Introduction
Hepatocellular carcinoma (HCC) is currently the
fifth most common type of solid tumor worldwide and the second
leading cause of cancer-associated mortality in China, accounting
for an estimated >650,000 mortalities annually (1). The high mortality associated with this
disease may be attributed primarily to the inability to diagnose
patients with HCC at an early stage, and its aggressive
invasiveness and metastasis (2).
Despite the fact that liver transplantation (LT) is considered the
best treatment option for progressive HCC in selected patients
accompanied by cirrhosis and hepatitis, the high postoperative
recurrence and metastasis of HCC, due to its invasion-associated
spreading, remains the biggest obstacle that affects the long-term
prognosis of patients following LT (3,4). This
unfavorable prognosis is primarily due to the fact that HCC is a
highly vascularized tumor type with frequent intra- or
extra-hepatic metastases. However, at present, the molecular
mechanisms underlying HCC recurrence and metastasis remain to be
elucidated. In addition, there is an urgent demand to identify
additional diagnostic and prognostic biomarkers of HCC, which may
be critical for developing novel therapeutic strategies.
Rho family GTPases, including Ras homolog gene
family, member A, Ras-related C3 botulinum toxin substrate 1 and
cell division control protein 42 homolog (Cdc42), belong to the
small GTPases of the Ras oncogene superfamily that are known to
have an essential role in cellular function. Their biological
activities are controlled through a tightly regulated GDP/GTP
cycle, which is stimulated by guanine nucleotide exchange factors
and terminated by GTPase-activating proteins (5–7). As the
pivotal suppressor of Rho GTPase, Rho GDP dissociation inhibitors
(GDIs) bind to the majority of Rho GTPases in the cytoplasm to
prevent the nucleotide exchange, and thus block their activation,
which leads to carcinogenesis and promotes the invasive phenotype
of tumor cells (8–10). At present, three human RhoGDIs have
been identified, termed RhoGDI1 (RhoGDI1 or RhoGDI-α), RhoGDI2
(D4-GDI or RhoGDI-β) and RhoGDI3 (RhoGDIG or RhoGDI-γ) (11). Dysregulation of RhoGDIs has been
explored in a variety of human tumors; however, the expression
levels exhibit opposite patterns depending on the tumor types.
RhoGDI1 is upregulated in advanced ovarian and colorectal cancers
and is associated with tumor progression (12,13).
However, the downregulation of RhoGDI1 has been identified in
breast cancer, prostate cancer and HCC (14–16).
RhoGDI2 is downregulated and identified as a putative metastasis
suppressor in bladder cancer, colorectal carcinoma and Hodgkin's
lymphoma, while the overexpression of RhoGDI2 has also been
detected in cases of ovarian, breast and gastric cancer (12,17–20).
However, RhoGDI3, which is preferentially expressed in the brain
and pancreas, is present at low levels in patients with breast
cancer metastasis (21). In addition,
a small number of studies are available on the expression pattern
and clinical implications of RhoGDIs in patients with HCC, while
the role of RhoGDIs in tumor progression and metastasis remains
controversial (22–24).
The purpose of the present study was to assess the
role RhoGDIs perform in the invasiveness and migration of HCC, to
investigate the expression of RhoGDIs in patients with HCC and to
determine their clinical prognostic significance in HCC following
LT. It was identified that the downregulation of RhoGDIs was
associated with decreased recurrence-free survival for patients
with HCC following LT, and promoted cancer progression by
facilitating cell migration and invasion, supporting its role as a
metastasis suppressor and its potential clinical utility for
biotherapy.
Materials and methods
Clinical samples
In total, 138 patients were enrolled in the present
study. A total of 80 hepatocellular carcinoma tissues were obtained
from patients treated with orthotopic LT (OLT) between January 2002
and December 2005 in a single group at the First Affiliated
Hospital of Zhejiang University School of Medicine (Hangzhou,
China), among which 56 patients (70%) were beyond the Milan
criteria (3) and 45 patients (56.3%)
developed tumor recurrence during a follow-up period of 2–46.9
months. The median follow-up time was 19.2 months. All diagnosed
cases were verified histologically prior or subsequent to surgery,
and the clinicopathological variables were summarized in Table I. All patients were monitored by serum
α fetoprotein (AFP) levels, abdominal ultrasonography, chest X-ray
and computed tomography scanning regularly following LT for the
surveillance of recurrence or metastases. Tumor recurrence was
confirmed by radiological techniques and aided by serum AFP
examination. An additional 58 matched pairs of HCC samples and
adjacent liver tissues were also included in the present study.
Tissues were immediately frozen in liquid nitrogen following
surgery, and then stored at −80°C until processing. The present
study was approved by the Ethics Committee of Zhejiang University
and written informed consent was obtained from all the patients,
according to the Declaration of Helsinki.
 | Table I.Association between RhoGDIs and
clinicopathological variables of patients with hepatocellular
carcinoma following liver transplantation. |
Table I.
Association between RhoGDIs and
clinicopathological variables of patients with hepatocellular
carcinoma following liver transplantation.
