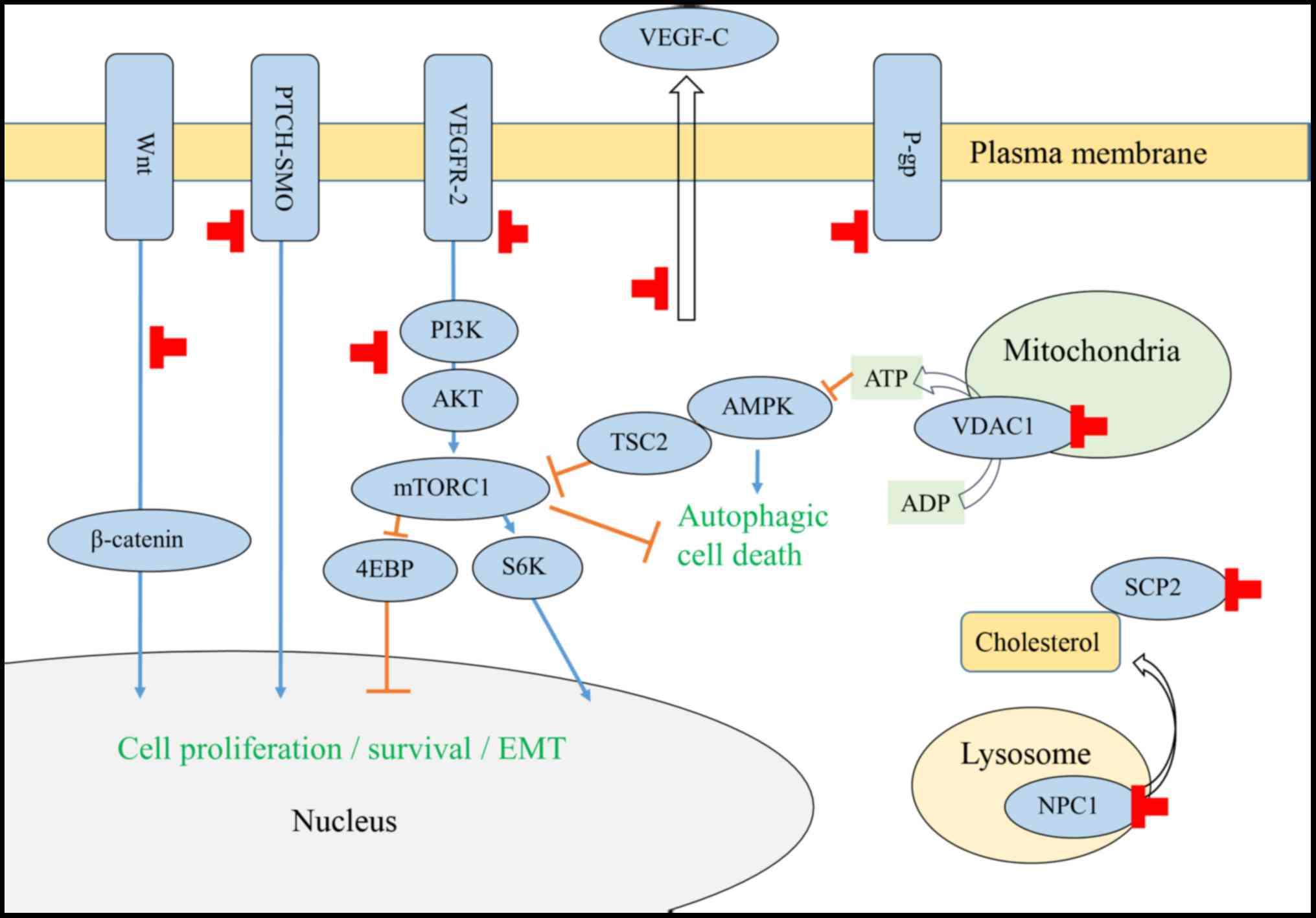
Certain chemotherapeutic drugs induce expression of
the drug efflux protein P-glycoprotein (P-gp), also known as
multi-drug resistance 1 or ATP-binding cassette (ABC) transporter
B1 (ABCB1). In the 1990s, itraconazole was demonstrated to reverse
chemoresistance in cancer cells overexpressing P-gp (Fig. 1; Table
I) (4–6). In addition, the human breast cancer
resistance protein is also inhibited by itraconazole (7).
