Introduction
The development of tyrosine kinase inhibitors (TKIs)
targeting the breakpoint cluster region (BCR)-Abelson kinase 1
(ABL1) oncoprotein has changed the landscape of treatment of
chronic myeloid leukemia (CML). In addition to targeting BCR-ABL1,
TKIs also affect other important signaling pathways including the
c-ABL, cellular mast/stem cell growth factor receptor Kit and
platelet-derived growth factor receptor signaling pathways.
Compared with imatinib, dasatinib, as a second-generation TKI,
inhibits a broader range of tyrosine kinases, including
proto-oncogene tyrosine protein kinase Src (Src), tyrosine protein
kinase Tec, ephrin type-A receptor 1, and other TKIs have important
roles in regulating various cellular functions (1,2). Leukocyte
C-terminal Src kinase (Lck) and tyrosine protein kinase Fyn (two
members of the Src kinase family) are involved in the early steps
of T-cell receptor (TCR) activation. A previous in vitro
study has suggested that Lck is more important in TCR signaling
(3). Therefore, it is not surprising
that TKIs are able to affect immune reconstitution as well as
proliferation, function and activation of T cells.
T lymphocytes are intimately involved in the
pathophysiology of autoimmune diseases, graft-vs. -host disease
(GVHD) and the graft-versus leukemia (GVL) effect. Cluster of
differentiation (CD) 4+CD25+ T cells
(regulatory T cells or Tregs) are a subset of T lymphocytes, which
have a crucial role in homeostasis for peripheral T-cells as well
as the maintenance of immune tolerance, particularly following
allogeneic hematopoietic stem cell transplantation (allo-HSCT)
(4–6).
The modulation of Tregs may be a novel means for treating
autoimmune diseases, including GVHD and GVL, as well as tumors
(7–10). There are two therapeutic options
available to patients with CML, who relapse following allo-HSCT:
Donor lymphocyte infusion and treatment with TKIs (11,12). The
combination of these treatments has yielded contradictory results
in clinical studies (13). An
improved understanding of the effect of TKIs on the biological
characteristics of Tregs is important for the development of
clinical applications. Recent studies have indicated that the
mechanism of suppression performed by Tregs can be divided
primarily into two aspects: i) Cell-cell contact dependent
mechanism; and ii) regulation by secretion of suppressive cytokines
(14). A number of vital surface
molecules are involved in the suppressive function of Tregs,
including forkhead box P3 (FOXP3), cytotoxic
T-lymphocyte-associated antigen 4 (CTLA-4), tumor necrosis factor
receptor (GITR), transforming growth factor (TGF)-β,
latency-associated peptide, CD4-related
lymphocyte-activation-gene-3, galectin-1 and CD39. Furthermore,
Tregs are also able to exhibit an immune suppressive role via the
production of interleukin (IL)-10, TGF-β, IL-4 and other
cytokines.
In vitro studies have demonstrated that
treatment with imatinib, dasatinib and nilotinib have inhibitory
effects on proliferation, suppressive capacity and cytokine
secretion of Tregs from healthy donors (15–17).
However, deficits in our knowledge remain concerning the effects of
imatinib, dasatinib and nilotinib treatment on Tregs in patients
with CML, particularly on the changes in Tregs in vivo and
on functional analysis of Tregs during long-term treatment with
TKIs.
To address these issues, in the present study, the
quantity and function of Tregs in patients with chronic-phase CML
(CML-CP) at the time of diagnosis and during treatment with TKIs
were evaluated.
Patients and methods
Patients
The inclusion criteria for the present study were:
i) Diagnosis of CML-CP, patients undergoing treatment with one type
of TKI (imatinib, dasatinib or nilotinib); ii) patients in the
novel diagnostic-phase and not under treatment of CML-associated
drugs, including hydroxyurea or TKIs; iii) preserved functioning of
major organs (lung, liver, heart and kidney) in patients; iv)
patients not undergoing treatment with immunomodulators; and v)
written informed consent from patients. The exclusion criteria
were: i) Presence of multiple tumors; ii) pregnant women and
juveniles (age <18 years); and iii) exclusion from enrollment at
the discretion of the physician.
The present study was performed in accordance with a
protocol approved by the Ethics Committee of Nanfang Hospital
(Guangzhou, China) according to The Declaration of Helsinki.
Written informed consent was obtained from each participant prior
to sample collection. A total of 108 peripheral blood (PB) samples
were obtained from participants between July 2014 and July 2015.
Samples were taken from patients at the time of diagnosis (n=31),
and at 3 and 6 months while treated with a TKI. TKI-treated
patients with CML were divided into three groups: Imatinib (400
mg/day; n=12), dasatinib (100 mg/day; n=11) and nilotinib (300 mg
twice daily; n=8). All TKIs were administered orally. The doses of
TKIs were decreased according to any side effects or toxicity. A
series of PB samples were taken from healthy volunteers (n=15) to
serve as the control group.
Cell isolation and culture
Peripheral blood mononuclear cells (PBMCs) from
patients and healthy donors were isolated using the Ficoll-Biocoll
separation solution (Biochrom, Ltd., Cambridge, UK) and stored in
liquid nitrogen until further use. CD4+CD25+
T cells and CD4+CD25− T cells were isolated
from total PBMCs using the CD4+CD25+
regulatory T cell isolation kit (Miltenyi Biotec, Inc., Cambridge,
MA, USA), according to the manufacturer's protocol. The cells were
cultured in RPMI-1640 (HyClone; GE Healthcare Life Sciences, Logan,
UT, USA) and supplemented with 10% human AB serum (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), 2 mmol/l L-glutamine
and 100 U/ml penicillin-streptomycin (Invitrogen; Thermo Fisher
Scientific, Inc.). The cells were subsequently incubated at 37°C
with 5% CO2 and at 95% humidity.
Cell proliferation assay
Prior to stimulation, purified
CD4+CD25+ T cells (1×106 cells/ml)
were labeled with vital dye carboxyfluorescein diacetate
succinimidyl ester (CFSE; Invitrogen; Thermo Fisher Scientific,
Inc.). Subsequently, the cells were cultured in 96-well U-bottomed
plates coated with 5 µg/ml anti-CD3 antibody (cat. no. 564001; BD
Biosciences, San Jose, CA, USA), 2 µg/ml soluble anti-CD28 antibody
(cat. no. 556620; BD Biosciences) and 300 U/ml IL-2 (R&D
Systems Inc., Minneapolis, MN, USA) with or without TKI stimulation
[500 nM imatinib (Novartis International AG, Basel, Switzerland),
10 nM dasatinib (Bristol-Myers Squibb, New York, NY, USA) or 10 µM
nilotinib (Novartis International AG, Basel, Switzerland)]. After 4
days, proliferation was analyzed by flow cytometry (MACSQuant
Analyzer 10, Miltenyi Biotec GmbH, Bergisch Gladbach, Germany).
Non-stimulated T cells served as a negative control in all of the
experiments.