|
| RhoGDI1 expression,
n |
| RhoGDI2 expression,
n |
| RhoGDI3 expression,
n |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Variables | Low | High | P-valuea | Low | High | P-valuea | Low | High | P-valuea |
|---|
| Age |
|
|
|
|
|
|
|
|
|
| ≤50
years | 24 | 21 |
0.795 | 27 | 19 |
0.062 | 19 | 21 |
0.118 |
| >50
years | 16 | 19 |
| 13 | 21 |
| 21 | 13 |
|
| Sex |
|
|
|
|
|
|
|
|
|
|
Female | 3 | 4 | >0.999 | 4 | 3 | >0.999 | 3 | 4 | >0.999 |
|
Male | 37 | 36 |
| 36 | 37 |
| 37 | 36 |
|
| PVTT |
|
|
|
|
|
|
|
|
|
|
Absent | 25 | 28 |
0.785 | 27 | 27 | >0.999 | 29 | 24 |
0.273 |
|
Present | 15 | 12 |
| 13 | 13 |
| 11 | 16 |
|
| Preoperative AFP
level |
|
|
|
|
|
|
|
|
|
| ≤400
ng/ml | 20 | 24 |
0.604 | 23 | 21 |
0.795 | 21 | 23 |
0.795 |
| >400
ng/ml | 20 | 16 |
| 17 | 19 |
| 19 | 17 |
|
| Histopathological
grading |
|
|
|
|
|
|
|
|
|
| Well +
moderate | 27 | 29 |
0.779 | 24 | 32 |
0.091 | 32 | 24 |
0.128 |
|
Poor | 13 | 11 |
| 16 | 8 |
| 8 | 16 |
|
| Tumor size |
|
|
|
|
|
|
|
|
|
| ≤5
cm | 16 | 19 |
0.795 | 19 | 16 |
0.795 | 12 | 21 |
0.063 |
| >5
cm | 24 | 21 |
| 21 | 24 |
| 28 | 19 |
|
| Tumor number |
|
|
|
|
|
|
|
|
|
|
Single | 13 | 13 | >0.999 | 13 | 13 | >0.999 | 16 | 11 |
0.273 |
|
Multiple | 27 | 27 |
| 27 | 27 |
| 24 | 19 |
|
| Tumor
recurrence |
|
|
|
|
|
|
|
|
|
| No | 9 | 24 |
0.004a | 11 | 23 |
0.018a | 20 | 13 |
0.190 |
|
Yes | 31 | 16 |
| 29 | 17 |
| 20 | 27 |
|
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from all specimens using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) while RT-PCR was performed using the MMLV Reverse
Transcriptase cDNA kit (Promega Corporation, Madison, WI, USA),
according to the manufacturer's protocol. RT-qPCR Amplification was
initiated with a 3-min pre-denaturation step at 93°C, followed by
40 cycles of 1 min at 93°C, 1 min at 55°C, and 1 min at 72°C and
then a final extension at 72°C for 5 min. SYBR-Green (Applied
Biosystems; Thermo Fisher Scientific, Inc.) was used as a
fluorophore. qPCR was performed in triplicate using an ABI7500
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
expression of RhoGDIs was normalized to β-actin, which was used as
an internal control according to the 2−ΔΔCq method
(25). All primer sequences used in
this study were as follows: RhoGDI1 sense,
5′-ACTACAAGCCCCCGGCCCAG-3′ and antisense,
5′-GTTGGGGACGTTGGGGTCTGC-3′; RhoGDI2 sense,
5′-GAGAGACAGAGGCACCCCGGA-3′ and antisense,
5′-GCTTTCGGATCTGTCACCACAGGA-3′; RhoGDI3 sense,
5′-TGTCGGAACAGGCTCCGGGG-3′ and antisense,
5′-GACGGTCTTGTCCACGCGCA-3′; and β-actin sense,
5′-TTGTTACAGGAAGTCCCTTGCC-3′ and antisense,
5′-ATGCTATCACCTCCCCTGTGTG-3′ (26).
All reactions were performed in duplicate.
Western blot analysis
Clinically frozen tissues were homogenized in
radioimmunoprecipitation lysis buffer and incubated at 4°C for 3 h.
For electrophoresis, total protein (40 µg per lane) was separated
by 12% SDS-PAGE (Invitrogen; Thermo Fisher Scientific, Inc.) and
then transferred onto polyvinylidene fluoride membranes. Following
blotting, the membranes were incubated with rabbit monoclonal
antibody against human RhoGDI1 (1:1,000, cat. no. ab133248; Abcam,
Cambridge, UK), rabbit polyclonal antibody against RhoGDI2
(1:2,000, cat. no. ab15198; Abcam) or mouse monoclonal antibody
against β-actin (1:2,000, cat. no. sc130065; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C. Following 3
washes with TBST buffer (20 mM Tris-HCl, 150 mM NaCl, and 0.05%
Tween-20) for 10 min, the membranes were incubated with second
antibody for 1 h at room temperature. Finally, immunocomplexes were
visualized by chemiluminescence using enhanced chemiluminescence
(Applygen Technologies, Inc., Beijing, China) according to the
manufacturer's protocol.
Immunohistochemical analysis
Immunohistochemical studies on RhoGDIs were
performed on formalin-fixed tissue sections obtained from tumor
tissues and their paired adjacent non-tumors tissues. In brief, 4
µm sections were heated at 60°C for 20 min followed by
deparaffinization with xylene for 20 min and rehydration with a
graded ethanol series (100, 95, 80 and 70%) and deionized distilled
water, for 5 min each. Subsequent to treatment with 3% hydrogen
peroxide to quench endogenous peroxidase activity, the sections
were submerged into potassium citrate antigenic retrieval buffer
and microwaved for antigenic retrieval, after which they were
incubated with 1% bovine serum albumin (Gibco; Thermo Fisher
Scientific, Inc.) to block non-specific binding sites. Then, the
sections were incubated with primary antibodies against RhoGDI1
(1:50; cat. no. ab133248; Abcam) or RhoGDI2 (1:100; cat. no.
ab15198; Abcam) for 1 h at 37°C. Following 3 washes with phosphate
buffered saline (PBS) for 5 min, tissue slides were treated with
biotinylated secondary anti-rabbit antibody (1:200; cat. no.
a11034; Invitrogen; Thermo Fisher Scientific, Inc.) and horseradish
peroxidase (HRP)-streptavidin complex (Yeasen, Shanghai, China) at
37°C for 30 min each. Finally, all sections were counterstained
with hematoxylin, dehydrated, and mounted. The immunostained scores
were independently interpreted and categorized using a
semi-quantitative scale as follows: 0, ≤5% positive staining; 1,
6–25% positive; 2, 26–50% positive; 3, 51–75% positive; and 4,
>75% positive by two pathologists blinded to the clinical
data.
Cell culture
Human liver cancer HepG2 and MHCC-97L cell lines
were purchased from the American Type Culture Collection (Manassas,
VA, USA) and Liver Cancer Institute of Fudan University (Shanghai,
China), respectively. All the cell lines were maintained in
Dulbecco's modified Eagle's Medium (DMEM) (Gibco; Thermo Fisher
Scientific, Inc.) with high glucose, supplemented with 10% fetal
bovine serum (FBS) (Gibco; Thermo Fisher Scientific, Inc.) at 37°C
in a 5% CO2 atmosphere at constant humidity.
