Introduction
Nasopharyngeal carcinoma (NPC) is a squamous cell
carcinoma caused by a combination of factors, such as viral,
dietary and genetic factors (1). It
is widely diffused in Southern China and Southeast Asia and it is
notorious for its local invasion and distant metastasis at the time
of diagnosis (2).
Chemotherapy and radiotherapy are the main
strategies to treat NPC patients (3).
However, poor prognosis and therapeutic failure are the most common
outcomes subsequent to treatment of NPC at an advanced stage, even
with concurrent chemoradiotherapy (4). Therefore, chemosensitization or
radiosensitization is essential for the successful treatment of
NPC.
Treatment with chemotherapeutic drugs activates
several biochemical signaling pathways, including the c-Jun
N-terminal kinase (JNK) pathway (5).
JNKs are serine/threonine kinases that belong to the
mitogen-activated protein kinase family (6), and are responsive to diverse stress
(7,8).
When phosphorylated by upstream kinases, JNK in turn phosphorylates
and activates three nuclear transcription factors, consisting of
c-Jun, activating transcription factor 2 and Elk-1 (9–11), which
regulate several important cellular functions, including cell
growth, differentiation, survival and apoptosis.
Previous studies have revealed the conflicting roles
of JNK, depending on tumor type (12). JNK pathway inactivation decreases
tumor growth in gastric or intestinal tumors (13,14),
indicating its pro-tumorigenic role. By contrast, JNK pathway
inactivation promotes tumor development in breast cancer (15,16), while
JNK activation can reduce cell proliferation and maintain
epithelial differentiation in non-small cell lung cancer (NSCLC)
cells (17), suggesting its
anti-tumorigenic role. The JNK pathway also mediates opposite
functions on apoptosis in a tumor type-dependent manner, such as a
pro-apoptotic role in human gastric cancer cells (18) vs. an anti-apoptotic role in SCLC cells
(19).
The present study explored the role of the JNK
pathway in NPC 5–8F cell line, as well as its influence on
chemotherapeutic sensitivity to Adriamycin. The present results may
suggest that the members of the JNK pathway could represent valid
therapeutic targets on NPC treatment.
Materials and methods
Cell culture
5-8F cells were cultured in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (GE Healthcare Life Sciences, Chalfont, UK),
100 U/ml penicillin and 100 µg/ml streptomycin (Beyotime Institute
of Biotechnology, Haimen, China) at 37°C in a 5% CO2
humidified atmosphere.
MTT assay
Briefly, cells were seeded at a density of
0.6×104 cells per well in 100 µl RPMI-1640 medium in a
96-well plate. Subsequent to incubation with different
concentrations of Adriamycin (0.1, 0.5, 1, 5 and 10 µg/ml; Zhejiang
Hisun Pharmaceutical Co. Ltd, Taizhou, China) and the JNK inhibitor
SP600125 (2.5, 5, 10, 20 and 40 µΜ; Beyotime Institute of
Biotechnology, Beijing, China) for 24 h, MTT assay was performed by
the MTT Cell Proliferation and Cytotoxicity Assay Kit (Beyotime
Institute of Biotechnology, Beijing, China). The absorbance was
measured at 490 nm using aN iMark Spectramax microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Western blot analysis
Total proteins from treated or untreated cells were
extracted by radioimmunoprecipitation buffer with protease
inhibitor (Beyotime Institute of Biotechnology), and separated by
electrophoresis in SDS-PAGE gels. Next, proteins were transferred
to polyvinylidene difluoride membranes (GE Healthcare Life
Sciences), followed by immunoblotting with 5% milk and incubation
with the following antibodies: Phosphorylated JNK (dilution,
1:1,000; catalog no., 4668; Cell Signaling Technology, Inc.,
Danvers, MA, USA); c-Jun (dilution, 1:1,000; catalog no., 9165;
Cell Signaling Technology, Inc.); phosphorylated c-Jun (dilution,
1:1,000; catalog no., 9164; Cell Signaling Technology, Inc.); and
GAPDH (dilution 1:2,000; catalog no., 2118; Cell Signaling
Technology, Inc.). The next day, membranes were washed with TBST
and incubated with secondary antibodies (dilution, 1:3,000; catalog
no., SA00001-2; ProteinTech, Chicago, IL, USA) for 1–2 h.