A screen of US Food and Drug Administration
(FDA)-approved drugs identified itraconazole as an anti-angiogenic
agent in 2007 and as an inhibitor of Hedgehog signaling in 2010
(8,9).
Itraconazole inhibits AKT (protein kinase B)/mechanistic target of
rapamycin (mTOR) signaling in human umbilical vein endothelial
cells (HUVECs), glioblastoma, endometrial carcinoma (EC) and
melanoma cells (10–14). Inhibition of Hedgehog signaling was
observed in basal cell carcinoma, medulloblastoma, pleural
mesothelioma, breast cancer and melanoma cells (9,14–17), but not in EC cells (13). Inhibition of Wnt/β-catenin signaling
was observed in basal cell and examined in melanoma cells (14). Itraconazole also induced autophagic
cell death in medulloblastoma cells as well as in breast cancer
cells (12,17), and suppressed lymphangiogenesis in
lung carcinoma cells (18).
In HUVECs, itraconazole induced the accumulation of
immature N-glycans on VEGFR2, which in turn inhibited
autophosphorylation and downstream activation (19); itraconazole also exhibited synergistic
effects with bevacizumab, a humanized monoclonal antibody against
VEGF (20). Additionally,
hypoglycosylation of the epidermal growth factor receptor was
observed in renal cell carcinoma cells (19).
Itraconazole directly binds to the mitochondrial
protein voltage-dependent anion channel 1 (VDAC1) and interferes
with mitochondrial ATP production, leading to the activation of the
AMP-activated protein kinase pathway and the subsequent inhibition
of mTOR activity (11).
The tumor microenvironment serves a key role in the
cell proliferation, invasion and metastasis in cancer (28); however, the exact underlying
mechanisms of cancer-stromal interactions are poorly understood.
Cancer-associated fibroblasts (CAFs) are essential for tumor growth
(29). Itraconazole inhibited the
proliferation of CAFs established from human colon cancer cells, as
well as the secretion of monocyte chemoattractant protein-1
(20). Monocyte/macrophage marker
CD14 is a glycosylphosphatidylinositol-anchored glycoprotein
present in cholesterol-rich lipid rafts, which contain a variety of
signaling proteins and receptors (30,31). In
mouse macrophages, itraconazole treatment altered the
N-glycosylation of CD14, and increased CD14 transcription and
protein expression (32).
In a randomized trial of leukemia, anti-fungal
prophylactic treatment with itraconazole was proven to be effective
and safe in patients receiving remission induction therapy,
including daunorubicin (33). Based
on preclinical data detailing the reversal of daunorubicin
resistance by itraconazole (34), a
sub-analysis of itraconazole anticancer activity was conducted in
27 patients with acute lymphoblastic leukemia (35), and itraconazole treatment was likely
to be associated with improved disease-free survival (Table II). The results of the clinical trial
(35), as well as preclinical data on
itraconazole reversing the resistance of taxane-resistant cancer
cells (5,6), supported the treatment of refractory
solid tumors with taxane-based chemotherapy in combination with
itraconazole. A prior retrospective study demonstrated that overall
survival (OS) was prolonged in 19 patients with refractory ovarian
cancer, who had been treated with taxane-based chemotherapy with
itraconazole (36). Additional
retrospective studies supported the survival advantage of
itraconazole treatment in refractory malignancies including ovarian
clear cell, triple-negative breast, pancreatic and biliary tract
cancer, as compared with the previous reports (37–40). In
pancreatic cancer, itraconazole treatment combined with
chemotherapy was conducted in progressive disease during
chemotherapy (39). A total of 38
patients received docetaxel (35 mg/m2), gemcitabine
(1,000 mg/m2) and carboplatin (4 mg/min/ml) in
combination with itraconazole (400 mg), following which a median OS
of 11.4 months was observed. In addition, 28 patients with biliary
tract cancer received itraconazole, and subsequently experienced a
median OS of 12 months (40).