RNA interference experiments
All small interfering RNAs (siRNAs) were purchased
from Shanghai GenePharma Co., Ltd. (Shanghai, China). Transient
transfection of each siRNA to knock down RhoGDIs using
Lipofectamine 2000 transfection reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. The
siRNA sequences were as follows: RhoGDI1-siRNA,
5′-AATTTAAGCAGTTAGAACT-3′; RhoGDI2-siRNA1,
5′-AATACGTTCAGCACACCTACA-3′; RhoGDI2-siRNA2,
5′-AAGGAAGGTTCTGAATATAGA-3′ and scrambled negative control-siRNA,
5′-AATCGCATAGCGTATGCCGTT-3′. The cells were harvested following 48
h of transfection, and the efficiency of each siRNA oligo duplex
was confirmed by qPCR.
In vitro cell migration/invasion
assay
Cell migration and invasion were measured by an
in vitro Transwell assay (Merck KGaA, Darmstadt, Germany),
according to the manufacturer's protocol. Tumor cells
(2.5×105) transfected with RhoGDI1/2-siRNA were
suspended in 250 µl serum-free DMEM were placed in the upper
insert, whose porous membrane was coated with (for invasion assay)
or without (for migration assay) Matrigel (BD Biosciences, Franklin
Lakes, NJ, USA). Subsequently, 600 µl medium containing 10% FBS was
added to the lower chamber as a chemoattractant. Following
migration for 24 h, or invasion for 48 h, the insert was removed
and added into dried methanol for 15 min at room temperature.
Finally, the penetrated cells on the filters were stained in 0.1%
crystal violet and counted in 5 fields under a ×100 objective lens
of an Olympus CX31 light microscope (Olympus Corporation, Tokyo,
Japan). Tumor cells transfected with negative control-siRNA were
used as control. Each experiment setting was repeated in
triplicate.
Statistical analysis
All statistical data were processed with SPSS v.16.0
(SPSS, Inc., Chicago, IL) and GraphPad Prism v5.0 (GraphPad
Software, La Jolla, CA) software. The differences between the two
groups were analyzed using an unpaired Student's t-test. The
χ2 or Fisher's exact tests were used for comparisons
between RhoGDI expression and clinicopathological factors.
Tumor-free survival curves were drawn by the Kaplan-Meier method,