Subsequent to washing with TBST, an electrochemiluminescence
Luminata™ Crescendo Western horseradish peroxidase
substrate (catalog no., WBLUR0100; EMD Millipore, Billerica, Ma,
USA) was performed (GE Healthcare Life Sciences) for the detection
of immunoblotting according to the protocol of the
manufacturer.
TUNEL assay
Cells were seeded onto a 24-well plate
(2×104 cells in 0.5 ml medium per well), and treated
with Adriamycin (0.1 µg/ml) in combination with SP600125 (2.5 µΜ),
as well as each agent alone. Twenty-four h later, TUNEL assay was
performed using an In Situ Cell Death Detection kit (Roche
Applied Science, Mannheim, Germany), according to the
manufacturer's instructions. In total, three fields per slice were
randomly selected and analyzed, and each experiment was repeated
three times. The apoptotic index was calculated as the ratio of the
TUNEL positive cell number to the total cell number in each
field.
Flow cytometry
Cells were seeded onto 6-well plate
(15×104 cells in 2 ml medium per well), and treated with
Adriamycin (0.5 µg/ml) in combination with SP600125 (10 µΜ), as
well as each agent alone. Twenty-four h later, cells were collected
and stained for Annexin V-fluorescein isothiocyanate (FITC) and
propidium iodide (PI) for 15 min at room temperature, according to
the manufacturer's instructions (Invitrogen; Thermo Fisher
Scientific, Inc.). Cells were subsequently analyzed by flow
cytometry (FACScan; Becton Dickinson, Franklin Lakes, NJ, USA).
Statistical analysis
Data were analyzed by SPSS 16.0 software (SPSS,
Inc., Chicago, IL, USA) and GraphPad Prism 5.0 (GraphPad Software,
Inc., La Jolla, CA, USA), and expressed as the mean ± standard
deviation. Statistical analysis was performed using paired
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Adriamycin caused dose-dependent
cytotoxicity on NPC cells
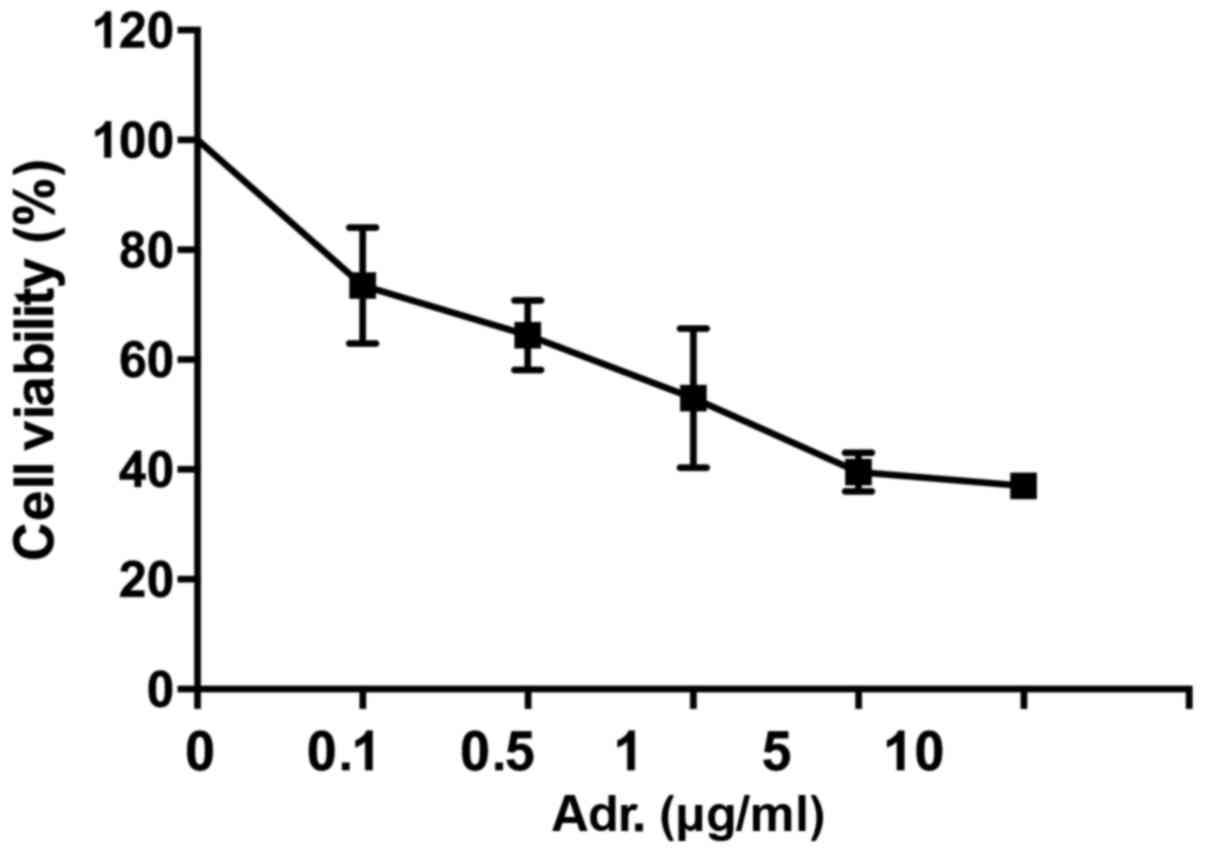
The effect of Adriamycin on NPC cells was evaluated
by MTT assay (Fig. 1). 5–8F cells
were treated with different concentrations of Adriamycin.