Suppression assay
In the suppression assay,
CD4+CD25− T cells (i.e., responder T cells;
1×105) and CD4+CD25+T cells (i.e.,
Tregs; 1×105) were co-cultured in 96-well flat bottom
plates, in triplicate. After 72 h of anti-CD3 and anti-CD28
treatment as previously described, the percentages of CFSE positive
cells were calculated by MACS Quant flow cytometry. Isolated
CD4+CD25− T cells were labeled with CFSE as
aforementioned. CD4+CD25+ T cells were
stimulated with 300 U/ml IL-2 for 24 h. Subsequently, the
CD4+CD25+ T cells were incubated with
CD4+CD25− T cells for 4 days in the presence
of anti-CD3 and anti-CD28 antibodies, as previously described, at a
ratio of 1:1.
Cytokine analysis
Purified CD4+CD25+ T cells
were incubated with anti-CD3 antibody, anti-CD28 antibody and IL-2,
as previously described, for 4 days. On the last day of culture,
the supernatants were collected and stored at −70°C. The levels of
IL-4, IL-10 and TGF-β were measured using ELISA kits (Human IL-4
ELISA kit, cat. no, 550614; human IL-10 ELISA set, cat. no. 555157;
human TGF-β1 ELISA set cat. no. 559119; all from BD Pharmingen, San
Diego, CA, USA), according to the manufacturer's protocol.
Flow cytometric analysis of GITR and FOXP3 in
CD4+CD25+ T cells.
CD4+CD25+ T cells were stimulated with
anti-CD3, anti-CD28 and IL-2, as previously described, with and
without TKI stimulation (500 nM imatinib, 10 nM dasatinib or 10 µM
nilotinib). Following stimulation, the cells were stained with
CD4-phycoerythrin (PE) -cyanine 7 (Cy7; dilution, 1:200; cat. no.
FAB3791P), CD25-allophycocyanin (APC) -Cy7 (dilution, 1:200; cat.
no. FAB1020A), and GITR-PE (dilution, 1:200; cat. no. FAB689P;
R&D Systems, Inc.). Following surface staining, the cells were
fixed in 2% paraformaldehyde at 37°C for 15 min and stained with
FOXP3-PE (dilution, 1:200; cat. no. 12-4776-41; eBioscience; Thermo
Fisher Scientific, Inc.) and CTLA-4-APC (dilution, 1:100; cat. no.
555855; BD Pharmingen), according to the manufacturer's protocol.
Flow cytometric analysis was performed and analyzed using MACS
Quant with CellQuest software.
Statistical analysis
The data are presented as the mean ± standard
deviation. The statistical analysis of the data was performed using
GraphPad Prism software (version 5.0 for Windows; GraphPad
Software, Inc., La Jolla, CA, USA). The statistical significance of
differences in continuous variables was assessed by a
non-parametric analysis of variance using the Kruskal-Wallis test,
and in the case of a significant main effect, pairwise comparisons
of patient groups were calculated using Dunn's multiple comparison
test. P<0.05 (two-sided) was considered to indicate a
statistically significant difference.
Results
Patient characteristics
Between July 2012 and July 2014, 31 patients with
CML-CP were enrolled into the study at the diagnostic phase (n=31;
mean age, 40 years, range, 25–56 years; males/females, 15/16) and
during treatment with a TKI (imatinib, n=12; mean age, 38 years,
range, 25–54 years; males/females, 7/5; total duration of therapy,
9 months, range, 7–12 months; dasatinib, n=11; mean age, 43 years,
range, 28–56 years; males/females, 5/6; total duration of therapy,
9 months, range, 7–14 months; nilotinib, n=8; mean age, 43 years,
range, 28–56 years; males/females, 3/5; total duration of therapy,
9 months, range, 6–12 months). Additionally, 15 healthy donors
(n=15, mean age, 30 years, range, 23–37 years; males/females, 9/6)
were enrolled. The clinical characteristics of the patients are
presented in Table I.
 | Table I.Characteristics of patients and
healthy donors. |
Table I.
Characteristics of patients and
healthy donors.
| Parameter | At diagnosis (no
therapy) | Imatinib treatment
group | Dasatinib treatment
group | Nilotinib treatment
group | Healthy donors |
|---|
| Group size, n | 31 | 12 | 11 | 8 | 25 |
| Age, years |
|
|
|
|
|
|
Median | 40 | 38 | 43 | 43 | 30 |
|
Range | 25–56 | 25–54 | 28–56 | 28–56 | 23–37 |
| Males/females,
n | 15/16 | 7/5 | 5/6 | 3/5 | 9/6 |
| Number of
samples | 31 | 24 | 22 | 16 | 15 |
All patients in the imatinib treatment group (n=12)
were only treated with imatinib. By contrast, 5 of the patients in
the dasatinib treatment group (n=11) were resistant or intolerant
to imatinib and switched to dasatinib. The remaining 6 patients
were only treated with dasatinib. In the nilotinib treatment group
(n=8), 4 patients switched to nilotinib treatment due to resistance
or intolerance to imatinib, and the remaining 4 patients were
treated only with nilotinib. All patients were in complete
cytogenetic remission [as defined by European LeukemiaNet (ELN)
recommendations (18)] at the time of
sampling.
Analysis of lymphocytes in patients
during treatment with TKIs
At the time of diagnosis, the patients from all
treatment groups exhibited a significantly lower median proportion
of lymphocytes compared with healthy controls (8 vs. 29%;
P<0.0001; Fig. 1A). There were no
significant differences in median absolute lymphocyte numbers
between diagnostic-phase patients and healthy controls (Fig. 1B). Patients in the TKI treatment
groups had an increased proportion of lymphocytes compared with
newly diagnosed patients with CML (25,45 and 25 vs. 8%, for
imatinib, dasatinib and nilotinib, respectively, P<0.0001,
Fig. 1A). However, following TKI
treatment, the median lymphocyte counts in the TKI treatment groups
were significantly decreased compared with diagnostic-phase
patients (1.18, 1.48 and 1.15 vs. 2.97×109 cells/l, for
imatinib, dasatinib and nilotinib, respectively, P<0.0001,
Fig. 1B). Additionally, there were no
significant differences between the three TKI treatment groups in
terms of median proportion and absolute numbers of lymphocytes
(Fig. 1A and B).
