved survival (P=0.0228 and
P=0.0048, respectively; Table IV).
Chemotherapy with molecular targeted agents was a borderline factor
for improved prognosis (P=0.0827). Although SBA may exhibit
different characteristics according to the location of the tumor,
the survival time between patients with duodenal SBA and patients
with non-duodenal SBA was not significantly different (data not
shown). Multivariate analysis adjusted for age identified that a
platinum-containing chemotherapy regimen was the most significant
predictive factor for improved survival time (P=0.0373, Table V).
 | Table IV.Univariate analysis of baseline and
clinicopathological characteristics as prognostic factors for
survival time in patients with small bowel adenocarcinoma. |
Table IV.
Univariate analysis of baseline and
clinicopathological characteristics as prognostic factors for
survival time in patients with small bowel adenocarcinoma.
| Clinicopathological
characteristic | HR | 95% CI | P-value |
|---|
| Age (years) |
|
| N.S. |
|
<60 | 1 |
|
|
|
≥60 | 0.85 | 0.32–2.36 |
|
| Sex |
|
| N.S. |
|
Male | 1 |
|
|
|
Female | 1.01 | 0.36–2.66 |
|
| Histological
type |
|
| N.S. |
|
Differentiated
adenocarcinoma | 1 |
|
|
|
Undifferentiated
adenocarcinoma | 0.57 | 0.16–1.64 |
|
| Performance
status |
|
| 0.0228 |
| 0 | 1 |
|
|
|
1/2 | 3.40 | 1.20–9.25 |
|
| Primary tumor
location |
|
| N.S. |
|
Duodenum | 1 |
|
|
|
Non-duodenum (Jejunum,
Ileum) | 1.16 | 0.40–4.17 |
|
| Reason for
chemotherapy |
|
|
|
|
Post-operative recurrence | 1 |
|
|
|
Non-curative resection | 2.69 | 0.72–17.5 | N.S. |
|
Unresectable | 3.92 | 0.64–30.0 | N.S. |
| CEA (ng/ml) |
|
| N.S. |
| ≤5 | 1 |
|
|
|
>5 | 0.78 | 0.28–2.05 |
|
| CA19-9 (ng/ml) |
|
| N.S. |
|
≤40 | 1 |
|
|
|
>40 | 0.72 | 0.22–1.95 |
|
| Liver
metastasis |
|
| N.S. |
|
Absent | 1 |
|
|
|
Present | 1.93 | 0.65–5.19 |
|
| Lung
metastasis |
|
| N.S. |
|
Absent | 1 |
|
|
|
Present | 0.92 | 0.05–4.60 |
|
| Number of
metastatic organs |
|
|
|
| 1 | 1 |
|
|
| 2 | 1.29 | 0.43–3.61 | N.S. |
| 3 | 2.48 | 0.11–10.4 | N.S. |
| Resection of
primary tumor |
|
| N.S. |
| No | 1 |
|
|
|
Yes | 0.79 | 0.29–2.22 |
|
| Platinum-containing
chemotherapy treatment |
|
| 0.0048 |
| No | 1 |
|
|
|
Yes | 0.08 | 0.01–0.41 |
|
| Molecular-targeted
agent-containing chemotherapy treatment |
|
| 0.0827 |
| No | 1 |
|
|
|
Yes | 0.38 | 0.10–1.12 |
|
| Combined
chemotherapy-radiotherapy treatment |
|
| N.S. |
| No | 1 |
|
|
|
Yes | 1.60 | 0.25–5.88 |
|
 | Table V.Multivariate analysis of
clinicopathological characteristics as prognostic factors for
survival time in small bowel adenocarcinoma. |
Table V.