Twenty-four h later, the cell growth was inhibited, with this being
statistically significant in cells treated with ≥0.1 µg/ml
Adriamycin (P<0.05). These data indicated 5–8F cells were
sensitive to Adriamycin and showed concentration-dependent
sensibility.
Adriamycin activated JNK signaling
pathway in NPC cells
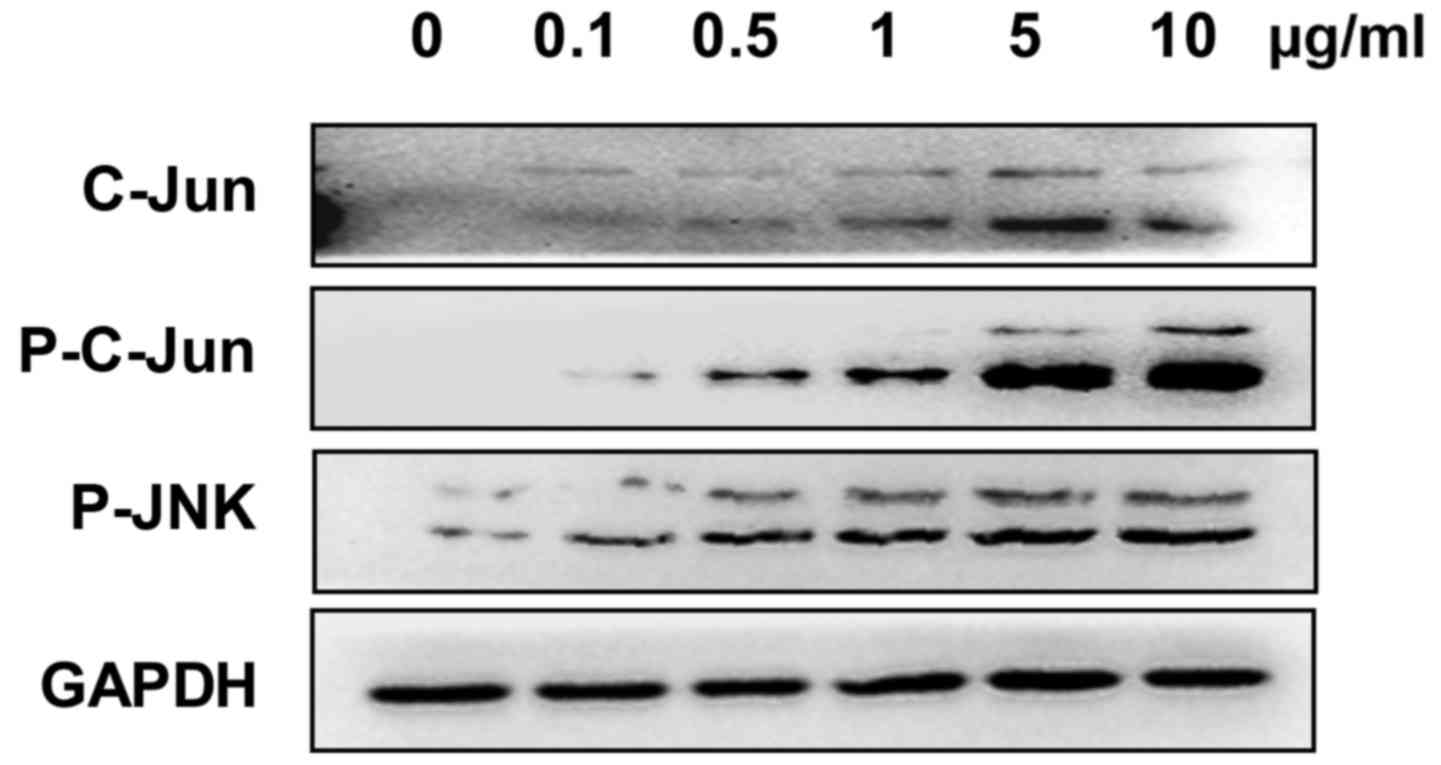
The expression of c-Jun, phosphorylated-JNK and
phosphorylated-c-Jun was analyzed to evaluate whether the JNK
signaling pathway was involved in Adriamycin-associated cell
inhibition in NPC cells. The present results showed an increased
expression of these genes in 5–8F cells treated with Adriamycin,
indicating an activation of the JNK pathway (Fig. 2).