and compared by the log-rank test. Cox regression analysis was used
for univariate and multivariate analysis to calculate the hazard
ratios for risk factors. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of RhoGDIs in HCC and
precancerous tissues
For the analysis of RhoGDIs expression in HCC, the
expression of RhoGDIs was first examined in 58 pairs of HCC tissues
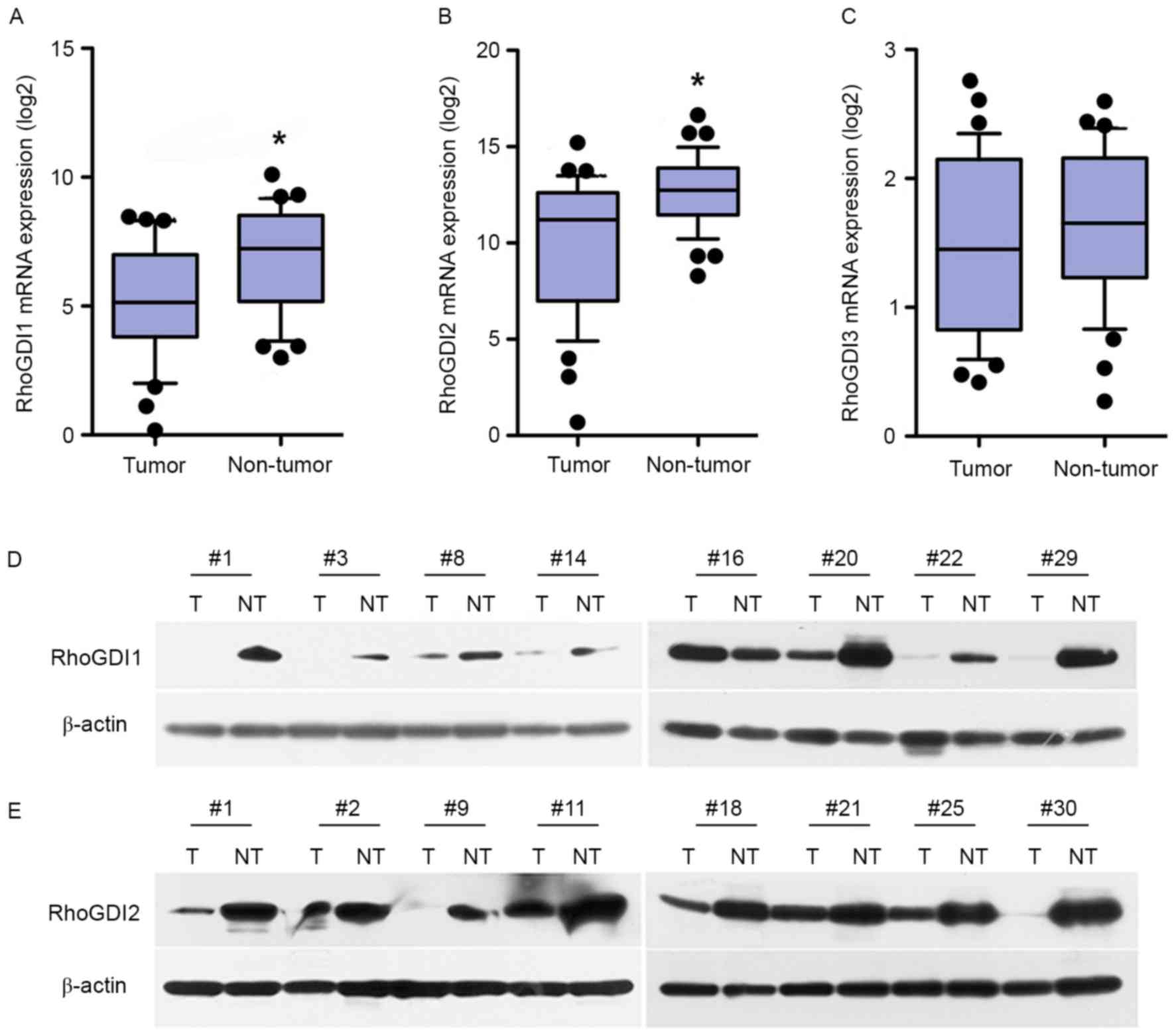
and adjacent noncancerous liver tissues by qPCR. As expected, the
downregulation of RhoGDI1 and RhoGDI2 mRNA was observed in 39
(67.2%) and 38 patients (65.5%), respectively. Overall, GDI1 and
GDI2 expression was significantly decreased in tumors compared with
matched non-tumorous tissues (P<0.05; Fig. 1A and B); however, RhoGDI3 expression
did not differ significantly between tumor and non-tumor tissue
(P>0.05; Fig. 1C). These results
were then confirmed in 30 pairs of primary HCC tissues by western
blot analysis. Compared with paired non-tumor liver tissues, the
downregulation of RhoGDI1 and RhoGDI2 were observed in 20/30
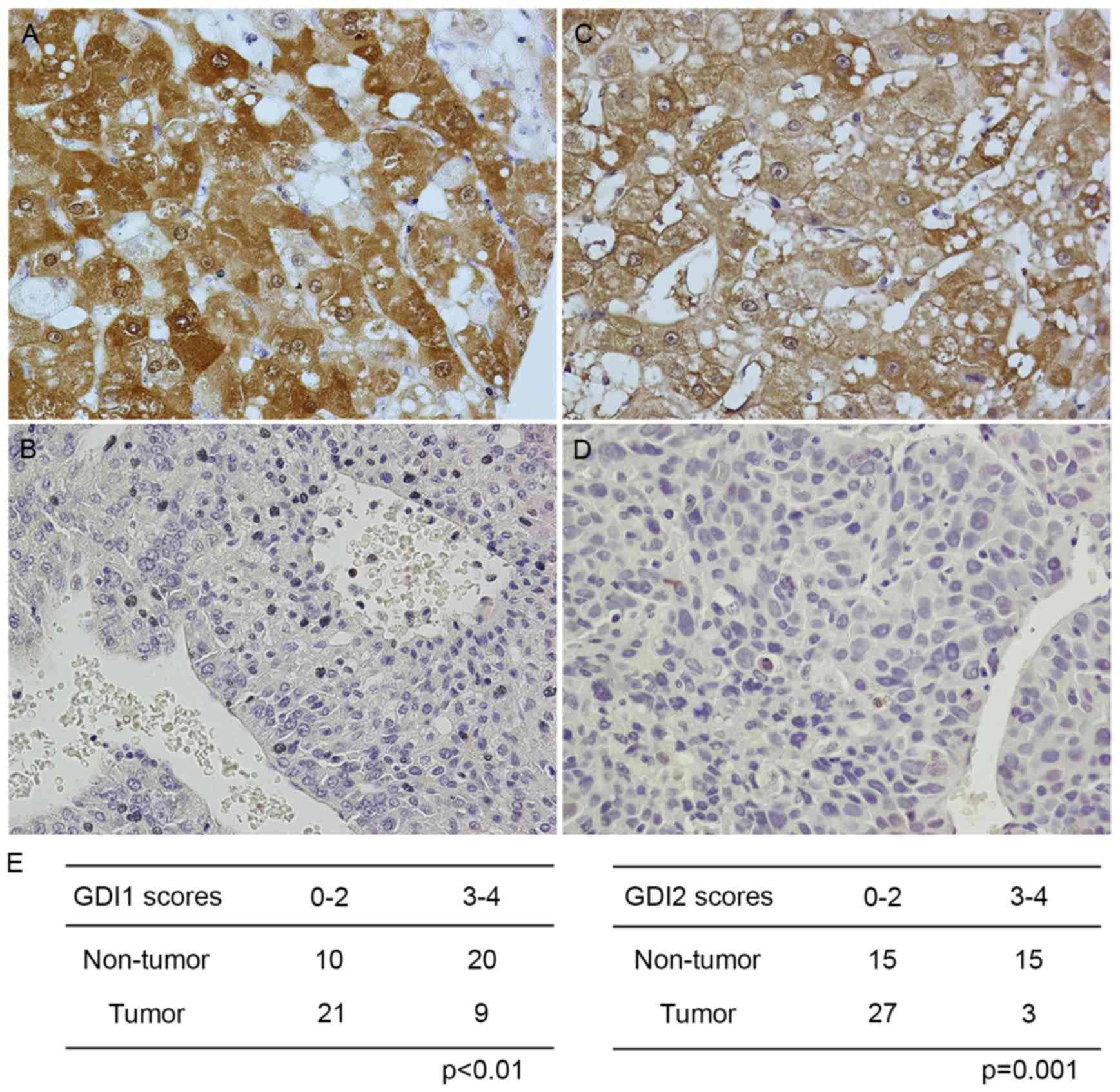
(66.7%) and 21/30 (70%) of HCCs, respectively (Fig. 1D and E). In addition, the expression
and subcellular localization of RhoGDIs protein was determined by
immunohistochemistry, and its expression intensity corresponded
closely with those of the RT-qPCR and immunoblotting analysis
(Fig. 2).
Association of RhoGDI expression with
clinicopathological variables
To determine the clinical significance of RhoGDIs in
HCC, the expression of RhoGDIs in 80 consecutive patients who
underwent LT was examined. When patients were segregated into
low/high expression groups with the median GDI expression level as
the cut-off line, the association study demonstrated that a low
expression of RhoGDI1 and RhoGDI2, but not RhoGDI3, was
significantly associated with tumor recurrence following LT
(Table I). However, no significant
association was observed between the downregulation of RhoGDIs and
clinicopathological characteristics, which included age, gender,
AFP level, histopathological grading, tumor number, tumor size and
portal vein tumor thrombus (Table
I).