Multivariate analysis of
clinicopathological characteristics as prognostic factors for
survival time in small bowel adenocarcinoma.
| Clinicopathological
characteristic | HR | 95% CI | P-value |
|---|
| Performance
status |
|
| 0.2016 |
| 0 | 1 |
|
|
|
1/2 | 2.34 | 0.60–7.78 |
|
| Platinum-containing
chemotherapy treatment |
|
| 0.0373 |
| No | 1 |
|
|
|
Yes | 0.14 | 0.02–0.88 |
|
Chemotherapy containing molecular
target agents for SBA
A total of 8 patients were treated with chemotherapy
regimens containing molecular targeted agents (Table III). Details of the regimens that
these patients received are provided in Table VI. The clinical outcomes of these
patients at the last follow-up period was CR in 1 patient, PR in 1
patient, SD in 1 patient, BSC in 2 patients and mortality in 3
patients (Table VI). The MST for the
8 patients treated with molecular targeted agents-containing
regimens was 27.2 months (data not shown).
 | Table VI.Details of the 8 patients with small
bowel adenocarcinoma treated with molecular targeted
agent-containing chemotherapy regimens. |
Table VI.
Details of the 8 patients with small
bowel adenocarcinoma treated with molecular targeted
agent-containing chemotherapy regimens.
|
|
|
|
| Chemotherapy
regimens (treatment periods, [months]) |
|
|---|
|
|
|
|
|
|
|---|
| Case | Age (years) | Sex | PS | 1st-line | 2nd-line | 3rd-line | 4th-line | 5th-line | 6th-line | Result |
|---|
| 1 | 62 | M | 0 | CAPOX + Bev
(26.7) |
|
|
|
|
| CR |
| 2 | 63 | F | 0 | mFOLFOX6 + Bev
(29.6) | CAPOX −23.2 |
|
|
|
| PR |
| 3 | 57 | M | 0 | mFOLFOX6 + Bev
(2.5) | FOLFILI + Bev
(12.1) | UFT + Bev −5.6 | Regorafenib
−0.2 |
|
| BSC |
| 4 | 81 | F | 0 | mFOLFOX6 + Bev
(6.0) | CPT-11+ Pani
−8.4 |
|
|
|
| Mortality |
| 5 | 74 | M | 0 | mFOLFOX6 + Bev
(2.0) | TS-1 (1.0) |
|
|
|
| Mortality |
| 6 | 62 | F | 1 | TS-1+CDDP
(7.7) | FOLFILI + Bev
(9.2) | mFOLFOX6+ Bev
(1.5) | mFOLFOX6 + Pani
(1.7) | PTX (1.5) |
| SD |
| 7 | 36 | F | 0 | mFOLFOX6 (3.0) | FOLFILI + Bev
(10.0) | Capecitabine + Bev
(5.0) | SOX + Bev −1 | SOX (6.0) | TS-1 + Bev
(3.0) | BSC |
| 8 | 38 | M | 0 | mFOLFOX6 + Cet
(6.8) | FOLFILI + Cet
(3.0) | FOLFILI + Bev
(2) | FOLFILI + Pani
(2) |
|
| Mortality |
The efficacy of molecular targeted agents in
patients treated with oxaliplatin-based chemotherapy, including
mFOLFOX6 and CAPOX as the first-line therapy was investigated. A
total of 8 patients were treated with molecular targeted agents as
the first or second line of treatment, including 7 patients with
bevacizumab and 1 patient with cetuximab (Table III) in 18 patients with SBA that
were treated with oxaliplatin-based chemotherapy (data not shown).
Following the exclusion of the 1 patient treated with cetuximab,
the prognostic factors for survival time in the 17 patients treated
with oxaliplatin-based chemotherapy with or without bevacizumab
were analyzed. A univariate analysis revealed that a PS of 0 and
treatment with bevacizumab were significant prognostic factors for
improved survival time (P=0.0256 and P=0.0121, respectively;
Table VII).
 | Table VII.Univariate analysis of baseline and
clinical characteristics as prognostic factors for survival time in
oxaliplatin-based chemotherapy. |
Table VII.