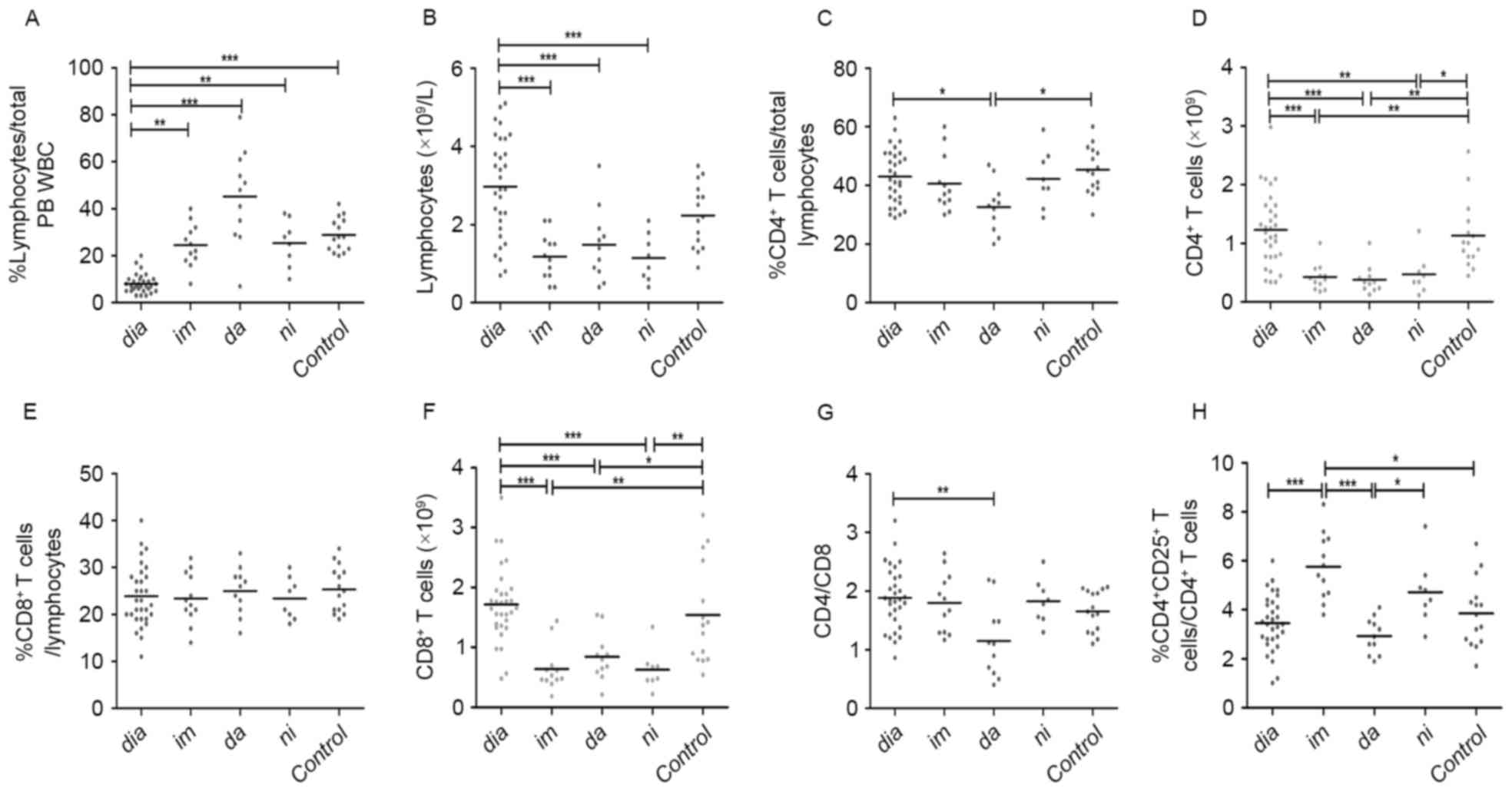 | Figure 1.Analysis of peripheral blood
lymphocytes from patients with CML at diagnosis and during
treatment with tyrosine kinase inhibitors. (A) Percentage and (B)
absolute numbers of lymphocytes. (C) Percentage and (D) absolute
numbers of CD4+ T cells. (E) Percentage and (F) absolute
numbers of CD8+ T cells. (G) CD4/CD8 ratio and (H)
percentage of CD4+CD25+ T cells. Statistical
significance of differences of continuous variables was assessed by
a non-parametric analysis of variance using the Kruskal-Wallis
test. In the case of a significant main effect, pairwise
comparisons of patient groups were calculated using Dunn's multiple
comparison test. *P<0.05. CML, chromic myeloid leukemia; CD,
cluster of differentiation; dia, diagnosis; im, imatinib treatment;
da, dasatinib treatment; ni, nilotinib treatment; Control, control
group; PB, peripheral blood; WBC, white blood cells. *P<0.05,
**P<0.01 and ***P<0.0001. |
Analysis of CD4+ T cells,
CD8+ T cells and CD4/CD8 in patients treated with
TKIs
The (median) proportion of CD4+ T cells
in lymphocytes were comparable among the diagnostic-phase patients,
healthy donors, and imatinib and nilotinib treatment groups
(Fig. 1C). By contrast, the median
proportion of CD4+ T cells in patients in the dasatinib
treatment groups was markedly decreased compared with newly
diagnosed patients or healthy controls (33, 43 and 45%, for
dasatinib, at diagnosis and control, respectively; P=0.0167). The
proportions of CD4+ T cells were similar among the three
TKI treatment groups (Fig. 1C). In
terms of median absolute counts of CD4+ T cells,
patients in the imatinib, nilotinib and dasatinib treatment groups
exhibited significantly decreased CD4+ T cells compared
with diagnostic-phase patients and controls (0.43, 0.38, 0.48, 1.23
and 1.13×109 cells/l, for imatinib, dasatinib,
nilotinib, at diagnosis and control, respectively; P<0.0001;
Fig. 1D). Notably, the (median)
proportions of CD8+ T cells in lymphocytes were similar
among all groups (Fig. 1E). The
median CD8+ T cells counts of the patients in the three
TKI treatment groups were decreased compared with patients at
diagnosis and in the control groups (0.64, 0.84, 0.63, 1.72 and
1.54×109 cells/l, for imatinib, dasatinib, nilotinib, at
diagnosis and control, respectively; P<0.0001; Fig. 1F). With the exception of the decreased
CD4/CD8 ratio of the dasatinib treatment group, the CD4/CD8 ratios
of other groups were similar and close to normal levels [normal
levels, 1.82±0.39 (1.18–2.6); 1.15 vs. 1.89, 1.66, for dasatinib,
at diagnosis and control, respectively; P=0.0197; Fig. 1G].
Analysis of
CD4+CD25+ T cells in CML patients treated
with TKIs
To assess whether imatinib, dasatinib, nilotinib
have different effects on CD4+CD25+ T cells,
the (median) proportions and absolute numbers of
CD4+CD25+ T cells in patients in the TKI
treatment groups were analyzed. The imatinib and nilotinib
treatment groups exhibited increased (median) proportions of
CD4+CD25+ T cells compared with the dasatinib
treatment group, the newly diagnosed group and the control (5.8,
4.7, 2.9, 3.5 and 3.9%, for imatinib, nilotinib, dasatinib, at
diagnosis and control, respectively; P<0.0001; Fig. 1H).
