Introduction
Epithelial ovarian cancer (EOC) is one of the most
common malignant gynecologic tumors. Debulking surgery followed by
a combination of platinum and taxane based chemotherapy are widely
used treatments for EOC at present. Although overall survival rates
have increased slightly over the past 25 years, 5-year survival
remains <50% (1). The high
mortality rate of ovarian cancer is due to late-stage diagnosis and
resistance to platinum-based chemotherapy. However, the mechanisms
underlying cisplatin resistance in EOC remain to be fully
understood.
MicroRNAs (miRNAs) are small non-coding RNAs of 8–23
nucleotides that post-transcriptionally regulate gene expression.
Multiple previous reports have indicated that dysregulation of
miRNA target genes promotes drug resistance, and inhibition of
miRNAs may reverse drug resistance (2,3). miRNA-21
is overexpressed in multiple types of cancer, and promotes the
initiation of cancer, progression and drug-resistance (4–9). miRNA-21
impacts tumorigenesis by negatively regulating several targets.
Phosphatase and tensin homolog (PTEN) is a tumor suppressor
molecule. Inactivating mutations and deletions of the PTEN gene
have been observed in multiple types of cancer. Notably,
bioinformatics tools have demonstrated that the 3′-untranslated
region of the PTEN gene harbors a putative binding site for
miRNA-21 (10). miRNA-21 expression
has been revealed to be markedly increased in ovarian cancer
compared with benign ovarian tumor tissues (11). miRNA-21 expression was also
demonstrated to be increased in drug-resistant ovarian cancer
compared with drug-sensitive ovarian cancer serum. In the present
study, miRNA-21 mimics, inhibitors and negative control were
transfected in to SKOV3 or SKOV3/DDP cells. The PTEN gene was
hypothesized to be regulated by miRNA-21 in ovarian cancer
cisplatin resistance.
Materials and methods
Cell lines and cell culture
The SKOV3 human ovarian cancer cell line was
purchased from Shanghai Institutes for Biological Sciences, Chinese
Academy of Sciences (Shanghai, China). The SKOV3/DDP human ovarian
cancer cell line was purchased from the Affiliated Hospital of
Qingdao University (Qingdao, China). The cells were cultured in
RPMI-1640 medium supplemented with 10% fetal bovine serum (both
from Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100
U/ml penicillin and 100 µg/ml streptomycin, in a humidified cell
incubator with an atmosphere of 5% CO2 and a temperature
of 37°C. Cells from the exponential growth phase were used for the
following experiments. The treatment groups of cells were as
follows: SKOV3/DDP group; SKOV3 group; the negative control group
(no drug); the blank control group (no cells). Cells were cultured
for 24 h in a humidified cell incubator with an atmosphere of 5%
CO2 and a temperature of 37°C.
A total of 200 µl of 4×104 cells/ml
SKOV3/DDP, SKOV3 or cell-free medium were seeded in a 96-well
plate. Cisplatin (Qilu Pharmaceutical Co., Ltd., Jinan, China) to a
concentration of 0, 3.125, 6.25, 12.5, 25, 50, 100 or 200 µmol/l
was added to each well. The cells were incubated in a humidified
cell incubator with an atmosphere of 5% CO2 and a
temperature of 37°C for 48 h. A total of 20 µl MTT was added to
each well with a concentration of 5 mg/ml. Following a 4-h
incubation at 37°C, the supernatant was discarded using pipettes. A
total of 150 µl DMSO was added to each well.
A microplate reader was used to analyze the
absorbance of each well at an optical density of 490 nm. The cells
inhibitory rate (IR) (%)=[(1-(group value-blank control group
value)/(negative control group value-blank control group
value)]x100%. Subsequently, the half-maximal inhibitory
concentration (IC50) was determined. Resistant factor (RF)=IC50 of
SKOV3 DDP/IC50 of SKOV3 (12).
RNA isolation
Total RNA was extracted and isolated from
2×105 cells/ml using the mirVana miRNA isolation kit
(Ambion; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
quality and quantity of the RNA samples were assessed by standard
electrophoresis and spectrophotometric methods (260/280
absorbance).
