Introduction
Squamous cell carcinoma (SCC) is the leading cancer
type affecting the head and neck region (HNSCC) representing
>90% of malignant neoplasms of the oral cavity and oropharynx
and 5 and 2% of all cancers in men and women, respectively
(1). Tobacco smoking and alcohol
consumption are the two major etiological factors of HNSCC and they
work in a synergistic manner to initiate SCC (1). Approximately 75% of HNSCC cases in
western countries are related to these two major risk factors,
while the remainder is predominantly associated with viral
infection, in particular infection with high-risk human
papillomavirus (HPV) (1). HPV has
been detected in a small fraction of oral cavity SCCs, and in
40–50% of oropharyngeal (mainly tonsillar) SCC (OPSCC) (1). While HPV-associated HNSCC may occur at
any site, including the sinonasal tract, the majority of
HPV-related SCCs originate in the lymphoid tissue bearing the
oropharynx, mainly the palatine tonsil and the base of the tongue
(1). Expression of p16 as a
consequence of oncogenic HPV infection has been increasingly used
as a surrogate marker for high-risk HPV infection, particularly in
the female genital tract and the oropharynx (2,3). The
sensitivity and specificity of p16 immunostaining for detecting
high-risk HPV in OPSCC patients has been in the range of 95–98%
(2,4–6). However,
the value of p16 immunohistochemistry as a surrogate marker for HPV
infection in head and neck sites other than the oropharynx,
including the oral cavity, larynx, hypopharynx and sinonasal tract,
has yet to be determined.
The human insulin-like growth factor II mRNA binding
protein 3 (IMP3), which was originally named KH domain-containing
protein overexpressed in cancer, plays an important role in early
human embryogenesis, although it is commonly expressed only at low
levels in adult tissues. IMP3 is considered an oncofetal protein
that is expressed in fetal and carcinogenic tissue; however, the
exact function of IMP3 remains unknown (7–12).
Expression of IMP3 has been studied in a wide variety of cancers,
including renal cell carcinoma (13,14),
adenocarcinoma of the uterine cervix (15), endometrial carcinoma (16), adenocarcinoma of the esophagus
(17), malignant melanoma (18), Merkel cell carcinoma (19), urothelial carcinoma (20), neuroendocrine carcinoma of the lung
(21), gastric adenocarcinoma
(22), hepatocellular carcinoma
(23), pancreatic (24–27) and
biliary tract (27) adenocarcinoma,
and triple negative breast cancer (28), where this marker has frequently been
associated with enhanced tumor aggressiveness and a worse
outcome.
However, only a few studies have previously
investigated the expression of IMP3 in head and neck SCC from
specific sites (29–34). In particular, the pattern of IMP3
expression in HNSCC with regards to the HPV status has not
previously been investigated. The aim of the current study was to
compare the expression pattern of IMP3 (as a ‘bad’ marker) with
that of p16 (as a ‘good’ marker) in HNSCC, with special reference
to a patient's HPV status.
Materials and methods
Patients and tissue samples
The present study involved 156 consecutive patients
diagnosed with HNSCC at the Institute of Pathology, University
Hospital, Friedrich-Alexander University Erlangen-Nuremberg
(Erlangen, Germany) and the OptiPath, Pathology Joint Practice
(Frankfurt, Germany) between January 1998 and December 2015. The
localizations of the SCCs were as follows: 81 (51.9%) from the
oropharynx, 44 (28.2%) from the oral cavity, 15 (9.6%) from the
larynx, 10 (6.4%) from the hypopharynx and 6 (3.9%) from the
nasopharynx. Only biopsy specimens were used in this study. The
present study was performed in accordance with accepted principles
of ethical and professional conduct for biomedical scientific
research, and it was approved by the ethical committee of the
medical faculty of the Friedrich-Alexander University
Erlangen-Nuremberg for retrospective translational research
activities.
Formalin-fixed (overnight at room temperature) and
paraffin-embedded biopsy specimens were cut into sections (3-µm)
and mounted onto Superfrost™ slides (Menzel-Gläser; Thermo Fisher
Scientific, Inc., Braunschweig, Germany). All biopsies were stained
with hematoxylin and eosin on eight step sections.