Univariate analysis of baseline and
clinical characteristics as prognostic factors for survival time in
oxaliplatin-based chemotherapy.
| Clinicopathological
characteristic | HR | 95% CI | P-value |
|---|
| Age |
|
| N.S. |
|
<60 | 1 |
|
|
|
≥60 | 1.19 | 0.38–3.65 |
|
| Sex |
|
| N.S. |
|
Male | 1 |
|
|
|
Female | 0.44 | 0.09–1.50 |
|
| Histological
type |
|
| N.S. |
|
Differentiated
adenocarcinoma | 1 |
|
|
|
Undifferentiated
adenocarcinoma | 0.81 | 0.26–3.08 |
|
| Performance
status |
|
| 0.0256 |
| 0 | 1 |
|
|
|
1/2 | 5.61 | 1.09–25.8 |
|
| Primary tumor
location |
|
| N.S. |
|
Duodenum | 1 |
|
|
|
Jejunum, Ileum | 1.09 | 0.28–7.20 |
|
| CEA (ng/ml) |
|
| N.S. |
| ≤5 | 1 |
|
|
|
>5 | 1.17 | 0.38–3.98 |
|
| CA19-9 (ng/ml) |
|
| N.S. |
|
≤40 | 1 |
|
|
|
>40 | 0.97 | 0.29–4.34 |
|
| Liver
metastasis |
|
| N.S. |
|
Absent | 1 |
|
|
|
Present | 1.07 | 0.29–3.24 |
|
| Lung
metastasis |
|
| N.S. |
|
Absent | 1 |
|
|
|
Present | 1.38 | 0.21–5.32 |
|
| Resection of
primary tumor |
|
| N.S. |
| No | 1 |
|
|
|
Yes | 0.40 | 0.12–1.27 |
|
| Bevacizumab
treatment |
|
| 0.0121 |
| No | 1 |
|
|
|
Yes | 0.16 | 0.02–0.69 |
|
Molecular targeted agent treatment
toxicity
A total of 8 patients suffered from toxicities
higher than Grade 3 due to chemotherapy, including 2 patients
treated with bevacizumab (data not shown). Dose reduction (40
mg/m2/day) was required due to neutropenia in 1 patient
treated with TS-1, in 1 patient treated with mFOLFOX6 (LV; 200
mg/m2, oxaliplatin 65 mg/m2 and bolus 5-FU;
200 mg/m2, infusion of 5-FU 2,000 mg/m2), and
due to thrombocytopenia in 1 patient treated with CAPOX
(capecitabine 1,500 mg/m2, oxaliplatin 100
mg/m2). Neurotoxicity was observed in 2 patients treated
with mFOLFOX6 and 1 patient treated with mFOLFOX6 and bevacizumab.
The 2 patients treated with mFOLFOX6 continued chemotherapy with
either 5-FU + LV + CPT-11 (FOLFILI) or uracil + tegafur (UFT) with
bevacizumab, and the 1 patient treated with mFOLFOX6 and
bevacizumab continued chemotherapy with FOLFILI with bevacizumab
due to oxaliplatin-induced neurotoxicity. Renal dysfunction was
observed in 1 patient treated with TS-1; however, chemotherapy with
TS-1 could be continued by reducing the dose to 60
mg/m2/day. Liver dysfunction was observed in 1 patient
treated with mFOLFOX6 and bevacizumab; however, an alteration of
the TS-1 dosage was effective (80 mg/m2/day). Although
bevacizumab treatment was discontinued due to the toxicity of
concomitant chemotherapies in 2 patients, no serious side effects
in response to the molecular targeted agents, including bleeding,
thrombosis, gastrointestinal perforation, allergic reactions and
rash, occurred in any of the patients included in the present
study. Therefore, chemotherapy with bevacizumab for SBA was
considered well tolerated by patients.