Transfection
The miRNA-21 mimics and miRNA-21 inhibitor were
purchased from Shanghai GenePharma Co., Ltd (Shanghai, China).
SKOV3 and SKOV3/DDP cells (2×105 cells/ml) were counted
and seeded onto 6-well plates the day prior to transfection to
ensure 50% cell confluence on the day of transfection. Transfection
of 10 nM miRNA-21 mimics into SKOV3 and transfection of 10 nM
miRNA-21 inhibitors, diluted in medium, into SKOV3/DDP cells were
performed using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) in accordance with the manufacturer's protocol.
Lipofectamine was used alone as a negative control. The miRNA-21
mimics/inhibitors were used at a final concentration of 100 nM.
Reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and western blot analysis were performed 48 h following
transfection.
RT-qPCR analysis
miRNA-21 and PTEN mRNA expression levels were
detected by stem-loop RT-qPCR. RT was performed using SYBR Premix
Ex Taq (Takara Bio, Inc., Otsu, Japan) at 16°C for 30 min, 42°C for
30 min and 85°C for 5 min. qPCR was performed using SYBR Premix Ex
Taq (Takara Bio, Inc.) according to the manufacturer's protocol,
with 1.33 µl cDNA from the RT reaction. The thermocycler conditions
were, an initial 15 min at 95°C, then 40 cycles of 15 sec at 95°C
and 60 sec at 60°C. The primers for miRNA-21 were as follows:
Stem-loop RT,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCAACA-3′; forward,
5′GCCCGCTAGCTTATCAGACTGATG-3′ and reverse, 5′-GTGCAGGGTCCGAGGT-3′.
The primers for U6 were as follows: Stem loop RT,
5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAAATATG-3′;
forward, 5′-GCGCGTCGTGAAGCGTTC-3′ and reverse,
5′-GTGCAGGGTCCGAGGT-3′. GAPDH was used to normalize PTEN mRNA
expression levels. The forward and reverse primer sequences for
PTEN mRNA were as follows: 5′-GAGGGATAAAACACCATG-3′ and
5′-AGGGGTAGGATGTGAACCAGTA-3′, respectively. The forward and reverse
primer sequences for GAPDH were 5′-AACTTTGGCATTGTGGAAGG-3′ and
5′-ACACATTGGGGGTAGGAACA-3′, respectively. All the experiments were
performed in triplicate. The relative expression ratios of miRNA-21
and PTEN mRNA in SKOV3 and SKOV3/DDP cell lines was calculated
using the 2−ΔΔCq method (13).
Western blot analysis
A total of 4×104 cultured cells were
lysed using RIPA buffer (Pierce; Thermo Fisher Scientific, Inc.) in
the presence of a Protease Inhibitor Cocktail (Pierce; Thermo
Fisher Scientific, Inc.). The protein concentration of the lysates
was measured using a BCA Protein Assay kit (Pierce; Thermo Fisher
Scientific, Inc.). Equivalent amounts of protein (0.5 mg/ml) were
resolved and mixed with 5X Lane Marker Reducing Sample Buffer
(Pierce; Thermo Fisher Scientific, Inc.), electrophoresed on 12.5%
SDS-acrylamide gel, and transferred to Immobilon-P transfer
membranes (Merck KGaA, Darmstadt, Germany). The membranes were
blocked with 5% non-fat milk in Tris-buffered saline at 4°C
overnight and then incubated with a rabbit anti-human PTEN
monoclonal antibody (cat. no., ab32199; dilution, 1:400; Abcam,
Cambridge, UK) at 4°C overnight followed by horseradish peroxidase
(HRP)-conjugated secondary antibody (cat. no., ab151499; dilution,
1:100; Abcam) at room temperature for 1 h. Signals were detected
using Immobilon western chemiluminescent HRP substrate (Merck
KGaA). β-actin (cat. no., ab8227; dilution, 1:100; Abcam) served as
the loading control.