Immunohistochemistry with anti-IMP3 (clone 69,1; dilution 1:100;
catalog no., M362629-2; Dako, Glostrup, Denmark) and anti-p16
(clone JC8; dilution 1:100; catalog no., sc-56330; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) antibodies was performed on
1-µm sections prepared from paraffin blocks using a fully automated
slide preparation system (BenchMark XT System; Ventana Medical
Systems Inc., Tucson, AZ, USA). The slides were evaluated by two
independent pathologists. IMP3 staining was evaluated
semi-quantitatively using a system for staining intensity, as
described previously (24): 0 (no
staining); 1+ (moderate to strong staining in <25% of cells or
weak staining to any extent); 2+ (moderate to strong staining in
25–50% of cells); and 3+ (moderate to strong staining in >50% of
cells).
Tumors were then grouped into two groups of
IMP3-(negative/weak staining, 0/1+) and IMP3+ (moderate/strong
staining, 2+/3+). Tumors were considered positive for p16 when
strong nuclear and cytoplasmic staining was present in >50% of
cells (5). The validity of the
anti-p16 antibody as a surrogate marker for HPV was confirmed by
comparing it to the CINtech protocol involving a large cohort of
routine specimens stained in parallel with both antibodies (the
anti-p16 antibody, clone JC8, and the CINtech protocol) at the
Institute of Pathology, University Hospital, Friedrich-Alexander
University Erlangen-Nuremberg (Erlangen, Germany) (Agaimy et
al, unpublished data).
HPV status
To determine whether there is an association between
the expression of the two biomarkers and HPV status, 38 tumors were
randomly selected to represent the four possible patterns of p16
and IMP3 expression: p16+/IMP3-(n=9), p16-/IMP3+ (n=10), p16+/IMP3+
(n=11) and p16-/IMP3- (n=8). Molecular testing for HPV in the 38
cases was performed as described previously (34).
Statistical analysis
Fisher's exact test was used to test for
associations in contingency tables (or alternatively the
χ2 test was used for tables with at least three rows and