RhoGDI expression is associated with
HCC prognosis
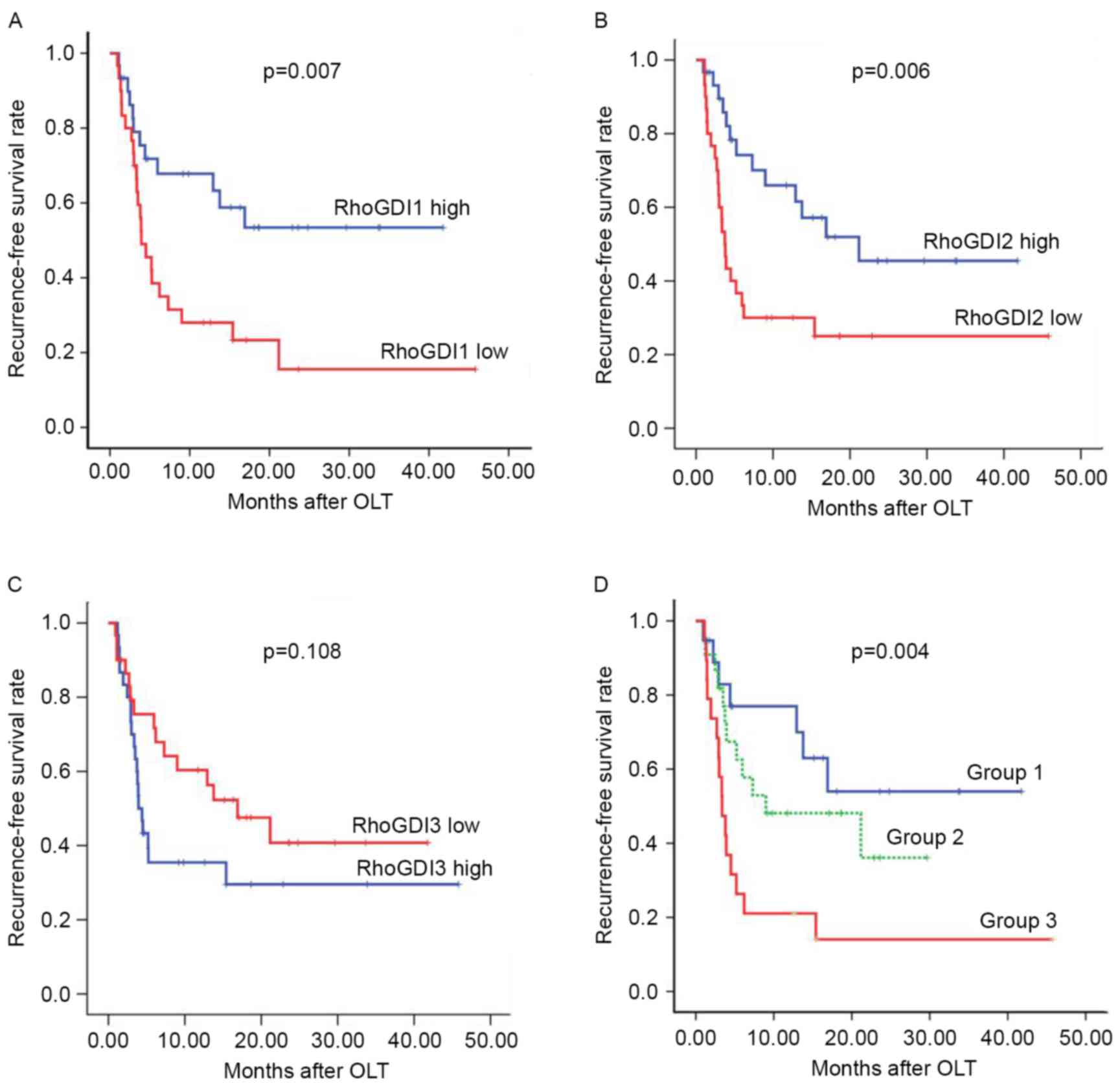
Whether the downregulation of RhoGDIs was associated
with the prognosis of patients with HCC was investigated. Notably,
Kaplan-Meier survival curves and the log-rank tests indicated that
the low expression of RhoGDI1 and RhoGDI2 were unfavorable
prognostic predictors for the disease-free survival (DFS) of HCC,
whereas RhoGDI3 expression exhibited no significant association
with HCC prognosis (RhoGDI1, P=0.02; RhoGDI2, P=0.006; RhoGDI3,
P=0.108; Fig. 3A-C). The combined
effect of RhoGDI1 and RhoGDI2 mRNA on DFS of HCC was also
evaluated. The GDI1 and GDI2 low expression group demonstrated
significantly shorter DFS compared with other groups (either GDI1
or GDI2 high expression group; GDI1 and GDI2 high group; Fig. 3D). Concurrently, univariate analyses
were performed to determine the prognostic factors for predicting
tumor recurrence of HCC by the Kaplan-Meier method. As demonstrated
in Table II, three of the well-known
factors associated with HCC recurrence were confirmed in the
present study (tumor size; preoperative AFP level; portal vein
tumor thrombus). In addition, the downregulation of RhoGDI1 or
RhoGDI2 exhibited significantly poorer DFS compared with those
patients with high expression of RhoGDI1 or RhoGDI2 (Table II). Ultimately, by the multivariate
Cox regression analysis, apart from preoperative serum AFP level,
low levels of expression RhoGDI2 were identified to be an
independent predictor of recurrence-free survival for HCC following
LT (hazard ratio, 3.306; 95% confidence interval, 1.610–6.790;
P=0.001; Table II). The data of the
present study indicate that RhoGDI2 expression may be an important
indicator for predicting HCC recurrence, which in turn may affect
the overall survival of patients following LT.
 | Table II.Downregulation of RhoGDI2 is an
independent prognostic factor for patients with hepatocellular
carcinoma following liver transplantation. |
Table II.