Statistical analysis
Independent and paired t-tests were used to compare
the data. All analyses were performed using SPSS19.0 software (IBM
SPSS, Armonk, NY, USA) and all tests were two-tailed. P<0.05 was
considered to indicate a statistically significant difference.
Results
miRNA-21 regulates the sensitivity of
SKOV3 and SKOV3/DDP cells to cisplatin
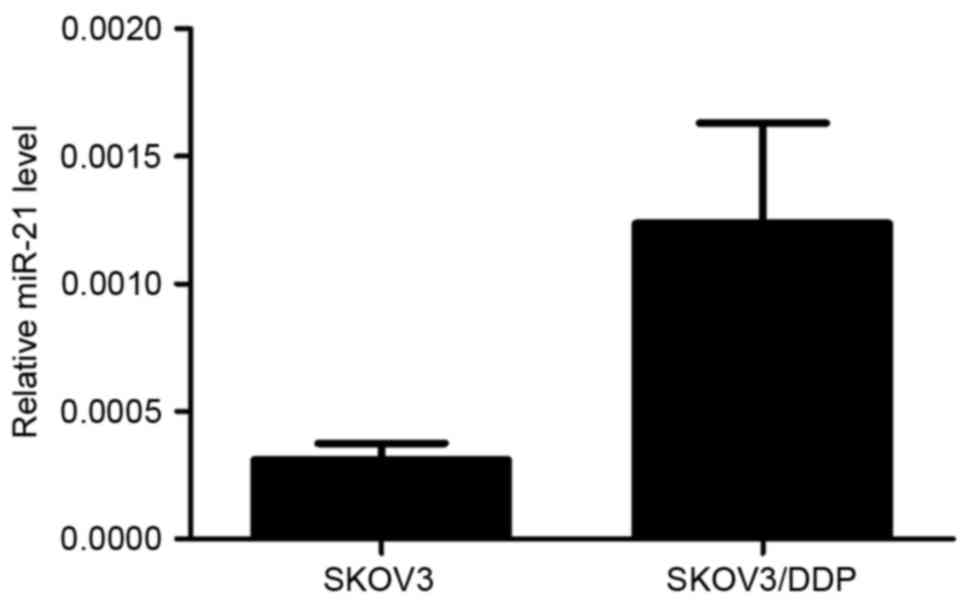
The RT-qPCR results revealed that SKOV3/DDP cells
had a higher endogenous miRNA-21 expression level than SKOV3 cells
(P<0.05; Fig. 1). The
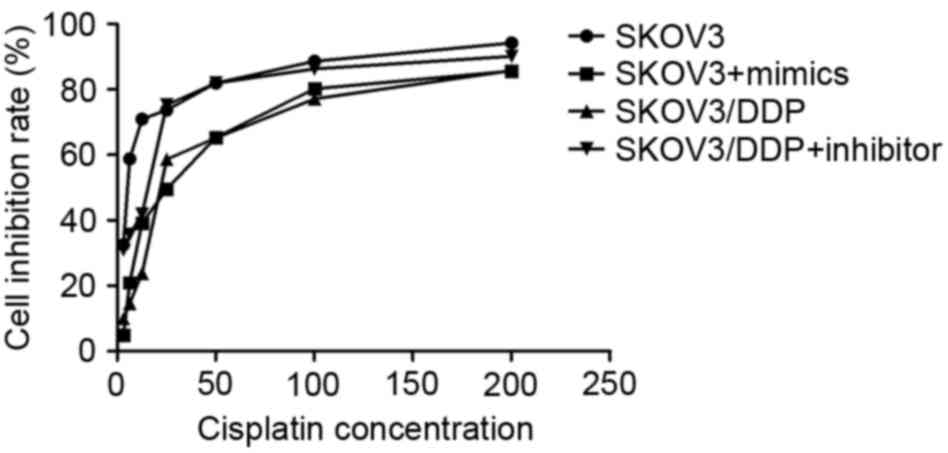
IC50 of control SKOV3 cells was 5.205 µmol/l cisplatin,
of SKOV3 cells with the miRNA-21 mimic, 25.763 µmol/l, of control
SKOV3/DDP cells, 21.914 µmol/l, and of SKOV3/DDP cells with the
inhibitor, 15.524 µmol/l. Thus, the relative cisplatin resistance
index of SKOV3 cells transfected with the miRNA-21 mimic was 4.9
compared to the control cells, of the SKOV3/DDP cells compared with
SKOV3/DDP cells with the inhibitor, 0.7, and of the control
SKOV3/DDP cells relative to the control SKOV3 cells, 4.2. The
sensitivity of SKOV3 cells transfected with miRNA-21 mimics to
cisplatin was significantly increased compared with the negative
control cells (P<0.05; Fig. 2). In
addition, SKOV3/DDP cells transfected with miRNA-21 inhibitors were
significantly less resistant to cisplatin compared with that of the
negative control cells (19.0; P<0.05; Fig. 2).