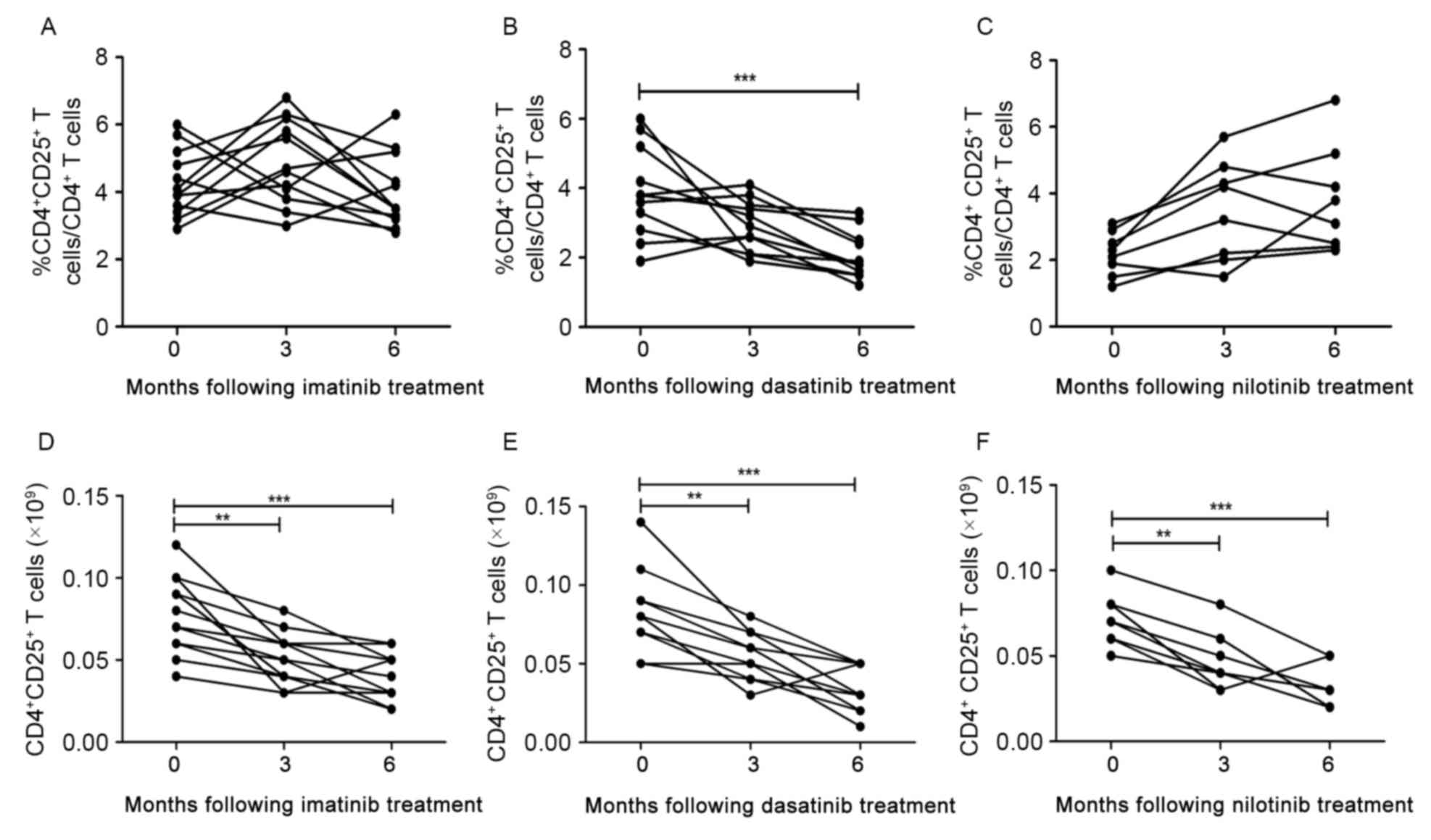
For patients in the imatinib treatment group, the
median proportion of CD4+CD25+ T cells
increased at 3 months following treatment and subsequently
decreased to diagnostic level at 6 months (Fig. 2A). However, for patients in the
dasatinib treatment group, a decrease in the median
CD4+CD25+ T cells proportion was observed at
3 months following treatment, and the decrease was significant at 6
months (3.9 vs. 2.0%, for at diagnosis and 6 months, respectively;
P=0.0009; Fig. 2B). No significant
increase was identified in the median proportion of
CD4+CD25+ T cells in the nilotinib treatment
group between 3 and 6 months following treatment (Fig. 2C).
Furthermore, median absolute numbers of
CD4+CD25+ T cells in the three TKI treatment
groups decreased significantly between 3 and 6 months following
treatment compared with at diagnosis (P<0.0001, P<0.0001 and
P=0.0004, for imatinib, dasatinib and nilotinib, respectively;
Fig. 2D-F).
Proliferation and suppressive capacity
of CD4+CD25+ T cells in patients treated with
TKIs
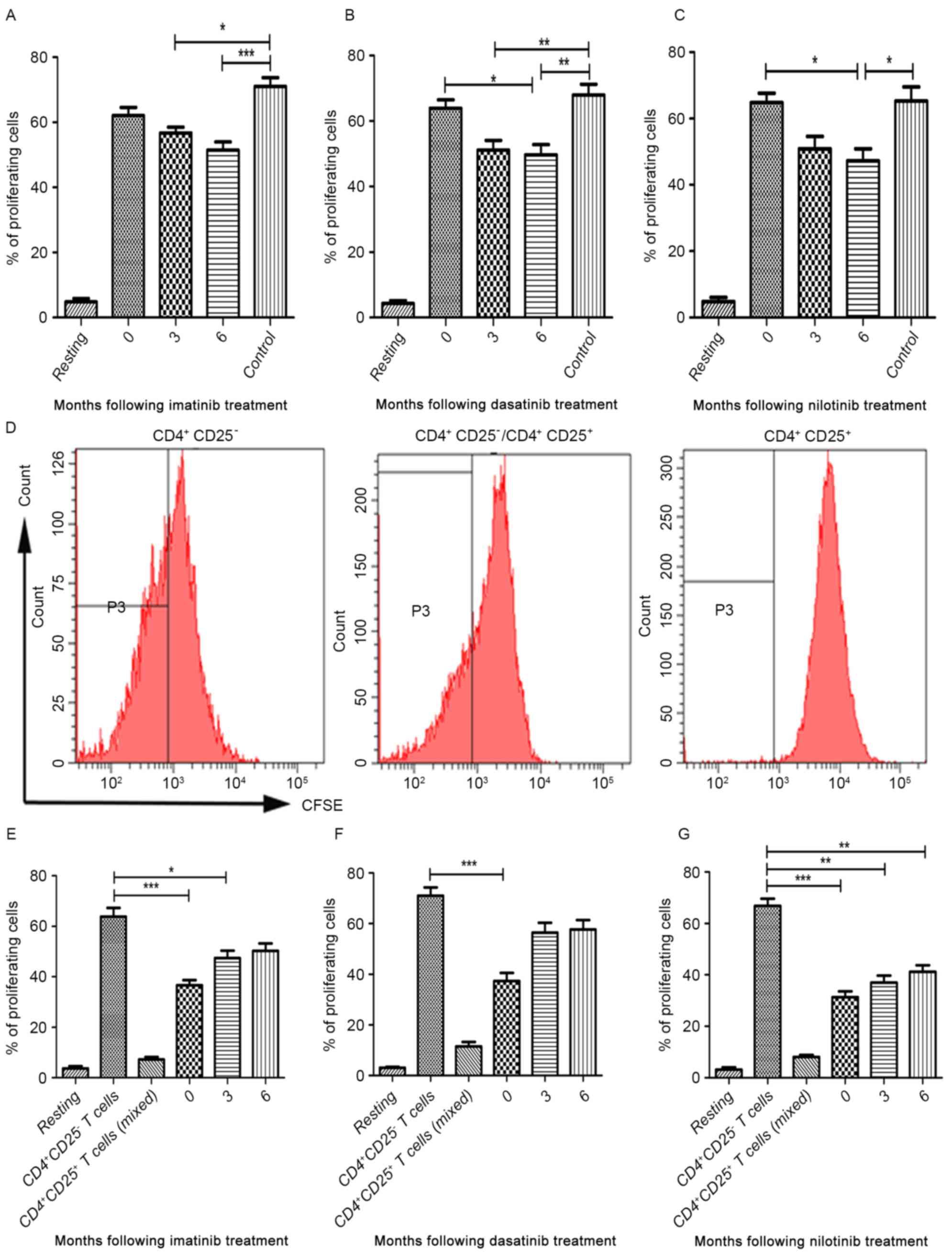
As demonstrated by proliferation assays, the
proportion of proliferating CD4+CD25+ T cells
in the three TKI treatment groups and healthy controls were
increased following stimulation with anti-CD3, anti-CD28 and IL-2
(Fig. 3A-C). No differences were
observed in the median proportion of proliferating
CD4+CD25+ T cells between diagnostic-phase
CML patients and controls (Fig.