Downregulation of RhoGDI2 is an
independent prognostic factor for patients with hepatocellular
carcinoma following liver transplantation.
| Variables | Hazard ratio (95%
confidence interval) |
P-valuea |
|---|
| Univariate
analysis | – | – |
| Tumor
size (>5 cm vs. ≤5 cm) | – | 0.034 |
| AFP
(>400 ng/ml vs. ≤400 ng/ml) | – | 0.006 |
| PVTT
(present vs. absent) | – | 0.028 |
| RhoGDI1
expression (low vs. high) | – | 0.007 |
| RhoGDI2
expression (low vs. high) | – | 0.006 |
| Multivariate
analysis | – | – |
| AFP
(>400 ng/ml vs. ≤400 ng/ml) | 2.482
(1.207–5.012) | 0.013 |
| RhoGDI2
expression (low vs. high) | 3.306
(1.610–6.790) | 0.001 |
Effects of RhoGDIs on cell migration
and invasion
It has been demonstrated that RhoGDI1 and RhoGDI2
function as metastasis suppressors and may block cell invasion in
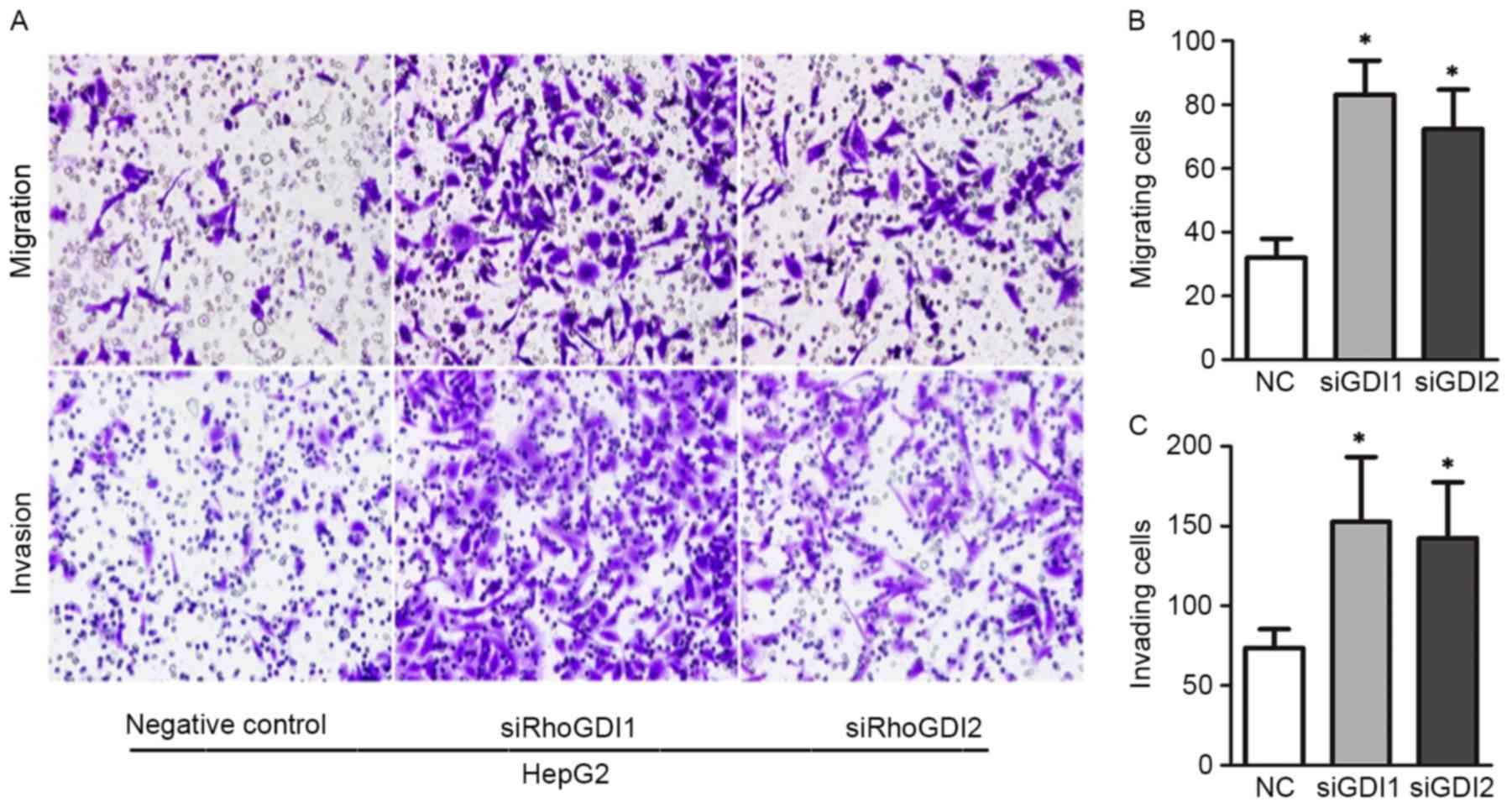
different types of tumors. To explore whether RhoGDI depletion
affects cell migration and invasion, a Transwell assay was
performed in GDI-RNAi cells. Following 48 h of RNA interference,
expression of RhoGDI1 and RhoGDI2 was efficiently suppressed by
specific siRNAs. As demonstrated in Fig.
4, HepG2 cells with RhoGDI1 knockout exhibited significantly
higher rates of migration and invasion compared with control
groups. Coincidently, silencing RhoGDI2 expression effectively
enhanced Matrigel invasion activity of HepG2 cells (P<0.05;
Fig. 4A and B). Similar results were
observed in MHCC-97L cells (data not shown). Overall, these in
vitro studies demonstrated that RhoGDIs perform crucial roles
in the regulation of HCC cell motility and invasiveness.
Discussion
Although marked progress has been made in previous
decades, the survival of patients with HCC remains at ~50% (range,
17–69) following 5 years (27,28). Tumor
recurrence remains one of the major challenges for patients with
HCC undergoing LT, constituting a major factor in its poor
prognosis. Diverse significant risk factors for recurrence have
been established, including tumor rupture, venous invasion and
cirrhosis, which have been implicated in early recurrence (29). Viral replication in viral hepatitis
and multiple tumors increases the risk for the late recurrence
(30,31). However, the molecular pathogenesis
underlying tumor recurrence remains largely unknown, which
seriously hinders the improvement of the clinical management of HCC
(32,33). In previous years, it was widely
accepted that abnormal gene expression constituted one of the major
characteristics of tumor invasion and metastasis. Thus, to
elucidate the mechanisms and identify novel biomarkers underlying
the early recurrence of HCC are considered to be significant for
improvements to the therapeutic interventions and overall prognosis
for patients with HCC.