miRNA-21 downregulates expression of
PTEN mRNA and protein in SKOV3 cells
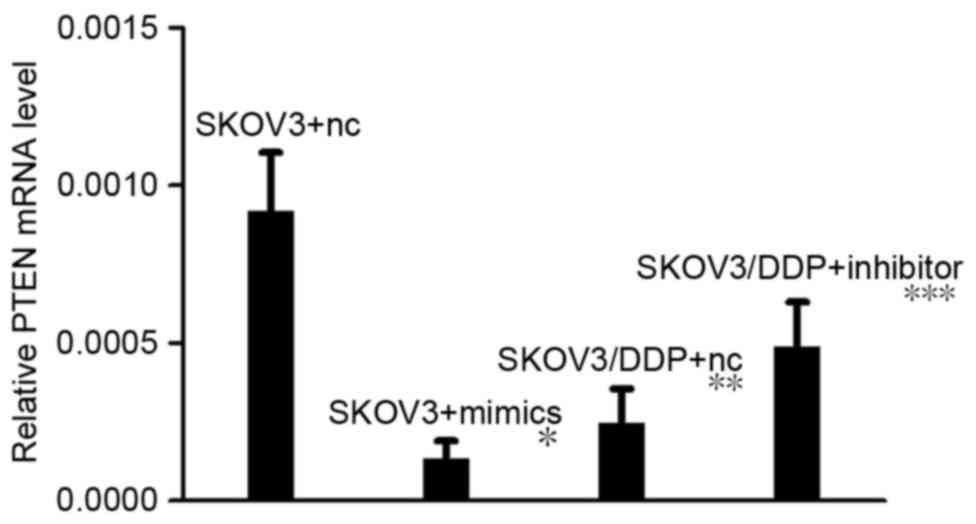
RT-qPCR revealed that, following transfection with
miRNA-21 mimics, PTEN mRNA expression levels in the SKOV3 cell line
were decreased compared with negative control cells (P<0.05;
Fig. 3). When the SKOV3/DDP cell line
was transfected with miR-21 inhibitors, PTEN mRNA expression levels
were not significantly altered. When miR-21 was transfected into
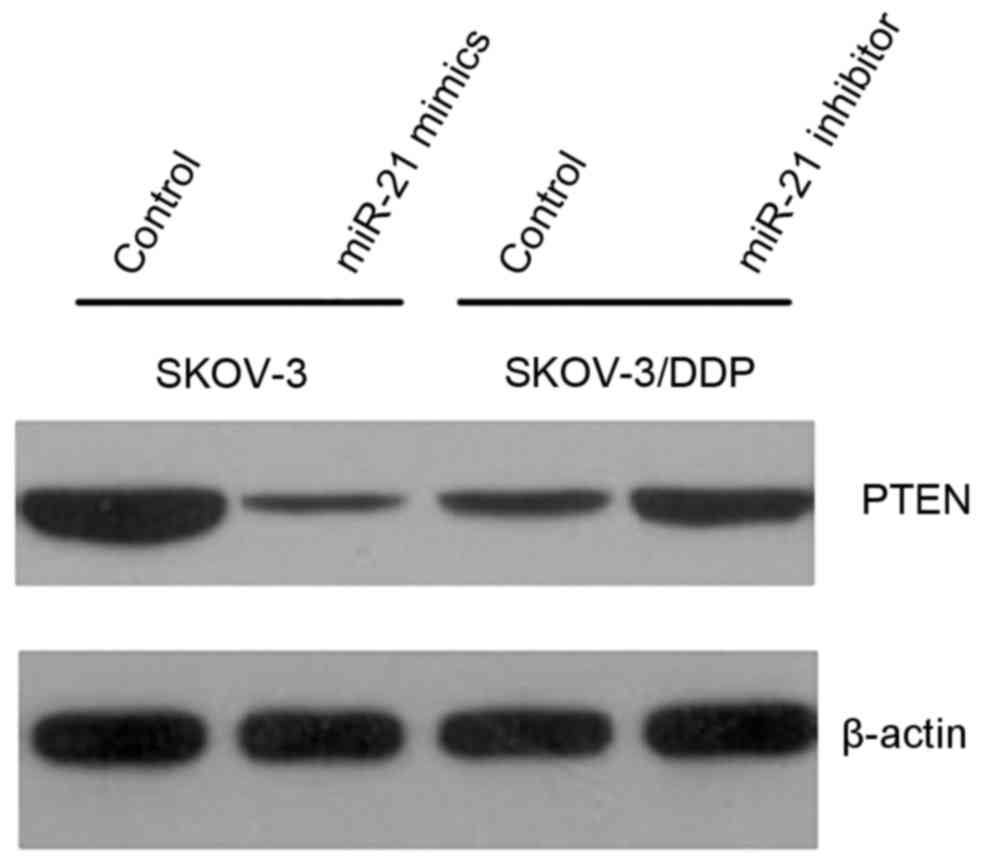
SKOV3 cells, western blot analysis revealed that PTEN protein
levels were visibly lower. It was also demonstrated that when
miR-21 was transfected into SKOV3/DPP cells line, PTEN protein
levels were visibly increased (Fig.
4.).
Discussion
In the present study, the drug resistance index of
SKOV3/DDP cells relative to SKOV3 cells was demonstrated to be 4.2
using the MTT assay. Thus, the present study selected the
appropriate cells for experimental study. Although platinum-based
chemotherapy has improved the prognosis of ovarian cancer,
drug-resistance remains the main obstacle to successful treatment.
The present study revealed that multiple miRNAs participate in
ovarian cancer drug-resistance, including miRNA-130a and miRNA-374a
(14). miRNA-152 and miRNA-185 were
demonstrated to be significantly downregulated in SKOV3/DDP and
A2780/DDP cells (14). Previous
studies have demonstrated that miRNA-21 is involved in drug
resistance in multiple types of cancer, including gastric, breast