3A-C). In the imatinib treatment group, the median proportion
of proliferating CD4+CD25+ T cells at 3 and 6
months following treatment were decreased compared with healthy
donors (P=0.0003; Fig. 3A). However,
no significant differences were identified in the median proportion
of proliferating CD4+CD25+ T cells between
patients in the imatinib treatment group and at the time of
diagnosis (Fig. 3A). In the dasatinib
treatment group, the median proportion of proliferating
CD4+CD25+ T cells at 3 months following
treatment was significantly decreased compared with the control
(P<0.0001). Furthermore, the median proportion of proliferating
CD4+CD25+ T cells at 6 months following
treatment was significantly decreased compared with the
diagnostic-phase group and the control (P=0.0004; Fig. 3B). A similar trend was observed in the
nilotinib treatment group (P=0.0004; Fig.
3C). However, no significant differences were identified in the
median proportion of proliferating CD4+CD25+
T cells between 3 and 6 months following treatment in all three TKI
treatment groups (Fig. 3A-C).
To further investigate the effect of imatinib,
dasatinib and nilotinib treatment on the suppressive capacity of
the CD4+CD25+ T cells,
CD4+CD25+ T cells from patients with CML
treated with TKIs were incubated in the presence of IL-2 prior to
being co-cultured with CD4+CD25− T cells at a
ratio of 1:1. Following incubation, the median proliferation
proportion of CD4+CD25− T cells was analyzed
using flow cytometry (Fig. 3D). In
the imatinib treatment group, imatinib treatment was able to
inhibit the suppressive capacity of the
CD4+CD25+ T cells at 6 months following
treatment (P<0.0001; Fig. 3E). In
dasatinib treatment group, there was a significant inhibitory
effect of dasatinib between 3 and 6 months following treatment
(P=0.0003; Fig. 3F). Notably,
nilotinib did not abrogate the suppressive capacity of
CD4+CD25+ T cells (Fig. 3G).
Analysis of cytokines secreted by
CD4+CD25+ T cells from patients treated with
TKIs
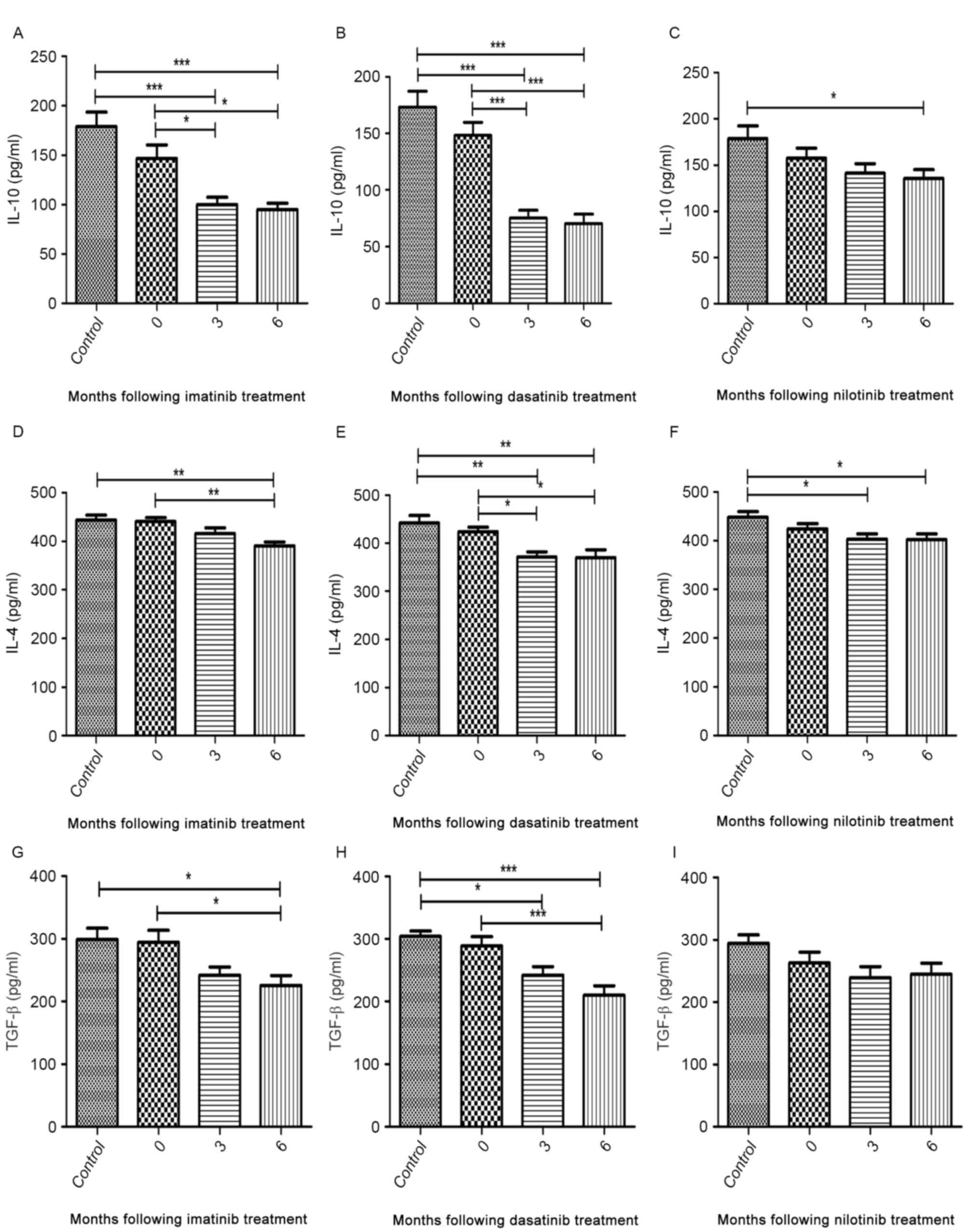
The median concentrations of IL-4, IL-10 and TGF-β
secreted by CD4+CD25+ T cells from
diagnostic-phase patients were comparable with those of healthy
controls (Fig. 4A-I). During imatinib
and dasatinib treatment, the production of cytokines was
significantly inhibited to various degrees (Fig. 4A, B, D, E, G and H). Nilotinib
treatment was able to significantly inhibit the secretion of IL-10
and IL-4 but not that of TGF-β (Fig. 4C,
F and I). Additionally, no significant difference was
identified in the median cytokine concentration detected between 3
and 6 months following treatment (Fig.