three columns). The sensitivity, specificity and predictive values
of potential markers were determined based on 2×2-tables and are
presented together with their exact 95% confidence intervals (CIs).
The statistical analyses were performed with SAS 9.3 (SAS Institute
INC., Cary, NC, USA).
Results
IMP3 expression in HNSCC
Of the 156 HNSCCs, 81 (51.9%) were positive for IMP3
(Table I). There was an association
between IMP3 positivity and HNSCC localization (P=0.022), with 50
(61.7%) of the OPSCCs being positive for IMP3 compared with only 16
(36.4%) of the oral cavity SCC cases (Table II). No positive association between
IMP3 expression and HPV status was observed; however, a trend
towards a negative association was apparent (P=0.053; Table III). Expression of IMP3 was strictly
cytoplasmic, as compared with the characteristic combined
nucleocytoplasmic expression of p16 (Fig.
1).
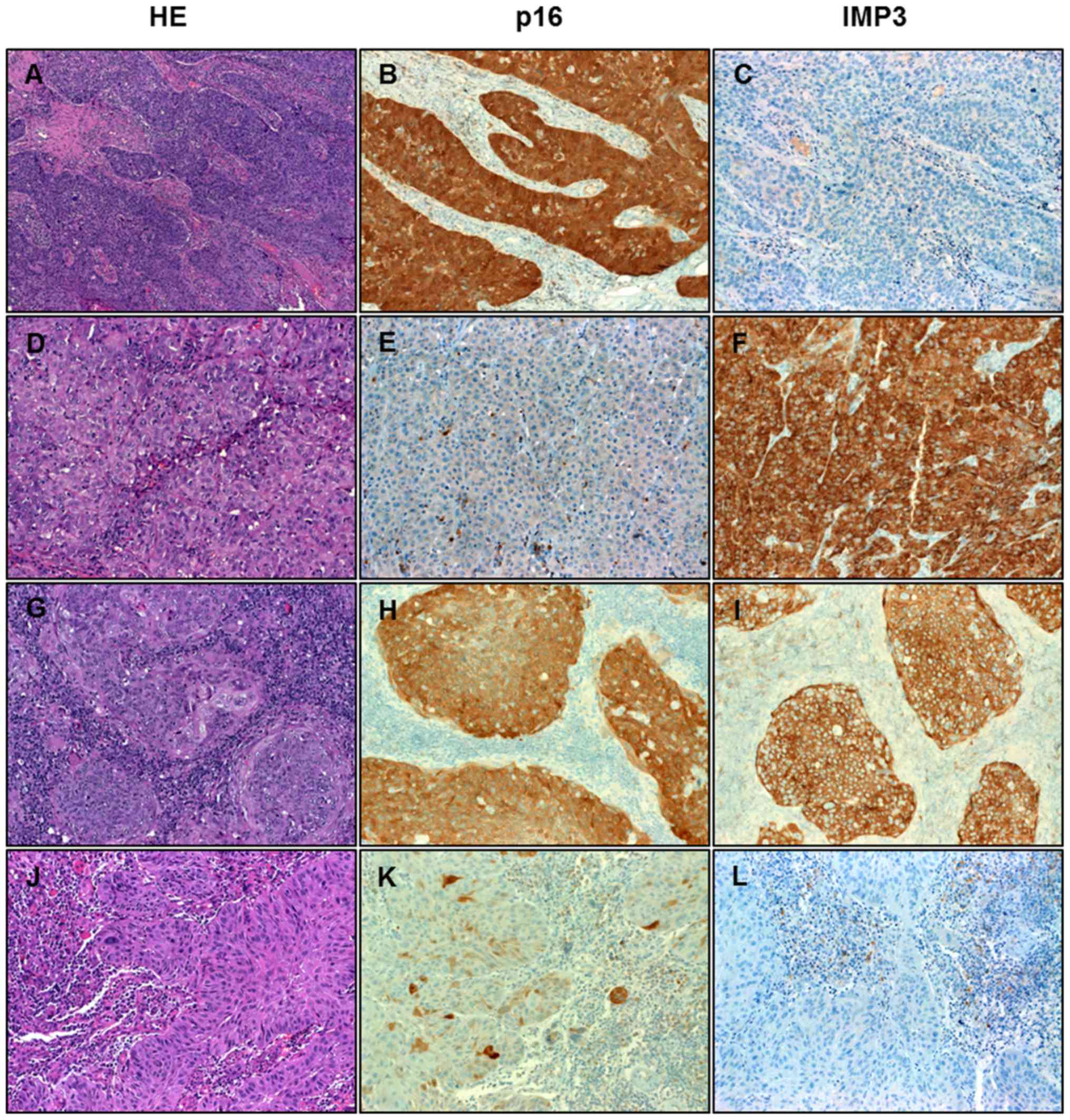 | Figure 1.Immunohistochemical analysis of HNSCC.
Representative examples of the four different expression patterns
of p16 and IMP3 in HNSCC. (A, B, C) HNSCC showing strong expression
of p16 but not IMP3. (D, E, F) HNSCC negative with p16 and strongly
positive for IMP3. (G, H, I) HNSCC expressing both markers. (J, K,
L) HNSCC negative for both markers. HNSCC, head and neck squamous
cell carcinoma; IMP3, insulin-like growth factor II mRNA binding
protein 3; HE, hematoxylin and eosin. Magnification, ×200. |
 | Table I.Association between IMP3 and p16
expression in 156 head and neck squamous cell carcinomas
(P=0.61). |
Table I.
Association between IMP3 and p16
expression in 156 head and neck squamous cell carcinomas
(P=0.61).
|
| IMP3 |
|
|---|
|
|
|
|
|---|
| p16 | Negative | Positive | Total |
|---|
| Negative | 51 (50) | 51 (50) | 102 (100) |
| Positive | 24 (44.4) | 30 (55.6) | 54
(100) |
| Total | 75 (48.1) | 81 (51.9) | 156 (100) |
 | Table II.Immunoprofiles of IMP3 and p16 in
association with localization of oral squamous cell carcinomas. |
Table II.