Discussion
SBA is the most common histological subtype of
carcinoma of the small bowel according to the National Cancer
Database, accounting for 36.9% of all small bowel malignancies
(27). The dominant immunophenotypic
pattern of SBA is cytokeratin (CK) 20+/CK7−
and this pattern is observed in 75–94% of colorectal cancer cases
(28). Caudal-type homeobox
transcription factor 2 (CDX2), which is highly expressed in
colorectal cancer, is also expressed in the majority of cases of
SBA, particularly in well-differentiated tumors (28). The molecular characteristics of SBA
are more similar to those of colorectal adenocarcinoma compared
with gastric adenocarcinoma, with low expression of receptor
tyrosine-protein kinase erbB-2 and high frequencies of GTPase KRAS
(KRAS) mutations (29). A genome-wide
DNA copy number analysis demonstrated that the profiles of SBA
overlapped more with colorectal adenocarcinoma compared with
gastric adenocarcinoma (26). These
results indicate that the characteristics of SBA resemble
colorectal cancer. A previous study demonstrated that there was no
significant difference in the immunophenotype determined by the
expression of cytokeratin (CK) 7, CK20 and CDX2 between duodenal
and non-duodenal SBA (28). Zaanan
et al (15,30) reported that OS was not significantly
different between duodenal and non-duodenal SBA. Consistent with
previous studies, OS was not significantly different between
duodenal and non-duodenal SBA in the present study.
Bevacizumab has been demonstrated to prolong the OS
of patients with colorectal cancer from 15.6 to 20.3 months
(20). Bevacizumab is a recombinant
Immunolobulin G1 humanized monoclonal antibody that targets
vascular endothelial growth factor (VEGF), which is a critical and
highly pleiotropic factor that promotes new blood vessel formation
in tumors. Bevacizumab inhibits tumor vessel hyperplasticity and
facilitates the intra-tumoral transition of anticancer drugs by
promoting tumor vessel permeability and decreasing intratumoral
stromal pressure (31,32). Willett et al (33) demonstrated that a single infusion of
bevacizumab decreases tumor perfusion, vascular volume,
microvascular density, interstitial fluid pressure and the number
of viable circulating endothelial and progenitor cells, but
increases the fraction of vessels with pericyte coverage in
patients with rectal carcinoma (33).
These results indicate that the inhibition of VEGF has a direct and
rapid antivascular effect in human tumors. In addition, bevacizumab
has also been suggested to be an effective treatment for SBA, as
the VEGF expression is detectable in 96% of SBA tumors (18).
Cetuximab is a monoclonal antibody directed against
the epidermal growth factor receptor. Cetuximab has been
demonstrated to improve first-line chemotherapy efficacy in
colorectal cancer; however, the benefit was limited to patients
with wild-type KRAS tumors (34).
There are only a small number of case reports about the use of
molecular targeted therapies, including bevacizumab and cetuximab,
for SBA (17,19,21,35).
Therefore, the results from the current multicenter retrospective
cohort study may aid in determining whether molecular targeted
agents for SBA are safe and effective. No serious side effects were
observed as a result of molecular targeted agents in the current
study. A univariate analysis of oxaliplatin-based chemotherapy
revealed that bevacizumab-containing chemotherapy was the most
significant positive prognostic factor. The current multicenter
study demonstrated that molecular targeted agent-containing
chemotherapy is safe and may improve survival times in patients
with SBA.
The current study had a number of limitations,
including a small sample size and the fact that the patients'
disease status was heterogeneous, despite conducting a multi-center
study, due to the rarity of SBA. In addition, there was no
standardized treatment regimen; the physicians in each hospital
selected the chemotherapy regimens for patients with SBA. The
current study also included patients that had undergone surgical
resection with varying degrees of success, which may have affected
the outcome of chemotherapy. Despite these limitations, preliminary
results from the present study indicate that molecular targeted
agents are effective and safe for the treatment of patients with
SBA. However, further studies are required to confirm these results
in a larger cohort of patients with SBA.