4A-I).
Expression of
CD4+CD25+ T cell-specific molecules in
patients treated with TKIs
The median expression levels of FOXP3, GITR and
CTLA-4 in patients at the time of diagnosis were similar to those
of the controls (Fig. 5A-I). In the
imatinib and dasatinib treatment groups, the median expression of
FOXP3, GITR and CTLA-4 decreased between 3 and 6 months following
treatment compared with patients at diagnosis and healthy
volunteers (Fig. 5A, B, D, E, G and
H). In the nilotinib treatment group, the median expression of
GITR and CTLA-4 significantly decreased at 6 months following
treatment compared with diagnostic-phase patients (P<0.0001 and
P=0.004, respectively; Fig. 5F and
I). However, nilotinib did not significantly inhibit the
expression of FOXP3 (Fig. 5C).
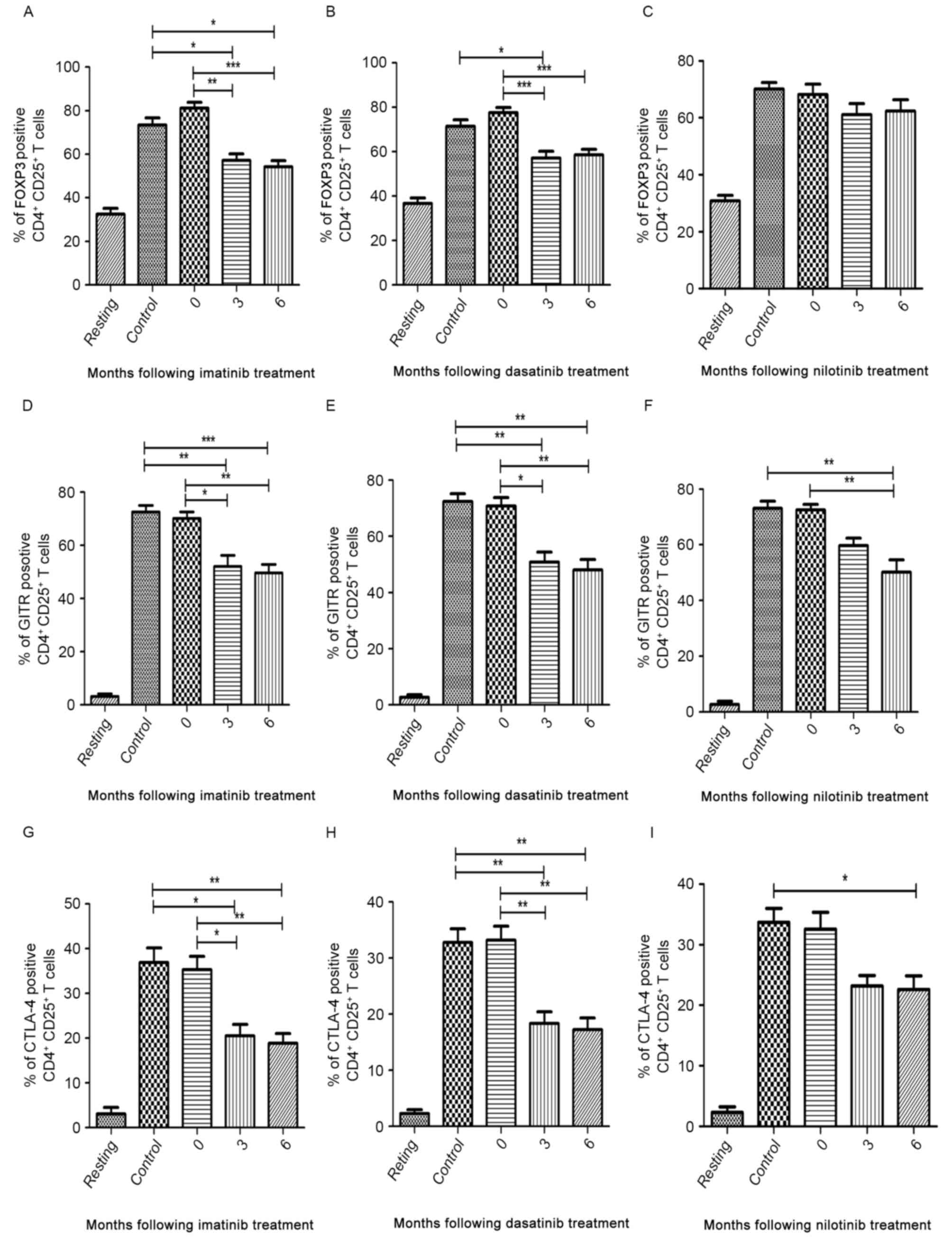 | Figure 5.Flow cytometric analysis of FOXP3,
GITR and CTLA-4 in CD4+CD25+ T cells in three
TKI treatment groups. ‘Resting’ is a negative control without
antibodies. Percentage of FOXP3-positive cells in (A) imatinib, (B)
dasatinib and (C) nilotinib treatment groups. Percentage of
GITR-positive cells in (D) imatinib, (E) dasatinib and (F)
nilotinib treatment groups. Percentage of CTLA-4-positive cells in
(G) imatinib, (H) dasatinib and (I) nilotinib treatment groups.