With the aim of enhancing the therapeutic efficacy
of anticancer drugs, P-gp inhibitors were investigated in a
clinical trial (41) that reported
unsatisfactory outcomes. The phase III study was conducted in the
ovarian cancer patients, in whom the paclitaxel dose was reduced
from 175 mg/m2 in control patients to 80
mg/m2 with valspodar (5 mg/kg every 6 h for 12 doses)
for patients undergoing the combination therpay (42). The addition of valspodar to standard
chemotherapy regimens did not significantly improve
progression-free survival (PFS) or OS, but increased the frequency
of adverse events experienced. Therefore, the survival advantage
conveyed by combination chemotherapy with itraconazole among
patients with various types of cancer could not be explained by
P-gp inhibition alone. Repurposing itraconazole for the targeting
of angiogenesis has been examined since 2009. In a randomized phase
II clinical trial of non-small cell lung cancer (43), 23 patients were enrolled in the
second-line setting. Of these, 15 patients who were treated with
pemetrexed (500 mg/m2, repeated every 21 days) and oral
itraconazole (200 mg, daily) exhibited a prolonged OS time, as
compared with the 8 patients who were treated with pemetrexed
alone. A meta-analysis of randomized trials demonstrated that the
VEGF inhibitor bevacizumab prolonged OS in colorectal, non-small
cell lung and cervical cancer, but not in breast or ovarian cancer
(44). Phase III trials of the VEGFR
inhibitor ramucirumab reported prolonged OS in non-small cell lung,
gastric and colorectal cancer, (45–48).
Considering the results of the clinical trials using P-gp inhibitor
or antiangiogenic agents (41,43–47),
the clinical efficacy of itracoanzole treatment in various types of
cancer (Table II) implicated the
additional anticancer activities, which was demonstrated in
preclinical studies (Table I).
In a randomized phase II clinical trial of
metastatic castration-resistant prostate cancer (49), 46 chemotherapy-naïve patients were
enrolled, of whom 29 received high-dose (600 mg/day) and 17
received low-dose (200 mg/day) itraconazole treatment.
Prostate-specific antigen PFS rates at 24 weeks were 48.0 and 11.8%
with median PFS of 11.9 and 35.9 weeks in the high- and low-dose
arm, respectively. Plasma VEGF levels remained unchanged following
itraconazole treatment in both arms, whereas the down-modulation of
GLI1 was significantly correlated with the decline of PSA.
Basal cell carcinoma, the most common type of skin
cancer, is associated with upregulated Hedgehog signaling, and two
Hedgehog inhibitors, vismodegib and sonidegib, which target
Smoothened have been approved by the FDA for treatment of basal
cell carcinoma (50). In a recent
study conducted on 29 patients with basal cell carcinoma (19
treated with itraconazole) (51), it
was observed that the tumor area decreased by an average of 24% in
8 of the itraconazole-treated patients with accessible lesions.
Among the vismodegib-naïve patients (n=8), the transcription of
GLI1 and Ki-67 activity was significantly decreased after
itraconazole treatment (51).
Following exposure to cytotoxic agents, the residual
tumors typically harbor cancer stem cells (CSCs) or develop
stemness (52). The concept of CSCs
was hypothesized to explain metastasis and recurrence following
exposure to chemotherapy (53); CSCs
are characterized by self-renewal, multi-differentiation and
chemoresistance. Additional potential mechanisms underlying
chemotherapy resistance may include dormant cell cycles, multidrug
resistance transporters and protection by niche cells. The current
focus is on the development of CSC-targeted therapy for preventing
cancer relapse and improving survival rates (54). Aberrant signaling pathways, including
AKT/mTOR, Hedgehog, and Wnt, have been reported in CSCs and
multi-targeting therapies have been proposed (54). The first-in-class cancer stemness
inhibitor napabucasin (BBI 608), which targets signal transducer
and activator of transcription 3 (Stat3), Nanog, and Wnt/β-catenin
pathways, has been reported to improve OS in patients with positive
phospho-STAT3 recurrent colorectal cancer (55,56).