References
|
1
|
Lowenfels AB: Why are small-bowel tumours
so rare? Lancet. 1:24–26. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Murray T, Ward E, Samuels A,
Tiwari RC, Ghafoor A, Feuer EJ and Thun MJ: Cancer statistics,
2005. CA Cancer J Clin. 55:10–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dabaja BS, Suki D, Pro B, Bonnen M and
Ajani J: Adenocarcinoma of the small bowel: Presentation,
prognostic factors and outcome of 217 patients. Cancer.
101:518–526. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mishkin DS, Chuttani R, Croffie J, Disario
J, Liu J, Shah R, Somogyi L, Tierney W, Song LM and Petersen BT;
Technology Assessment Committee, American Society for
Gastrointestinal Endoscopy, : ASGE technology status evaluation
report: Wireless capsule endoscopy. Gastrointest Endosc.
63:539–545. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamamoto H, Sekine Y, Sato Y, Higashizawa
T, Miyata T, Iino S, Ido K and Sugano K: Total enteroscopy with a
nonsurgical steerable double-balloon method. Gastrointest Endosc.
53:216–220. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamamoto H, Yano T, Kita H, Sunada K, Ido
K and Sugano K: New system of double-balloon enteroscopy for
diagnosis and treatment of small intestinal disorders.
Gastroenterology. 125:1556–1557. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chow JS, Chen CC, Ahsan H and Neugut AI: A
population-based study of the incidence of malignant small bowel
tumours: SEER, 1973–1990. Int J Epidemiol. 25:722–728. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hatzaras I, Palesty JA, Abir F, Sullivan
P, Kozol RA, Dudrick SJ and Longo WE: Small-bowel tumors:
Epidemiologic and clinical characteristics of 1260 cases from the
connecticut tumor registry. Arch Surg. 142:229–235. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ouriel K and Adams JT: Adenocarcinoma of
the small intestine. Am J Surg. 147:66–71. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barnes G Jr, Romero L, Hess KR and Curley
SA: Primary adenocarcinoma of the duodenum: Management and survival
in 67 patients. Ann Surg Oncol. 1:73–78. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bauer RL, Palmer ML, Bauer AM, Nava HR and
Douglass HO Jr: Adenocarcinoma of the small intestine: 21-year
review of diagnosis, treatment, and prognosis. Ann Surg Oncol.
1:183–188. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rose DM, Hochwald SN, Klimstra DS and
Brennan MF: Primary duodenal adenocarcinoma: A ten-year experience
with 79 patients. J Am Coll Surg. 183:89–96. 1996.PubMed/NCBI
|
|
13
|
Raghav K and Overman MJ: Small bowel
adenocarcinomas-existing evidence and evolving paradigms. Nat Rev
Clin Oncol. 10:534–544. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Haan JC, Buffart TE, Eijk PP, van de Wiel
MA, van Wieringen WN, Howdle PD, Mulder CJ, van de Velde CJ, Quirke
P, Nagtegaal ID, et al: Small bowel adenocarcinoma copy number
profiles are more closely related to colorectal than to gastric
cancers. Ann Oncol. 23:367–374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zaanan A, Costes L, Gauthier M, Malka D,
Locher C, Mitry E, Tougeron D, Lecomte T, Gornet JM, Sobhani I, et
al: Chemotherapy of advanced small-bowel adenocarcinoma: A
multicenter AGEO study. Ann Oncol. 21:1786–1793. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang L, Wang LY, Deng YM, Wang FH, Feng
F, Chen YC, An X, Chen C, Xu RH and Li YH: Efficacy of the
FOLFOX/CAPOX regimen for advanced small bowel adenocarcinoma: A
three-center study from China. J BUON. 16:689–696. 2011.PubMed/NCBI
|
|
17
|
Tsushima T, Taguri M, Honma Y, Takahashi
H, Ueda S, Nishina T, Kawai H, Kato S, Suenaga M, Tamura F, et al:
Multicenter retrospective study of 132 patients with unresectable
small bowel adenocarcinoma treated with chemotherapy. Oncologist.