JNK signaling pathway inhibition
caused cytotoxicity on NPC cells
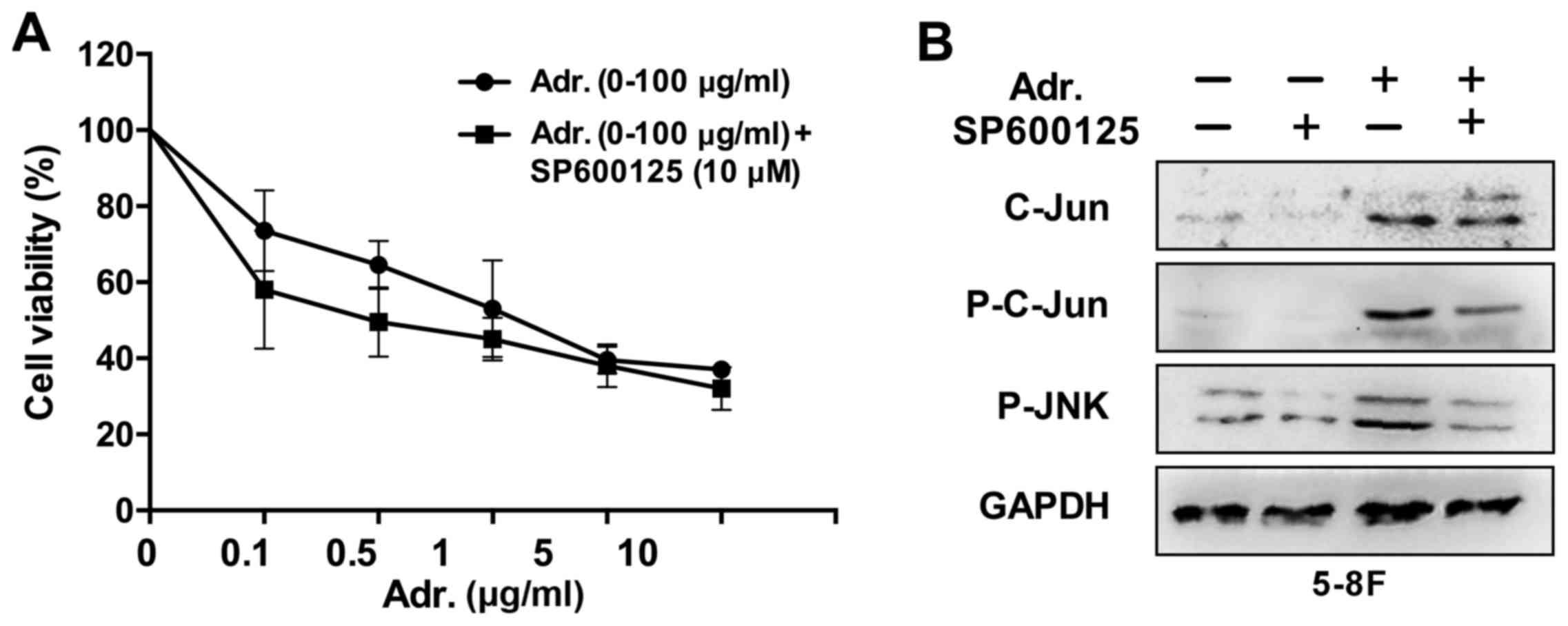
To investigate the effect of the JNK pathway on the
viability of NPC cells, 5–8F cells were treated with the JNK
pathway inhibitor SP600125 to block the signaling pathway. Western
blot analysis demonstrated high inhibition efficiency (Fig. 3B). Following treatment for 24 h at a
concentration of 5 µM, the number of 5–8F cells was 79%
(P<0.05), indicating that JNK signaling pathway inhibition can
reduce NPC cells growth (Fig.