Rho GTPases, including Rho, Rac and Cdc42, that bind
RhoGDIs regulate numerous cellular functions, including cell
polarity, cell cycle progression, apoptosis, vesicular trafficking
and tumorigenesis in several types of cancer (5,13,16). Changes in the levels of the different
RhoGDIs markedly affects the overall levels and Rho GTPase
activity, primarily by their ability to prevent nucleotide exchange
and membrane association (9). This
indicates that the deregulation of RhoGDIs may be involved in
progression and metastasis for a number of types of human
cancer.
To the best of our knowledge, the role of RhoGDIs in
cancer remains controversial. A small number of studies mentioned
the expression of RhoGDIs in HCC. Ding et al (22) identified that miR-151 may directly
target RhoGDI1, which is frequently downregulated in HCC and
functions as a metastasis suppressor, leading to the activation of
Rac1, Cdc42 and Rho GTPases, thus facilitating HCC cell invasion
and spreading (22). In a small study
using transcriptomic and proteomic approaches, the downregulation
of RhoGDI2 was identified in patients with HCC with hepatitis B
infection (24). In the present
study, the expression of RhoGDI1 and RhoGDI2, but not RhoGDI3, was
significantly lower in HCC tissues compared with para-cancerous
tissues at mRNA and protein levels, and these results are
consistent with a previous study (16). In addition, a lower expression of
RhoGDI1 was negatively associated with disease-free survival in
patients with HCC who underwent LT (P=0.013). Since Rho-GDI2 and
Rho-GDI1 exhibit similar abilities to inhibit Rho GTPase, it was
hypothesized whether Rho-GDI2 would exert the same effect on HCC
prognosis. According to the present study, the same trend was also
observed at the RhoGDI2 gene (P=0.006). The most valuable result of
the present study is that RhoGDI2 expression is an independent
prognostic factor for HCC recurrence following LT. To the best of
our knowledge, this is the first study demonstrating that RhoGDI2
is frequently downregulated in HCC, and functions as a metastasis
suppressor in HCC. It also possesses negative associations with
recurrence-free survival of patients with HCC following LT. Thus,
detecting the expression levels of RhoGDI1, in combination with
RhoGDI2 and Rho GTPases, may be valuable in predicting the
prognosis of patients with HCC prior to LT more accurately compared
with current measures. Future studies will determine whether the
deregulation of Rho GTPase activity is also associated with the
prognosis of patients with HCC following LT. By contrast, Rho-GDI3
did not affect the clinical outcome of HCC, implying that besides
sharing functions, the three RhoGDIs may also have individual, not
interchangeable, activities in tumor progression.
Based on the trend of decreasing RhoGDI expression
in HCC and poorer clinical prognosis, it was considered that lower
expression levels of RhoGDI in tumor cells may contribute to more
aggressive tumor behavior. To confirm this hypothesis, the
biological significance of RhoGDI in cancer invasion and migration
was then investigated in vitro. It was observed that the
knockdown of RhoGDI1 and RhoGDI2 by siRNA accelerated the cell
migration rate, and the invasive ability of HepG2 and MHCC-97L
cells. Despite the critical molecular mechanism underlying the
biological function of GDIs, which has not been described in the
present study, to a certain extent it indicates that the loss of
RhoGDIs promotes the malignant behavior of HCC cells. In the
future, additional molecular and animal model studies are also
necessary to confirm these data.
In summary, the results of the present study
demonstrate the important roles RhoGDIs perform, particularly
RhoGDI2, in liver cancer biology. The downregulation of RhoGDI1 and
RhoGDI2 may significantly promote HCC cell invasion and migration
in vitro. Concurrently, RhoGDI2 may be a novel biomarker and
independent prognostic factor in predicting the recurrence of HCC
following LT. These data may enable an exploration of the potential
use of RhoGDIs as a target for cancer therapy.
Acknowledgements
The present study was supported by grants from the
National S&T Major Project (no. 2012ZX10002017) and the
National Natural Science Foundation of China (nos. 81101840 and
81302074). The abstract of the present study was presented at the
International Liver Transplantation Society 20th Annual
International Congress (June 2014; London, United Kingdom).
References
|
1
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hainaut P and Plymoth A: Targeting the
hallmarks of cancer: Towards a rational approach to next-generation
cancer therapy. Curr Opin Oncol. 25:50–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mazzaferro V, Regalia E, Doci R, Andreola
S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A and
Gennari L: Liver transplantation for the treatment of small
hepatocellular carcinomas in patients with cirrhosis. N Engl J Med.
334:693–699. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng SS, Xu X, Wu J, Chen J, Wang WL,
Zhang M, Liang TB and Wu LM: Liver transplantation for
hepatocellular carcinoma: Hangzhou experiences. Transplantation.
85:1726–1732. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu J, Zhang D, Luo W, Yu Y, Yu J, Li J,
Zhang X, Zhang B, Chen J, Wu XR, et al: X-linked inhibitor of
apoptosis protein (XIAP) mediates cancer cell motility via Rho GDP
dissociation inhibitor (RhoGDI)-dependent regulation of the
cytoskeleton. J Biol Chem. 286:15630–15640. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Olofsson B: Rho guanine dissociation
inhibitors: Pivotal molecules in cellular signalling. Cell Signal.
11:545–554. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moissoglu K, McRoberts KS, Meier JA,
Theodorescu D and Schwartz MA: Rho GDP dissociation inhibitor 2
suppresses metastasis via unconventional regulation of RhoGTPases.