Immunoprofiles of IMP3 and p16 in
association with localization of oral squamous cell carcinomas.
| Marker | Oral cavity | Oro-pharynx | Naso-pharynx | Hypo-pharynx | Larynx | P-value |
|---|
| IMP3 |
|
|
|
|
| 0.022 |
|
Negative | 28 (63.6) | 31 (38.3) | 1 (16.7) | 6 (60.0) | 9 (60.0) |
|
|
Positive | 16 (36.4) | 50 (61.7) | 5 (83.3) | 4 (40.0) | 6 (40.0) |
|
| p16 |
|
|
|
|
| <0.001 |
|
Negative | 39 (88.6) | 38 (46.9) | 5 (83.3) | 7 (70.0) | 13 (86.7) |
|
|
Positive | 5 (11.4) | 43 (53.1) | 1 (16.7) | 3 (30.0) | 2 (13.3) |
|
 | Table III.Sensitivities, specificities,
negative predictive values and positive predictive values of IMP3
and/or p16 expression for predicting the human papillomavirus
status in head and neck squamous cell carcinoma. |
Table III.
Sensitivities, specificities,
negative predictive values and positive predictive values of IMP3
and/or p16 expression for predicting the human papillomavirus
status in head and neck squamous cell carcinoma.
| Marker
combination | P-value | Sensitivity (95%
CI) | Specificity (95%
CI) | Negative predictive
value (95% CI) | Positive predictive
value (95% CI) |
|---|
| IMP3 | 0.053 | 0.47
(0.28–0.66) | 0.13
(0.01–0.53) | 0.06
(0.01–0.29) | 0.67
(0.43–0.85) |
| p16 | 0.017 | 0.63
(0.44–0.80) | 0.88
(0.47–0.99) | 0.39
(0.17–0.64) | 0.95
(0.75–0.99) |
| Combination
IMP3/p16 | 0.391 | 0.13
(0.01–0.53) | 0.77
(0.58–0.90) | 0.13
(0.01–0.53) | 0.77
(0.58–0.90) |
To address the question of whether IMP3 is a
suitable surrogate marker for HPV infection, we calculated the
sensitivity (i.e. the proportion of HPV positives that were
correctly identified) specificity (i.e. the proportion of HPV
negatives that were correctly identified) negative predictive value
(NPV; i.e. the proportion of IMP3 negatives that were also HPV
negative) and positive predictive value (PPV; i.e. the proportion
of IMP3 positives that were also HPV positive) were calculated. For
IMP3, the sensitivity was 0.47 (95% CI=0.28–0.66), the specificity
was 0.13 (95% CI=0.01–0.53), the NPV was 0.06 (95% CI=0.01–0.29)
and the PPV was 0.67 (95% CI=0.43–0.85) (Table III).
p16 expression in HNSCC
Of the 156 HNSCCs, 54 (34.6%) were positive for p16
(Table I). The expression of p16 was
observed most frequently in OPSCCs (53.1 vs. ≤30% for other
localization; P<0.001; Table II).
p16 expression was also significantly associated with HPV status
(P=0.017). The specificity of p16 for HPV status in the present
study was 0.88 (95% CI=0.47–0.99), combined with a sensitivity of
0.63 (95% CI=0.44–0.80), an NPV of 0.39 (95% CI=0.17–0.64) and a
PPV of 0.95 (95% CI=0.75–0.99) (Table
III). Although a cutoff of >50% for positive p16 results was
applied, as in a previous study (5),
all positive cases showed expression of 80–100% in tumor cells,
which is in line with the known pattern of p16 in OPSCC (5).
Comparative expression of IMP3 and p16
in OPSCC vs
non-OPSCC and correlation with HPV status. IMP3 and
p16 were expressed in 81 (51.9%) and 54 (34.6%) of the HNSCC cases,
respectively (Table I). When
combining the results of IMP3 and p16, it was found that 75 (48.1%)
were positive for either one of the markers, 51 (32.7%) were
negative for both markers and 30 (19.2%) were positive for both
markers. These findings were associated with the localization of
the tumors (P<0.001; Table IV).
Notably, 25/44 (56.8%) of the oral cavity SCCs were negative for
IMP3 and p16, as compared with only 12/81 (14.81%) of the OPSCCs
(Table IV).
 | Table IV.Immunoprofiles of the combination of
IMP3 and p16 in association with the localization of oral squamous
cell carcinomas (P<0.001). |
Table IV.