Itraconazole may be a promising agent for targeting CSCs in
relapsed disease of multiple types of cancer; therefore, further
preclinical studies on CSCs and the surrounding stroma cells are
warranted.
Ongoing clinical trials with itraconazole (as an
anticancer agent) were identified from ClincalTrials.gov (https://clinicaltrials.gov/ct2/home) and UMIN-CTR
Search Clinical Trials (http://www.umin.ac.jp/ctr/index.htm), as well as
Google search (Table III). No
ongoing clinical trials were registered at the EU Clinical Trial
Register (https://www.clinicaltrialsregister.eu/ctr-search/search).
Obtaining cancer tissues and blood from patients prior to and
following itraconazole treatment is essential for exploring and
characterizing novel targets in the tumor and the microenvironment,
as well as for identifying biomarkers predictive of patient
response for future enrichment clinical trials.
|
1
|
Siddiqui M and Rajkumar SV: The high cost
of cancer drugs and what we can do about it. Mayo Clin Proc. 87:pp.
935–943. 2012; View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pantziarka P, Bouche G, Meheus L, Sukhatme
V, Sukhatme VP and Vikas P: The repurposing drugs in oncology
(ReDO) project. Ecancermedicalscience. 8:4422014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pantziarka P, Sukhatme V, Bouche G, Meheus
L and Sukhatme VP: Repurposing drugs in oncology
(ReDO)-itraconazole as an anti-cancer agent. Ecancermedicalscience.
9:5212015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kurosawa M, Okabe M, Hara N, Kawamura K,
Suzuki S, Sakurada K and Asaka M: Reversal effect of itraconazole
on adriamycin and etoposide resistance in human leukemia cells. Ann
Hematol. 72:17–21. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takara K, Tanigawara Y, Komada F,
Nishiguchi K, Sakaeda T and Okumura K: Cellular pharmacokinetic
aspects of reversal effect of itraconazole on
P-glycoprotein-mediated resistance of anticancer drugs. Biol Pharm
Bull. 22:1355–1359. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shirakawa K, Takara K, Tanigawara Y,
Aoyama N, Kasuga M, Komada F, Sakaeda T and Okumura K: Interaction
of docetaxel (‘Taxotere’) with human P-glycoprotein. Jpn J Cancer
Res. 90:1380–1386. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gupta A, Unadkat JD and Mao Q:
Interactions of azole antifungal agents with the human breast
cancer resistance protein (BCRP). J Pharm Sci. 96:3226–3235. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chong CR, Xu J, Lu J, Bhat S, Sullivan DJ
Jr and Liu JO: Inhibition of angiogenesis by the antifungal drug
itraconazole. ACS Chem Biol. 2:263–270. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim J, Tang JY, Gong R, Kim J, Lee JJ,
Clemons KV, Chong CR, Chang KS, Fereshteh M, Gardner D, et al:
Itraconazole, a commonly used antifungal that inhibits Hedgehog
pathway activity and cancer growth. Cancer Cell. 17:388–399. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu J, Dang Y, Ren YR and Liu JO:
Cholesterol trafficking is required for mTOR activation in
endothelial cells. Proc Natl Acad Sci USA. 107:pp. 4764–4769. 2010;
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Head SA, Shi W, Zhao L, Gorshkov K,
Pasunooti K, Chen Y, Deng Z, Li RJ, Shim JS, Tan W, et al:
Antifungal drug itraconazole targets VDAC1 to modulate the
AMPK/mTOR signaling axis in endothelial cells. Proc Natl Acad Sci
USA. 112:pp. E7276–E7285. 2015; View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu R, Li J, Zhang T, Zou L, Chen Y, Wang
K, Lei Y, Yuan K, Li Y, Lan J, et al: Itraconazole suppresses the
growth of glioblastoma through induction of autophagy: Involvement
of abnormal cholesterol trafficking. Autophagy. 10:1241–1255. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsubamoto H, Inoue K, Sakata K, Ueda T,
Takeyama R, Shibahara H and Sonoda T: Itraconazole inhibits
Akt/mTOR signalling and proliferation in endometrial cancer cells.