Cancer Res. 69:2838–2844. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garcia-Mata R, Boulter E and Burridge K:
The ‘invisible hand’: Regulation of RHO GTPases by RHOGDIs. Nat Rev
Mol Cell Biol. 12:493–504. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
DerMardirossian C and Bokoch GM: GDIs:
Central regulatory molecules in Rho GTPase activation. Trends Cell
Biol. 15:356–363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fang KP, Zhang JL, Ren YH and Qian YB:
Talin-1 correlates with reduced invasion and migration in human
hepatocellular carcinoma cells. Asian Pac J Cancer Prev.
15:2655–2661. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu W, Wang J and Wen T: Downregulation of
Rho-GDI gamma promotes differentiation of neural stem cells. Mol
Cell Biochem. 311:233–240. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhen H, Yang S, Wu H, Wang S, Lv J, Ma L
and Zhang X: LyGDI is a promising biomarker for ovarian cancer. Int
J Gynecol Cancer. 20:316–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao L, Wang H, Sun X and Ding Y:
Comparative proteomic analysis identifies proteins associated with
the development and progression of colorectal carcinoma. FEBS J.
277:4195–4204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ronneburg H, Span PN, Kantelhardt E,
Dittmer A, Schunke D, Holzhausen HJ, Sweep FC and Dittmer J: Rho
GDP dissociation inhibitor α expression correlates with the outcome
of CMF treatment in invasive ductal breast cancer. Int J Oncol.
36:379–386. 2010.PubMed/NCBI
|
|
15
|
Yamashita T, Okamura T, Nagano K, Imai S,
Abe Y, Nabeshi H, Yoshikawa T, Yoshioka Y, Kamada H, Tsutsumi Y and
Tsunoda S: Rho GDP-dissociation inhibitor alpha is associated with
cancer metastasis in colon and prostate cancer. Pharmazie.
67:253–255. 2012.PubMed/NCBI
|
|
16
|
Li W, Wang H, Jin X and Zhao L: Loss of
RhoGDI is a novel independent prognostic factor in hepatocellular
carcinoma. Int J Clin Exp Pathol. 6:2535–2541. 2013.PubMed/NCBI
|
|
17
|
Wu Y, McRoberts K, Berr SS, Frierson HF
Jr, Conaway M and Theodorescu D: Neuromedin U is regulated by the
metastasis suppressor RhoGDI2 and is a novel promoter of tumor
formation, lung metastasis and cancer cachexia. Oncogene.
26:765–773. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma L, Xu G, Sotnikova A, Szczepanowski M,
Giefing M, Krause K, Krams M, Siebert R, Jin J and Klapper W: Loss
of expression of LyGDI (ARHGDIB), a rho GDP-dissociation inhibitor,
in Hodgkin lymphoma. Br J Haematol. 139:217–223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moon HG, Jeong SH, Ju YT, Jeong CY, Lee
JS, Lee YJ, Hong SC, Choi SK, Ha WS, Park ST and Jung EJ:
Up-regulation of RhoGDI2 in human breast cancer and its prognostic
implications. Cancer Res Treat. 42:151–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cho HJ, Baek KE, Park SM, Kim IK, Choi YL,
Cho HJ, Nam IK, Hwang EM, Park JY, Han JY, et al: RhoGDI2
expression is associated with tumor growth and malignant
progression of gastric cancer. Clin Cancer Res. 15:2612–2619. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y and Zhang B: D4-GDI, a Rho GTPase
regulator, promotes breast cancer cell invasiveness. Cancer Res.
66:5592–5598. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ding J, Huang S, Wu S, Zhao Y, Liang L,
Yan M, Ge C, Yao J, Chen T, Wan D, et al: Gain of miR-151 on
chromosome 8q24.3 facilitates tumour cell migration and spreading
through downregulating RhoGDIA. Nat Cell Biol. 12:390–399. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang H, Wang B, Liao Q, An H, Li W, Jin X,
Cui S and Zhao L: Overexpression of RhoGDI, a novel predictor of
distant metastasis, promotes cell proliferation and migration in
hepatocellular carcinoma. FEBS Lett. 588:503–508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li C, Tan YX, Zhou H, Ding SJ, Li SJ, Ma
DJ, Man XB, Hong Y, Zhang L, Li L, et al: Proteomic analysis of
hepatitis B virus-associated hepatocellular carcinoma:
Identification of potential tumor markers. Proteomics. 5:1125–1139.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lai MC, Yang Z, Zhou L, Zhu QQ, Xie HY,
Zhang F, Wu LM, Chen LM and Zheng SS: Long non-coding RNA MALAT-1
overexpression predicts tumor recurrence of hepatocellular
carcinoma after liver transplantation. Med Oncol. 29:1810–1816.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yuan SX, Yang F, Yang Y, Tao QF, Zhang J,
Huang G, Yang Y, Wang RY, Yang S, Huo XS, et al: Long noncoding RNA
associated with microvascular invasion in hepatocellular carcinoma
promotes angiogenesis and serves as a predictor for hepatocellular
carcinoma patients' poor recurrence-free survival after
hepatectomy. Hepatology. 56:2231–2241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livraghi T, Makisalo H and Line PD:
Treatment options in hepatocellular carcinoma today. Scand J Surg.
100:22–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fernandez M, Semela D, Bruix J, Colle I,
Pinzani M and Bosch J: Angiogenesis in liver disease. J Hepatol.
50:604–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rosmorduc O and Housset C: Hypoxia: A link
between fibrogenesis, angiogenesis, and carcinogenesis in liver
disease. Semin Liver Dis. 30:258–270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shi L, Wu LL, Yang JR, Chen XF, Zhang Y,
Chen ZQ, Liu CL, Chi SY, Zheng JY, Huang HX, et al: Serum
peroxiredoxin3 is a useful biomarker for early diagnosis and
assessemnt of prognosis of hepatocellular carcinoma in Chinese
patients. Asian Pac J Cancer Prev. 15:2979–2986. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thomas MB, Jaffe D, Choti MM, Belghiti J,
Curley S, Fong Y, Gores G, Kerlan R, Merle P, O'Neil B, et al:
Hepatocellular carcinoma: Consensus recommendations of the national
cancer institute clinical trials planning meeting. J Clin Oncol.
28:3994–4005. 2010. View Article : Google Scholar : PubMed/NCBI
|