3A).
JNK pathway inhibition sensitized NPC
cells to Adriamycin
Since the aforementioned results revealed that
Adriamycin was an effective agent against NPC cells and activated
the JNK pathway, which was a growth promoter in NPC cells, it was
examined whether JNK pathway inhibition enhanced chemosensitivity
of NPC cells to Adriamycin. In the presence of 10 µM SP600125, the
toxic effect of Adriamycin was evidently increased by 15% for 5–8F
cells (P<0.05) compared with Adriamycin alone (Fig. 4). These results provide an indication
that JNK pathway inhibition sensitized NPC cells to the cytotoxic
effects of Adriamycin.
Adriamycin combined with JNK pathway
inhibition induced apoptosis in NPC cells
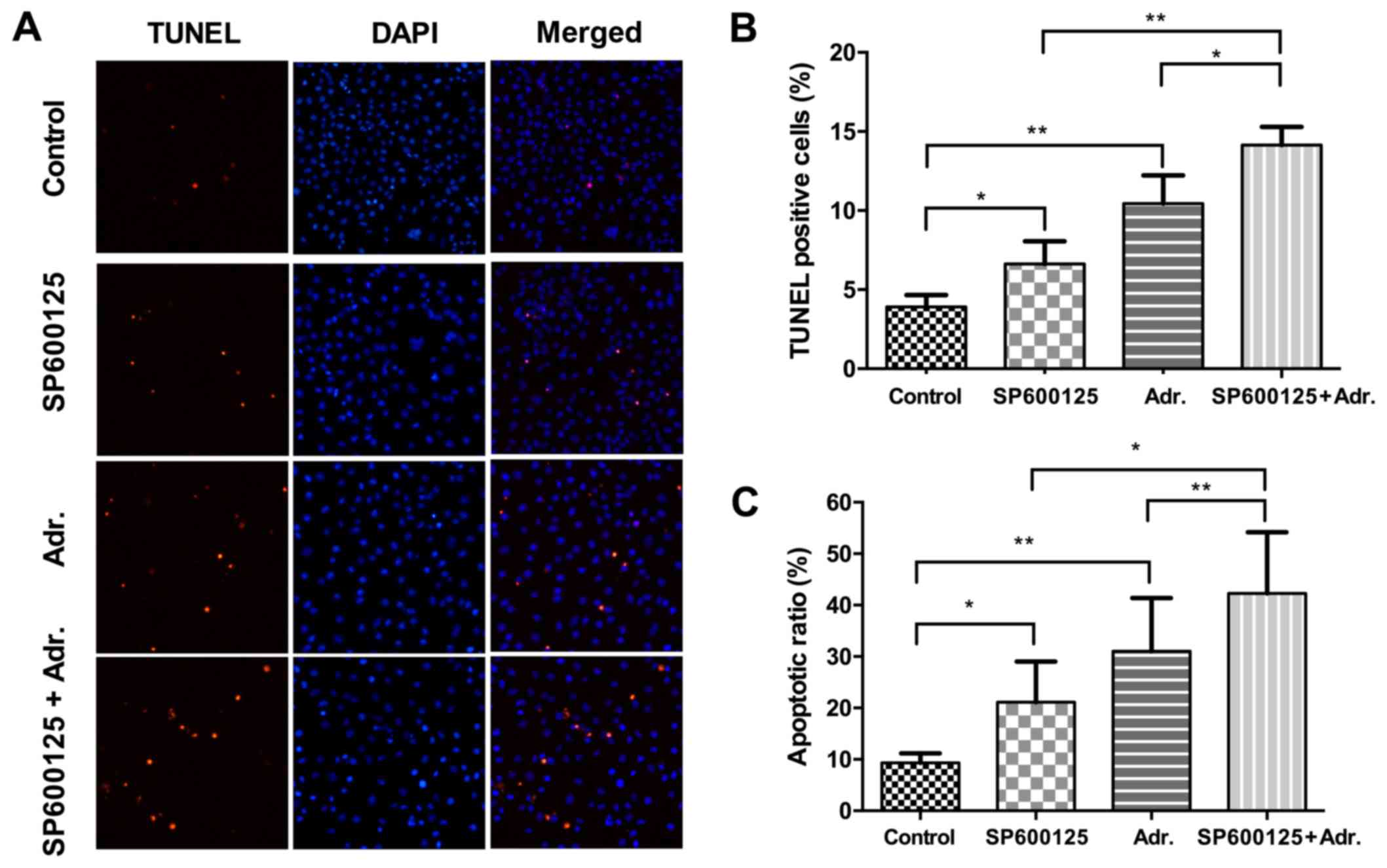
To investigate the effect of Adriamycin and JNK
pathway inhibition on apoptotic cell death, NPC cells treated with
either agent or a combination of the two were stained with Annexin
V FITC/PI and TUNEL, which were analyzed by flow cytometry and
fluorescent microscopy, respectively. As shown in Fig. 5, following treatment with low
concentrations of Adriamycin (0.1 µg/ml) or SP600125 (2.5 µM)
alone, the cellular apoptotic rate in 5–8F cells increased between
3.9 and 6.6% (P<0.05), and 3.9 and 10.4% (P<0.01),
respectively, while the combination treatment increased between 3.9
and 14.1% (P<0.001). The results were also confirmed by
treatment with increased concentrations of Adriamycin (0.5 µg/ml)
or SP600125 (10 µM), which were analyzed by flow cytometry
(Fig. 5C). These data demonstrated
increased efficiency in the apoptosis induction by Adriamycin,
combined with JNK pathway inhibition.
Discussion
Chemotherapy is the main strategy for cancer
treatment, but drug resistance occurs to limit the efficacy in
clinical use. Currently, the screening of agents inducing
chemosensitivity is the main method to overcome this problem
(20). In the present study, JNK
pathway inhibition was shown to cause cytotoxicity on NPC cells,
indicating that this signaling pathway exerts a growth promotion
function in NPC cells. 5–8F cells treated with Adriamycin showed
activated JNK signaling, which may resist Adriamycin-induced cell