Anticancer Res. 37:515–519. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liang G, Liu M, Wang Q, Shen Y, Mei H, Li
D and Liu W: Itraconazole exerts its anti-melanoma effect by
suppressing Hedgehog, Wnt, and PI3K/mTOR signaling pathways.
Oncotarget. 8:28510–28525. 2017.PubMed/NCBI
|
|
15
|
Kim J, Aftab BT, Tang JY, Kim D, Lee AH,
Rezaee M, Kim J, Chen B, King EM, Borodovsky A, et al: Itraconazole
and arsenic trioxide inhibit Hedgehog pathway activation and tumor
growth associated with acquired resistance to smoothened
antagonists. Cancer Cell. 23:23–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
You M, Varona-Santos J, Singh S, Robbins
DJ, Savaraj N and Nguyen DM: Targeting of the Hedgehog signal
transduction pathway suppresses survival of malignant pleural
mesothelioma cells in vitro. J Thorac Cardiovasc Surg. 147:508–516.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X, Wei S, Zhao Y, Shi C, Liu P, Zhang
C, Lei Y, Zhang B, Bai B, Huang Y and Zhang H: Anti-proliferation
of breast cancer cells with itraconazole: Hedgehog pathway
inhibition induces apoptosis and autophagic cell death. Cancer
Lett. 385:128–136. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y, Yao Y, Liu H, Ma X, Lv T, Yuan D,
Xiao X, Yin J and Song Y: Itraconazole can inhibit malignant
pleural effusion by suppressing lymphangiogenesis in mice. Trans
Lung Cancer Res. 4:27–35. 2015.
|
|
19
|
Nacev BA, Grassi P, Dell A, Haslam SM and
Liu JO: The antifungal drug itraconazole inhibits vascular
endothelial growth factor receptor 2 (VEGFR2) glycosylation,
trafficking, and signaling in endothelial cells. J Biol Chem.
286:44045–44056. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hara M, Nagasaki T, Shiga K and Takeyama
H: Suppression of cancer-associated fibroblasts and endothelial
cells by itraconazole in bevacizumab-resistant gastrointestinal
cancer. Anticancer Res. 36:169–177. 2016.PubMed/NCBI
|
|
21
|
White CP: On the occurrence of crystals in
tumours. J Pathol Bacteriol. 13:3–10. 1909. View Article : Google Scholar
|
|
22
|
Kuwano M, Kamiya T, Endo H and Komiyama S:
Potentiation of 5-fluorouracil, chromomycin A3, and bleomycin by
amphotericin B or polymyxin B in transformed fibroblastic cells.
Antimicrob Agents Chemother. 3:580–584. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ikezaki K, Akiyama S, Miyazaki C, Kimura G
and Kuwano M: Imidazole-resistant phenotype and virus
transformation in cultured rat cells. Cancer Res. 44:1791–1795.
1984.PubMed/NCBI
|
|
24
|
Beloribi-Djefaflia S, Vasseur S and
Guillaumond F: Lipid metabolic reprogramming in cancer cells.
Oncogenesis. 5:e1892016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Calay D, Vind-Kezunovic D, Frankart A,
Lambert S, Poumay Y and Gniadecki R: Inhibition of Akt signaling by
exclusion from lipid rafts in normal and transformed epidermal
keratinocytes. J Invest Dermatol. 130:1136–1145. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Head SA, Shi WQ, Yang EJ, Nacev BA, Hong
SY, Pasunooti KK, Li RJ, Shim JS and Liu JO: Simultaneous targeting
of NPC1 and VDAC1 by itraconazole leads to synergistic inhibition
of mTOR signaling and angiogenesis. ACS Chem Biol. 12:174–182.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gallegos AM, Atshaves BP, Storey SM,
Starodub O, Petrescu AD, Huang H, McIntosh AL, Martin GG, Chao H,
Kier AB and Schroeder F: Gene structure, intracellular
localization, and functional roles of sterol carrier protein-2.