FOXP3, forkhead box P3; GITR, tumor necrosis factor receptor;
CTLA-4, cytotoxic T-lymphocyte-associated antigen 4; CD, cluster of
differentiation; TKI, tyrosine kinase inhibitor. *P<0.05,
**P<0.01 and ***P<0.0001. |
Discussion
The clinical benefits obtained with TKIs in the
treatment of solid cancers and Philadelphia-positive
(Ph+) CML have led to a new era in oncology, creating
possibilities for advances in tumor-targeted therapies (19). TKIs are usually tolerable to the
patient (relative to other forms of therapy), and therapy
discontinuation due to side effects is uncommon. Although for the
majority of the time TKIs are well-tolerated, they are not entirely
selective for oncogenic kinases (20). Therefore, long-term off-target effects
in normal cells and tissues may occur. Recently, a number of in
vitro studies have investigated the inhibitory effects of TKIs
on immune response. Treatment with imatinib and dasatinib has been
demonstrated to reversibly inhibit T-cell proliferation in
vitro, and the effect of dasatinib treatment is greater than
that of imatinib (21,22). Treatment with dasatinib was able to
inhibit LCK at low concentrations. By contrast, higher
concentrations of imatinib were required to be effective (23). Furthermore, treatment with nilotinib
inhibited T cells approximately twice as markedly as imatinib
(24). Therefore, dasatinib and
nilotinib have been suggested to be potent T cell activation
inhibitors.
In the present study, patients with CML had similar
absolute numbers of lymphocytes compared with healthy donors.
During treatment with TKIs, the absolute number of total T cells,
CD4+ T and CD8+ T cells were decreased
compared with diagnostic-phase patients. Absolute numbers and
proportion of lymphocytes did not differ significantly between TKI
treatment groups. The results of the present study were consistent
with the idea that TKIs have immune suppressive effects, and the
clinical complications induced by these effects require further
study with larger samples and longer follow-up periods.
Tregs constitute a naturally occurring subpopulation
of T cells with immunosuppressive capacity. Consistently, the
results of the present study indicated that imatinib dasatinib and
nilotinib attenuate the quantity of Tregs from patients with CML.
In the present study, a number of patients in the imatinib and
nilotinib treatment groups exhibited increased proportion and
absolute numbers of Tregs compared with those in the dasatinib
treatment group. Previous studies have indicated that increased
numbers of Tregs in tumor tissue are associated with an improved
prognosis (25). TKIs have been
reported to be able to affect the quantity of Tregs and their
function. As an indispensable molecule, which mediates suppression,
FOXP3 is highly expressed in Tregs (26). When FOXP3 genes mutate, the
probability of the occurrence of autoimmune diseases and/or
allergies increases (27,28). In humans,
FOXP3highCD25highCD4+ T cells have
marked suppression, and this is accompanied by high levels of
CTLA-4 expression, which may be promoted by FOXP3 (29). Results from prior experiments have
demonstrated that anti-GITR antibodies may reverse the suppressive
effects of Tregs in vitro and indicated that GITR exerts an
important effect on the function of Tregs (30). Apart from being dependent on
cell-to-cell contact, Tregs also exhibit immunosuppressive activity
by secreting suppressive cytokines, including IL-4, IL-10 and
TGF-β. Inhibition of the IL-10 receptor and the neutralization of
TGF-β are also able to abolish Treg-mediated inhibition of
autoimmune disease (31). Taken
together, cytokines and molecules expressed on the cell surface of
Tregs synergistically contribute to their suppressive functions
(32). Therefore, it is necessary to
detect suppressive cytokines and key suppressive-associated
molecules in order to comprehensively evaluate the effect of TKIs
on functions of Tregs.
In the present study prior to treatment with TKIs,
Tregs from patients with CML-CP have similar levels of
proliferation, suppression, concentrations of suppressive cytokines
(IL-4, IL-10 and TGF-β) and cell-specific molecules (FOXP3, GITR
and CTLA-4) compared with healthy donors. During treatment with
TKIs, cytokines and the expression of cell-specific molecules of
Tregs in the imatinib and dasatinib treatment groups decreased to
different degrees. The inhibitory effect of imatinib and dasatinib
treatment on Tregs was observed for 3 months after treatment. The
results of the present study have demonstrated that the inhibitory
effects of imatinib and dasatinib treatment remain for up to 6
months. By contrast, the inhibitory effect of nilotinib treatment
on Tregs was not significant.
The results of the present study differ from a
previous study, which demonstrated that nilotinib treatment was
more potent compared with imatinib treatment in its inhibition of T
cells (24). The discrepancy may be
due to factors, including, but not limited to, differences in the
treatment dosage and duration of TKIs, individual drug metabolism
and the in vivo environment. The inhibitory effects of
imatinib and dasatinib treatment may impair the immunosuppressive
role of Tregs, which in turn may induce a relative immune
hyper-reactivity. It should be noted that careful follow-up is
warranted in patients with impaired Tregs to prevent the
possibility of autoimmune diseases.
In conclusion, TKIs exhibit different off-target
effects in T lymphocytes, particularly Tregs in patients with
CML-CP. The results of the present study have demonstrated that
imatinib and dasatinib treatments have more marked inhibitory
effects on the number and functions of Tregs compared with
nilotinib treatment. These results indicate that personalized
treatment and follow-up are warranted. The immune system is
relevant for infection control and for the mediation of tumor cell
expansion. In the near future, strategies may be developed for
controlling CML by combining TKIs with adoptive immunotherapy.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81170521) and the
Science Foundation of Nanfang Hospital (grant no. 2012Z013). The
abstract of the present study was presented at the 57th American
Society of Hematology Annual Meeting, 5–8 December 2015, Orlando,
FL, USA and was published as Abstract no. 4036 in Blood (126),
2015.
References
|
1
|
Lombardo LJ, Lee FY, Chen P, Norris D,
Barrish JC, Behnia K, Castaneda S, Cornelius LA, Das J, Doweyko AM,
et al: Discovery of
N-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide
(BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor
activity in preclinical assays. J Med Chem. 47:6658–6661. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rix U, Hantschel O, Dürnberger G, Rix LL
Remsing, Planyavsky M, Fernbach NV, Kaupe I, Bennett KL, Valent P,
Colinge J, et al: Chemical proteomic profiles of the BCR-ABL
inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase
and nonkinase targets. Blood. 110:4055–4063. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Oers NS, Killeen N and Weiss A: Lck
regulates the tyrosine phosphorylation of the T cell receptor
subunits and ZAP-70 in murine thymocytes. J Exp Med. 183:1053–1062.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meignin V, de Latour R Peffault, Zuber J,
Régnault A, Mounier N, Lemaître F, Dastot H, Itzykson R, Devergie
A, Cumano A, et al: Numbers of Foxp3-expressing CD4+CD25high T
cells do not correlate with the establishment of long-term
tolerance after allogeneic stem cell transplantation. Exp Hematol.
33:894–900. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zorn E, Kim HT, Lee SJ, Floyd BH, Litsa D,
Arumugarajah S, Bellucci R, Alyea EP, Antin JH, Soiffer RJ and Ritz
J: Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in
patients with chronic graft-versus-host disease. Blood.