death. According to these findings, 5–8F cells treated with a
combination of the JNK pathway inhibitor SP600125 at a fixed
concentration and Adriamycin at increasing concentrations showed
elevated cell death compared with cells treated with each agent
alone.
In previous years, studies have revealed multiple
complicated signaling networks in NPC growth inhibition (21,22). The
present study found that the JNK pathway was activated 24 h
subsequent to Adriamycin administration, which was consistent with
a study reporting that the JNK pathway was sensitive to cytotoxic
drugs (23).
Previous studies have described the conflicting
roles of the JNK pathway in tumor growth (13,14). In
the present study, 5–8F cells were treated with a JNK pathway
inhibitor, resulting in significantly decreased cell viability and
increased apoptotic rate, suggesting its pro-tumorigenic role and
anti-apoptotic activity.
Overall, the present study showed that the
inhibition of the JNK pathway sensitized NPC cells to the cytotoxic
effects of Adriamycin, which resulted in cell growth inhibition and
apoptosis. Since the function of the JNK pathway in cancer
pathogenesis is dependent on cancer cell type, whether this
strategy may be applied to other cancers remains unknown, and
additional studies are required.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation (grant no. 81201784), Scientific
Research Foundation of the Education Department of Sichuan Province
(grant no. 15ZA0163), and The Affiliated Hospital Of Southwest
Medical University Foundation (grant no. 201519#).
References
|
1
|
Zhang F and Zhang J: Clinical hereditary
characteristics in nasopharyngeal carcinoma through Ye-Liang's
family cluster. Chin Med J (Engl). 112:185–187. 1999.PubMed/NCBI
|
|
2
|
Li XJ, Ong CK, Cao Y, Xiang YQ, Shao JY,
Ooi A, Peng LX, Lu WH, Zhang Z, Petillo D, et al: Serglycin is a
theranostic target in nasopharyngeal carcinoma that promotes
metastasis. Cancer Res. 71:3162–3172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee AW, Lin JC and Ng WT: Current
management of nasopharyngeal cancer. Semin Radiat Oncol.