Prog Lipid Res. 40:498–563. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Plaks V, Kong N and Werb Z: The cancer
stem cell niche: How essential is the niche in regulating stemness
of tumor cells? Cell Stem Cell. 16:225–238. 2915. View Article : Google Scholar
|
|
29
|
Kalluri R and Zeisberg M: Fibroblasts in
cancer. Nat Rev Cancer. 6:392–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schmitz G and Orsó E: CD14 signalling in
lipid rafts: New ligands and co-receptors. Curr Opin Lipidol.
13:513–521. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martinez-Outschoorn UE, Sotgia F and
Lisanti MP: Caveolae and signalling in cancer. Nat Rev Cancer.
15:225–237. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Frey T and De Maio A: The antifungal agent
itraconazole induces the accumulation of high mannose glycoproteins
in macrophages. J Biol Chem. 284:16882–16890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vreugdenhil G, Van Dijke BJ, Donnelly JP,
Novakova IR, Raemaekers JM, Hoogkamp-Korstanje MA, Koster M and de
Pauw BE: Efficacy of itraconazole in the prevention of fungal
infections among neutropenic patients with hematologic malignancies
and intensive chemotherapy. A double blind, placebo controlled
study. Leuk Lymphoma. 11:353–358. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gupta S, Kim J and Gollapudi S: Reversal
of daunorubicin resistance in P388/ADR cells by itraconazole. J
Clin Invest. 87:1467–1469. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vreugdenhil G, Raemaekers JM, van Dijke BJ
and de Pauw BE: Itraconazole and multidrug resistance: Possible
effects on remission rate and disease-free survival in acute
leukemia. Ann Hematol. 67:107–109. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsubamoto H, Sonoda T, Yamasaki M and
Inoue K: Impact of combination chemotherapy with itraconazole on
survival of patients with refractory ovarian cancer. Anticancer
Res. 34:2481–2487. 2014.PubMed/NCBI
|
|
37
|
Tsubamoto H, Sonoda T, Yamasaki M and
Inoue K: Impact of combination chemotherapy with itraconazole on
survival for patients with recurrent or persistent ovarian clear
cell carcinoma. Anticancer Res. 34:2007–2014. 2014.PubMed/NCBI
|
|
38
|
Tsubamoto H, Sonoda T and Inoue K: Impact
of itraconazole on the survival of heavily pre-treated patients
with triple-negative breast cancer. Anticancer Res. 34:3839–3844.
2014.PubMed/NCBI
|
|
39
|
Tsubamoto H, Sonoda T, Ikuta S, Tani S,
Inoue K and Yamanaka N: Combination chemotherapy with itraconazole
for treating metastatic pancreatic cancer in the second-line or
additional setting. Anticancer Res. 35:4191–4196. 2015.PubMed/NCBI
|
|
40
|
Tsubamoto H, Sonoda T, Ikuta S, Tani S,
Inoue K and Yamanaka N: Impact of itraconazole after first-line
chemotherapy on survival of patients with metastatic biliary tract
cancer. Anticancer Res. 35:4923–4927. 2015.PubMed/NCBI
|
|
41
|
Chung FS, Santiago JS, Jesus MF, Trinidad
CV and See MF: Disrupting P-glycoprotein function in clinical
settings: What can we learn from the fundamental aspects of this
transporter? Am J Cancer Res. 6:1583–1598. 2016.PubMed/NCBI
|
|
42
|
Lhommé C, Joly F, Walker JL, Lissoni AA,
Nicoletto MO, Manikhas GM, Baekelandt MM, Gordon AN, Fracasso PM,
Mietlowski WL, et al: Phase III study of valspodar (PSC 833)
combined with paclitaxel and carboplatin compared with paclitaxel
and carboplatin alone in patients with stage IV or suboptimally
debulked stage III epithelial ovarian cancer or primary peritoneal
cancer. J Clin Oncol. 26:2674–2682. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rudin CM, Brahmer JR, Juergens RA, Hann
CL, Ettinger DS, Sebree R, Smith R, Aftab BT, Huang P and Liu JO:
Phase 2 study of pemetrexed and itraconazole as second-line therapy
for metastatic nonsquamous non-small-cell lung cancer. J Thorac
Oncol. 8:619–623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Roviello G, Bachelot T, Hudis CA,
Curigliano G, Reynolds AR, Petrioli R and Generali D: The role of
bevacizumab in solid tumours: A literature based meta-analysis of
randomised trials. Eur J Cancer. 75:245–258. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Garon EB, Ciuleanu TE, Arrieta O, Prabhash
K, Syrigos KN, Goksel T, Park K, Gorbunova V, Kowalyszyn RD, Pikiel
J, et al: Ramucirumab plus docetaxel versus placebo plus docetaxel
for second-line treatment of stage IV non-small-cell lung cancer
after disease progression on platinum-based therapy (REVEL): A
multicentre, double-blind, randomised phase 3 trial. Lancet.