17:1163–1170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Overman MJ, Varadhachary GR, Kopetz S,
Adinin R, Lin E, Morris JS, Eng C, Abbruzzese JL and Wolff RA:
Phase II study of capecitabine and oxaliplatin for advanced
adenocarcinoma of the small bowel and ampulla of Vater. J Clin
Oncol. 27:2598–2603. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiang XJ, Liu YW, Zhang L, Qiu F, Yu F,
Zhan ZY, Feng M, Yan J, Zhao JG and Xiong JP: A phase II study of
modified FOLFOX as first-line chemotherapy in advanced small bowel
adenocarcinoma. Anticancer Drugs. 23:561–566. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsang H, Yau T, Khong PL and Epstein RJ:
Bevacizumab-based therapy for advanced small bowel adenocarcinoma.
Gut. 57:1631–1632. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okubo K, Yoshioka S, Asukai K, Hata T,
Nakanishi M, Maekawa T, Hama N, Kashiwazaki M, Taniguchi M, Tsujie
M, et al: A case report of primary adenocarcinoma of small
intestine. Gan To Kagaku Ryoho. 37:2792–2794. 2010.(In Japanese).
PubMed/NCBI
|
|
23
|
Nagaraj G, Zarbalian Y, Flora K and Tan BR
Jr: Complete response and prolonged disease-free survival in a
patient with recurrent duodenal adenocarcinoma treated with
bevacizumab plus FOLFOX6. J Gastrointest Oncol. 5:E1–E6.
2014.PubMed/NCBI
|
|
24
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
National Institutes of health national
cancer institute, . Common Terminology criteria for adverse events
(CTCAE) Version 4.0. U.S. Department of Health and Human Services;
2009
|
|
27
|
Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY,
Bennett CL and Talamonti MS: Small bowel cancer in the United
States: Changes in epidemiology, treatment, and survival over the
last 20 years. Ann Surg. 249:63–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Overman MJ, Pozadzides J, Kopetz S, Wen S,
Abbruzzese JL, Wolff RA and Wang H: Immunophenotype and molecular
characterisation of adenocarcinoma of the small intestine. Br J
Cancer. 102:144–150. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aparicio T, Svrcek M, Zaanan A, Beohou E,
Laforest A, Afchain P, Mitry E, Taieb J, Di Fiore F, Gornet JM, et
al: Small bowel adenocarcinoma phenotyping, a clinicobiological
prognostic study. Br J Cancer. 109:3057–3066. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zaanan A, Gauthier M, Malka D, Locher C,
Gornet JM, Thirot-Bidault A, Tougeron D, Taïeb J, Bonnetain F and
Aparicio T; Association des Gastro Entérologues Oncologues, :
Second-line chemotherapy with fluorouracil, leucovorin, and
irinotecan (FOLFIRI regimen) in patients with advanced small bowel
adenocarcinoma after failure of first-line platinum-based
chemotherapy: A multicenter AGEO study. Cancer. 117:1422–1428.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dvorak HF: Vascular permeability
factor/vascular endothelial growth factor: A critical cytokine in
tumor angiogenesis and a potential target for diagnosis and
therapy. J Clin Oncol. 20:4368–4380. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Willett CG, Boucher Y, di Tomaso E, Duda
DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, et
al: Direct evidence that the VEGF-specific antibody bevacizumab has
antivascular effects in human rectal cancer. Nat Med. 10:145–147.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
van Cutsem E, Köhne CH, Hitre E, Zaluski
J, Chien CR Chang, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G,
et al: Cetuximab and chemotherapy as initial treatment for
metastatic colorectal cancer. N Engl J Med. 360:1408–1417. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
de Dosso S, Molinari F, Martin V, Frattini
M and Saletti P: Molecular characterisation and cetuximab-based
treatment in a patient with refractory small bowel adenocarcinoma.
Gut. 59:1587–1588. 2010. View Article : Google Scholar : PubMed/NCBI
|