106:2903–2911. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Theil A, Tuve S, Oelschlägel U, Maiwald A,
Döhler D, Oßmann D, Zenkel A, Wilhelm C, Middeke JM, Shayegi N, et
al: Adoptive transfer of allogeneic regulatory T cells into
patients with chronic graft-versus-host disease. Cytotherapy.
17:473–486. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bluestone JA, Trotta E and Xu D: The
therapeutic potential of regulatory T cells for the treatment of
autoimmune disease. Expert Opin Ther Targets. 19:1091–1103. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Demirkiran A, Hendrikx TK, Baan CC and van
der Laan LJ: Impact of immunosuppressive drugs on CD4+CD25+FOXP3+
regulatory T cells: Does in vitro evidence translate to the
clinical setting? Transplantation. 85:783–789. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hoffmann P, Ermann J, Edinger M, Fathman
CG and Strober S: Donor-type CD4(+)CD25(+) regulatory T cells
suppress lethal acute graft-versus-host disease after allogeneic
bone marrow transplantation. J Exp Med. 196:389–399. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Trenado A, Charlotte F, Fisson S, Yagello
M, Klatzmann D, Salomon BL and Cohen JL: Recipient-type specific
CD4+CD25+ regulatory T cells favor immune reconstitution and
control graft-versus-host disease while maintaining
graft-versus-leukemia. J Clin Invest. 112:1688–1696. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Savani BN, Montero A, Kurlander R, Childs
R, Hensel N and Barrett AJ: Imatinib synergizes with donor
lymphocyte infusions to achieve rapid molecular remission of CML
relapsing after allogeneic stem cell transplantation. Bone Marrow
Transplant. 36:1009–1015. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weisser M, Tischer J, Schnittger S, Schoch
C, Ledderose G and Kolb HJ: A comparison of donor lymphocyte
infusions or imatinib mesylate for patients with chronic
myelogenous leukemia who have relapsed after allogeneic stem cell
transplantation. Haematologica. 91:663–666. 2006.PubMed/NCBI
|
|
13
|
Chunduri S, Dobogai LC, Bruno A, Kadkol S
and Rondelli D: Does post-transplant treatment with imatinib
mesylate inhibit graft-versus-leukemia? Leukemia. 19:456–457. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de Rezende LC, Silva IV, Rangel LB and
Guimarães MC: Regulatory T cell as a target for cancer therapy.
Arch Immunol Ther Exp (Warsz). 58:179–190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Larmonier N, Janikashvili N, LaCasse CJ,
Larmonier CB, Cantrell J, Situ E, Lundeen T, Bonnotte B and
Katsanis E: Imatinib mesylate inhibits CD4+ CD25+ regulatory T cell
activity and enhances active immunotherapy against BCR-ABL- tumors.
J Immunol. 181:6955–6963. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fei F, Yu Y, Schmitt A, Rojewski MT, Chen
B, Götz M, Döhner H, Bunjes D and Schmitt M: Dasatinib inhibits the
proliferation and function of CD4+CD25+ regulatory T cells. Br J
Haematol. 144:195–205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fei F, Yu Y, Schmitt A, Rojewski MT, Chen
B, Greiner J, Götz M, Bunjes D and Schmitt M: Effects of nilotinib
on regulatory T cells: The dose matters. Mol Cancer. 9:222010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baccarani M, Deininger MW, Rosti G,
Hochhaus A, Soverini S, Apperley JF, Cervantes F, Clark RE, Cortes
JE, Guilhot F, et al: European LeukemiaNet recommendations for the
management of chronic myeloid leukemia: 2013. Blood. 122:872–884.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shah K, Parikh S and Rawal R: Tyrosine
kinase inhibitors in Ph+ chronic myeloid leukemia therapy: A
review. Asian Pac J Cancer Prev. 17:3025–3033. 2016.PubMed/NCBI
|
|
20
|
Hughes TP, Hochhaus A, Branford S, Müller
MC, Kaeda JS, Foroni L, Druker BJ, Guilhot F, Larson RA, O'Brien
SG, et al: Long-term prognostic significance of early molecular
response to imatinib in newly diagnosed chronic myeloid leukemia:
An analysis from the International Randomized Study of Interferon
and STI571 (IRIS). Blood. 116:3758–3765. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seggewiss R, Loré K, Greiner E, Magnusson
MK, Price DA, Douek DC, Dunbar CE and Wiestner A: Imatinib inhibits
T-cell receptor-mediated T-cell proliferation and activation in a
dose-dependent manner. Blood. 105:2473–2479. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schade AE, Schieven GL, Townsend R,
Jankowska AM, Susulic V, Zhang R, Szpurka H and Maciejewski JP:
Dasatinib, a small-molecule protein tyrosine kinase inhibitor,
inhibits T-cell activation and proliferation. Blood. 111:1366–1377.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Das J, Chen P, Norris D, Padmanabha R,
Linc J, Moquin RV, Shen Z, Cook LS, Doweyko AM, Pitt S, et al:
2-Aminothiazole as a novel kinase inhibitor template.
Structure-activity relationship studies toward the discovery of
N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-
piperazinyl)]-2-methyl-4-pyrimidinyl]amino)]-1,3-thiazole-5-carbox-amide
(dasatinib, BMS-354825) as a potent pan-Src kinase inhibitor. J Med
Chem. 49:6819–6832. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Blake SJ, Lyons AB and Hughes TP:
Nilotinib inhibits the Src-family kinase LCK and T-cell function in
vitro. J Cell Mol Med. 13:599–601. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mustjoki S, Lundán T, Knuutila S and
Porkka K: Appearance of bone marrow lymphocytosis predicts an
optimal response to imatinib therapy in patients with chronic
myeloid leukemia. Leukemia. 21:2363–2368. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hori S, Nomura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1057–1061. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fontenot JD and Rudensky AY: A well
adapted regulatory contrivance: Regulatory T cell development and
the forkhead family transcription factor Foxp3. Nat Immunol.
6:331–337. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Geiger TL and Sharyn T: Nature and nurture
in Foxp3(+) regylatory T cell development, stability, and function.
Hum Immunol. 73:232–239. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miyara M, Yoshioka Y, Kitoh A, Shima T,
Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, et al:
Functional delineation and differentiation dynamics of human CD4+ T
cells expressing the FoxP3 transcription factor. Immunity.
30:899–911. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shimizu J, Yamazaki S, Takahashi T, Ishida
Y and Sakaguchi S: Stimulation of CD25(+)CD4(+) regulatory T cells
through GITR breaks immunological self-tolerance. Nat Immunol.
3:135–142. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Suri-Payer E and Cantor H: Differential
cytokine requirements for regulation of autoimmune gastritis and
colitis by CD4(+)CD25(+) T cells. J Autoimmun. 16:115–123. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen X, Du Y, Lin X, Qian Y, Zhou T and
Huang Z: CD4+CD25+ regulatory T cells in tumor immunity. Int
Immunopharmacol. 34:244–249. 2016. View Article : Google Scholar : PubMed/NCBI
|