22:233–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang L, Chen QY, Liu H, Tang LQ and Mai
HQ: Emerging treatment options for nasopharyngeal carcinoma. Drug
Des Devel Ther. 7:37–52. 2013.PubMed/NCBI
|
|
5
|
Chang PY, Wu ZZ, Sun NK and Chao CC:
EBV-encoded LMP-1 sensitizes nasopharyngeal carcinoma cells to
genotoxic drugs by down-regulating Cabin1 expression. J Cell
Physiol. 229:309–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1923. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kyriakis JM and Avruch J: Mammalian MAPK
signal transduction pathways activated by stress and inflammation:
A 10-year update. Physiol Rev. 92:689–737. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vlahopoulos S and Zoumpourlis VC: JNK: A
key modulator of intracellular signaling. Biochemistry (Mosc).
69:844–854. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hibi M, Lin A, Smeal T, Minden A and Karin
M: Identification of an oncoprotein- and UV-responsive protein
kinase that binds and potentiates the c-Jun activation domain.
Genes Dev. 7:2135–2148. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gupta S, Campbell D, Dérijard B and Davis
RJ: Transcription factor ATF2 regulation by the JNK signal
transduction pathway. Science. 267:389–393. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cavigelli M, Dolfi F, Claret FX and Karin
M: Induction of c-fos expression through JNK-mediated TCF/Elk-1
phosphorylation. EMBO J. 14:5957–5964. 1995.PubMed/NCBI
|
|
12
|
Tournier C: The 2 Faces of JNK Signaling
in Cancer. Genes Cancer. 4:397–400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shibata W, Maeda S, Hikiba Y, Yanai A,
Sakamoto K, Nakagawa H, Ogura K, Karin M and Omata M: c-Jun
NH2-terminal kinase 1 is a critical regulator for the development
of gastric cancer in mice. Cancer Res. 68:5031–5039. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nateri AS, Spencer-Dene B and Behrens A:
Interaction of phosphorylated c-Jun with TCF4 regulates intestinal
cancer development. Nature. 437:281–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cellurale C, Weston CR, Reilly J, Garlick
DS, Jerry DJ, Sluss HK and Davis RJ: Role of JNK in a
Trp53-dependent mouse model of breast cancer. PLoS One.
5:e124692010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cellurale C, Girnius N, Jiang F,
Cavanagh-Kyros J, Lu S, Garlick DS, Mercurio AM and Davis RJ: Role
of JNK in mammary gland development and breast cancer. Cancer Res.
72:472–481. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Winn RA, Marek L, Han SY, Rodriguez K,
Rodriguez N, Hammond M, Van Scoyk M, Acosta H, Mirus J, Barry N, et
al: Restoration of Wnt-7a expression reverses non-small cell lung
cancer cellular transformation through frizzled-9-mediated growth
inhibition and promotion of cell differentiation. J Biol Chem.
280:19625–19634. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim R, Ohi Y, Inoue H and Toge T:
Enhancement of chemotherapeutic agents induced-apoptosis associated
with activation of c-Jun N-terminal kinase 1 and caspase 3 (CPP32)
in bax-transfected gastric cancer cells. Anticancer Res.
20:439–444. 2000.PubMed/NCBI
|
|
19
|
Levresse V, Marek L, Blumberg D and
Heasley LE: Regulation of platinum-compound cytotoxicity by the
c-Jun N-terminal kinase and c-Jun signaling pathway in small-cell
lung cancer cells. Mol Pharmacol. 62:689–697. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vadlapatla RK, Vadlapudi AD, Pal D and
Mitra AK: Mechanisms of drug resistance in cancer chemotherapy:
Coordinated role and regulation of efflux transporters and
metabolizing enzymes. Curr Pharm Des. 19:7126–7140. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin L, Deng W, Tian Y, Chen W, Wang J, Fu
L, Shi D, Zhao M and Luo W: Lasiodin inhibits proliferation of
human nasopharyngeal carcinoma cells by simultaneous modulation of
the Apaf-1/caspase, AKT/MAPK and COX-2/NF-κB signaling pathways.
PLoS One. 9:e977992014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chou J, Lin YC, Kim J, You L, Xu Z, He B
and Jablons DM: Nasopharyngeal carcinoma-review of the molecular
mechanisms of tumorigenesis. Head Neck. 30:946–963. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kyriakis JM and Avruch J: Mammalian
mitogen-activated protein kinase signal transduction pathways
activated by stress and inflammation. Physiol Rev. 81:807–869.
2001.PubMed/NCBI
|