and lung cancer, via regulation of PTEN (7,8,15). In the present study, the expression
level of miRNA-21 in SKOV3/DDP cells was significantly higher than
in SKOV3 cells, as detected by qPCR. It was demonstrated that
miR-21 may participate in ovarian cancer drug-resistance. In the
present study, miRNA-21 mimics were transfected into SKOV3 cells,
and miRNA-21 inhibitors were transfected into SKOV3/DDP cells.
Following transfection, the upregulation of miRNA-21 in SKOV3 cells
transfected with the miRNA-21 mimics resulted in an increased
cisplatin drug resistance. The downregulation of miRNA-21 in
SKOV3/DDP cells transfected with the miRNA-21 inhibitor resulted in
decreased cisplatin drug resistance. Thus, the present study
further demonstrated miRNA-21 participated in cisplatin resistance
in EOC. PTEN mRNA expression levels in SKOV3 cells transfected with
the miRNA-21 mimics was significantly decreased compared with
negative control cells (cells treated with Lipofectamine alone).
However, PTEN mRNA expression levels in SKOV3/DDP cells transfected
with the miRNA-21 inhibitors revealed no significant increase. The
effect of miR-21 regulated the expression of PTEN mRNA was not
clear. The upregulation of miRNA-21 in SKOV3 cells was concurrent
with the downregulation of PTEN protein in these cells. The
downregulation of miRNA-21 in SKOV3/DDP cells was also concurrent
with the upregulation of PTEN protein in these cells. The present
study demonstrated that miRNA-21 may have regulated cisplatin
resistance by negatively targeting PTEN protein in EOC.