Immunoprofiles of the combination of
IMP3 and p16 in association with the localization of oral squamous
cell carcinomas (P<0.001).
| Marker
combination | Oral cavity | Oro-pharynx | Naso-pharynx | Hypo-pharynx | Larynx | Total |
|---|
| IMP3 & p16
negative | 25 (56.8) | 12 (14.8) | 1 (16.7) | 5
(50.0) | 8
(53.3) | 51 (32.7) |
| Either IMP3
positive or p16 positive | 17 (38.6) | 45 (55.6) | 4 (66.7) | 3
(30.0) | 6
(40.0) | 75 (48.1) |
| IMP3 & p16
positive | 2 (4.6) | 24 (29.6) | 1 (16.6) | 2
(20.0) | 1
(16.7) | 30 (19.2) |
| Total | 44 (100) | 81 (100) | 6 (100) | 10 (100) | 15 (100) | 156 (100) |
Although the combination of the expression of IMP3
and p16 was not significantly associated with the HPV status
(P=0.391), it is interesting to note that 10/11 (90.9%) of the
tumors coexpressing IMP3 and p16 were HPV positive by polymerase
chain reaction (Table III).
In the present study, the specificity for the
combination of p16 and IMP3 in predicting the HPV status was 0.77
(95% CI=0.58–0.90), combined with a sensitivity of 0.13 (95%
CI=0.01–0.53), an NPV of 0.13 (95% CI=0.01–0.53) and a PPV of 0.77
(95% CI=0.58–0.90). These results suggest that the variable
combinations of p16 and/or IMP3 expression do not qualify as
predictive markers for HPV infection in HNSCC (Table III).
HPV status
In the subgroup analyzed for HPV status (n=38
cases), 30 (79.0%) were HPV positive, which showed an association
with the localization (P=0.029); all 19 (100%) OPSCCs were HPV
positive (data not shown).
Discussion
Since its discovery in 1997, several studies have
investigated the expression status of IMP3 in various neoplasms and
in normal tissues in order to understand its role in diseases
(9). IMP3 is overexpressed in human
cancer, but it also has a ubiquitous role in early embryogenesis,
including in the development of the intestine, thymus, pancreas and
kidneys (8). Functional studies have
shown that IMP3 possibly influences tumor cell adhesion and
enhances invasive growth in cancer (11,12).
However, currently, the actual role of IMP3 in the modulation of
tumor cell function and invasiveness remains poorly understood.
Although extensively studied in several cancer
types, only a few studies have previously investigated the
expression status of IMP3 in HNSCC. IMP3 overexpression in SCC of
the oral cavity was shown to be an independent predictive marker
for an advanced clinical stage, lymph node metastasis and as a
prognostic indicator (29–31). Furthermore, it has been reported to be
an indicator of a poor prognosis and a marker of enhanced
malignancy in SCC of the tongue and larynx (32–35). In
addition, it has been shown to be expressed in certain malignant
salivary gland tumors (36).
Little is known about the interactions between IMP3
and p16 expression in cancer. A few studies investigating
carcinomas of the uterine cervix focused on this subject. Li et
al (16) reported that a
combination of these markers may be helpful in the distinction of
adenocarcinoma from benign endocervical glands in situ.
Furthermore, another study found that the combination of p16/IMP3
expression improved the discrepant results between cytological and
histological diagnoses (37). In
HIV-positive patients, IMP3 showed a higher sensitivity than p16
for identifying patients at risk of progression and recurrence of
cervical intraepithelial neoplasia (38).
To the best of our knowledge, no previous study has
investigated the expression of IMP3 in comparison with p16 and/or
HPV status in HNSCC, which was the aim of the present pilot study.
The present study analyzed and compared the expression status of
IMP3 and p16 in a cohort of HNSCCs enriched with HPV-positive
OPSCCs. The current study showed that IMP3 and p16 were frequently
expressed in SCCs of the head and neck; in particular, more
frequently in oropharyngeal carcinomas, as compared with other
sites.
The present study demonstrated that IMP3 was more
frequently expressed in HNSCC than p16 (51.9 vs. 34.6%,
respectively). Furthermore, IMP3 expression was significantly
associated with localization to the oropharynx (P=0.022), as it was
expressed in 61.7% of OPSCC cases compared with only 41.3% of SCCs
from other head and neck sites.