384:665–673. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fuchs CS, Tomasek J, Yong CJ, Dumitru F,
Passalacqua R, Goswami C, Safran H, dos Santos LV, Aprile G, Ferry
DR, et al: Ramucirumab monotherapy for previously treated advanced
gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An
international, randomised, multicentre, placebo-controlled, phase 3
trial. Lancet. 383:31–39. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wilke H, Muro K, Van Cutsem E, Oh SC,
Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, et
al: Ramucirumab plus paclitaxel versus placebo plus paclitaxel in
patients with previously treated advanced gastric or
gastro-oesophageal junction adenocarcinoma (RAINBOW): A
double-blind, randomised phase 3 trial. Lancet Oncol. 15:1224–1235.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tabernero J, Yoshino T, Cohn AL,
Obermannova R, Bodoky G, Garcia-Carbonero R, Ciuleanu TE, Portnoy
DC, Van Cutsem E, Grothey A, et al: Ramucirumab versus placebo in
combination with second-line FOLFIRI in patients with metastatic
colorectal carcinoma that progressed during or after first-line
therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine
(RAISE): A randomised, double-blind, multicentre, phase 3 study.
Lancet Oncol. 16:499–508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Antonarakis ES, Heath EI, Smith DC,
Rathkopf D, Blackford AL, Danila DC, King S, Frost A, Ajiboye AS,
Zhao M, et al: Repurposing itraconazole as a treatment for advanced
prostate cancer: A noncomparative randomized phase II trial in men
with metastatic castration-resistant prostate cancer. Oncologist.
18:163–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
NCCN: Clinical practice guidelines in
oncology, basal cell skin cancer, version 1. 2017.https://www.nccn.org/professionals/physician_gls/pdf/nmsc.pdfApril
20–2017
|
|
51
|
Kim DJ, Kim J, Spaunhurst K, Montoya J,
Khodosh R, Chandra K, Fu T, Gilliam A, Molgo M, Beachy PA and Tang
JY: Open-label, exploratory phase II trial of oral itraconazole for
the treatment of basal cell carcinoma. J Clin Oncol. 32:745–751.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhou BB, Zhang H, Damelin M, Geles KG,
Grindley JC and Dirks PB: Tumour-initiating cells: Challenges and
opportunities for anticancer drug discovery. Nat Rev Drug Discov.
8:806–823. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhao J: Cancer stem cells and
chemoresistance: The smartest survives the raid. Pharmacol Ther.
160:145–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ahmed M, Chaudhari K, Babaei-Jadidi R,
Dekker LV and Nateri A Shams: Concise Review: Emerging drugs
targeting epithelial cancer stem-like cells. Stem Cells.
35:839–850. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li Y, Rogoff HA, Keates S, Gao Y,
Murikipudi S, Mikule K, Leggett D, Li W, Pardee AB and Li CJ:
Suppression of cancer relapse and metastasis by inhibiting cancer
stemness. Proc Natl Acad Sci USA. 112:pp. 1839–1844. 2015;
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jonker DJ, Nott L, Yoshino T, Gill S,
Shapiro J, Ohtsu A, Zalcerg J, vickers MM, Wei A, Gao Y, et al: A
randomized phase III study of napabucasin [BBI608] (NAPA) vs
placebo (PBO) in patients (pts) with pretreated advanced colorectal
cancer (ACRC): The CCTG/AGITG CO.23 trial. Ann Oncol. 27 Suppl
6:S454O2016. View Article : Google Scholar
|