In conclusion, the present study demonstrated that
miRNA-21 may have participated in cisplatin resistance in EOC.
Furthermore, miRNA-21 may have regulated cisplatin resistance by
negatively targeting PTEN protein in EOC. Future study should
additionally consider cells with a higher resistance factor in
order to further the study of cisplatin resistance. The association
between the PTEN/PI3K/Akt signaling pathway, miRNA-21 and cisplatin
resistance also requires further study.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81101973/H1621).
References
|
1
|
Vargas-Hernández VM, Moreno-Eutimio MA,
Acosta-Altamirano G and Vargas-Aguilar VM: Management of recurrent
epithelial ovarian cancer. Gland Surg. 3:198–202. 2014.PubMed/NCBI
|
|
2
|
Chen PS, Su JL and Hung MC: Dysregulation
of microRNAs in cancer. J Biomed Sci. 19:902012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Garofalo M and Croce CM: MicroRNAs as
therapeutic targets in chemoresistance. Drug Resist Updat.
16:47–59. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Song WF, Wang L, Huang WY, Cai X, Cui JJ
and Wang LW: MiR-21 upregulation induced by promoter zone histone
acetylation is associated with chemoresistance to gemcitabine and
enhanced malignancy of pancreatic cancer cells. Asian Pac J Cancer
Prev. 14:7529–7536. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hong L, Han Y, Zhang Y, Zhang H, Zhao Q,
Wu K and Fan D: MicroRNA-21: A therapeutic target for reversing
drug resistance in cancer. Expert Opin Ther Targets. 17:1073–1080.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang ZX, Lu BB, Wang H, Cheng ZX and Yin
YM: MicroRNA-21 modulates chemosensitivity of breast cancer cells
to doxorubicin by targeting PTEN. Arch Med Res. 42:281–290. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang SM, Huang C, Li XF, Yu MZ, He Y and
Li J: miR-21 confers cisplatin resistance in gastric cancer cells
by regulating PTEN. Toxicology. 306:162–168. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Komatsu S, Ichikawa D, Kawaguchi T,
Miyamae M, Okajima W, Ohashi T, Imamura T, Kiuchi J, Konishi H,
Shiozaki A, et al: Circulating miR-21 as an independent predictive
biomarker for chemoresistance in esophageal squamous cell
carcinoma. Am J Cancer Res. 6:1511–1523. 2016.PubMed/NCBI
|
|
9
|
Shi GH, Ye DW, Yao XD, Zhang SL, Dai B,
Zhang HL, Shen YJ, Zhu Y, Zhu YP, Xiao WJ and Ma CG: Involvement of
microRNA-21 in mediating chemo-resistance to docetaxel in
androgen-independent prostate cancer PC3 cells. Acta Pharmacol Sin.
31:867–873. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Milella M, Falcone I, Conciatori F, Incani
U Cesta, Del Curatolo A, Inzerilli N, Nuzzo CM, Vaccaro V, Vari S,
Cognetti F and Ciuffreda L: PTEN: Multiple Functions in Human
Malignant Tumors. Front Oncol. 5:242015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lou Y, Yang X, Wang F, Cui Z and Huang Y:
MicroRNA-21 promotes the cell proliferation, invasion and migration
abilities in ovarian epithelial carcinomas through inhibiting the
expression of PTEN protein. Int J Mol Med. 26:819–827. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li N, Yang L, Wang H, Yi T, Jia X, Chen C
and Xu P: MiR-130a and MiR-374a Function as novel regulators of
cisplatin resistance in human ovarian cancer A2780 Cells. PLoS One.
10:e01288862015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu ZL, Wang H, Liu J and Wang ZX:
MicroRNA-21 (miR-21) expression promotes growth, metastasis, and
chemo- or radioresistance in non-small cell lung cancer cells by
targeting PTEN. Mol Cell Biochem. 372:35–45. 2013. View Article : Google Scholar : PubMed/NCBI
|