In contrast to the results observed for p16, a trend
towards a negative association between IMP3 expression and HPV
status was observed in the present study. Therefore, the potential
role of IMP3 as a marker for aggressiveness in HPV-related and
non-HPV-related HNSCC, as well as its prognostic impact, require
verification in larger cohorts with an extended follow-up
period.
A previous study showed promising results in support
of the use of IMP3 as a potential vaccination therapy in patients
with HNSCC (39). A new Phase II
study using IMP3 as a vaccination therapy in patients with HNSCC
showed an immune response and an improved overall survival
(39). These developments underline
the relevance of further in-depth studies in the future to
determine the exact roles of IMP3 in different molecular and
etiological subtypes of HNSCC.
In summary, the present study analyzed and compared
the expression status of p16 and IMP3, as well as the HPV status,
in a cohort of HNSCCs enriched with HPV-positive OPSCC. The results
of the present study highlighted a potential role for IMP3 as a
biomarker in HPV-positive HNSCC. Further studies involving larger
cohorts with an extended follow-up are required to validate the
value of combined p16/IMP3 expression as predictive prognostic
and/or therapeutic biomarkers in HNSCC.
References
|
1
|
Barnes L, Eveson JW, Reichart P and
Sidransky D: World Health Organization Classification of Tumours.
Pathology and Genetics of Head and Neck Tumours. Lyon, France: IARC
Press; pp. 46–50. 2005
|
|
2
|
Jo VY, Mills SE, Stoler MH and Stelow EB:
Papillary squamous cell carcinoma of the head and neck: Frequent
association with human papillomavirus infection and invasive
carcinoma. Am J Surg Pathol. 33:1720–1724. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Benevolo M, Mottolese M, Marandino F,
Vocaturo G, Sindico R, Piperno G, Mariani L, Sperduti I, Canalini
P, Donnorso RP and Vocaturo A: Immunohistochemical expression of
p16(INK4a) is predictive of HR-HPV infection in cervical low-grade
lesions. Mod Pathol. 19:384–391. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lewis JS Jr, Thorstad WL, Chernock RD,
Haughey BH, Yip JH, Zhang Q and El-Mofty SK: p16 positive
oropharyngeal squamous cell carcinoma: An entity with a favorable
prognosis regardless of tumor HPV status. Am J Surg Pathol.
34:1088–1096. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mehrad M, Carpenter DH, Chernock RD, Wang
H, Ma XJ, Luo Y, Luo J, Lewis JS Jr and El-Mofty SK: Papillary
squamous cell carcinoma of the head and neck: Clinicopathologic and
molecular features with special reference to human papillomavirus.
Am J Surg Pathol. 37:1349–1356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cai C, Chernock RD, Pittman ME, El-Mofty
SK, Thorstad WL and Lewis JS Jr: Keratinizing-type squamous cell
carcinoma of the oropharynx: p16 overexpression is associated with
positive high-risk HPV status and improved survival. Am J Surg
Pathol. 38:809–815. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Müeller-Pillasch F, Lacher U, Wallrapp C,
Micha A, Zimmerhackl F, Hameister H, Varga G, Friess H, Büchler M,
Beger HG, et al: Cloning of a gene highly overexpressed in cancer
coding for a novel KH-domain containing protein. Oncogene.
14:2729–2733. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mueller-Pillasch F, Pohl B, Wilda M,
Lacher U, Beil M, Wallrapp C, Hameister H, Knöchel W, Adler G and
Gress TM: Expression of the highly conserved RNA binding protein
KOC in embryogenesis. Mech Dev. 88:95–99. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nielsen FC, Nielsen J and Christiansen J:
A family of IGF-II mRNA binding proteins (IMP) involved in RNA
trafficking. Scand J Clin Lab Invest Suppl. 234:93–99. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Monk D, Bentley L, Beechey C, Hitchins M,
Peters J, Preece MA, Stanier P and Moore GE: Characterisation of
the growth regulating gene IMP3, a candidate for Silver-Russell
syndrome. J Med Genet. 39:575–581. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liao B, Hu Y, Herrick DJ and Brewer G: The
RNA-binding protein IMP-3 is a translational activator of
insulin-like growth factor II leader-3 mRNA during proliferation of
human K562 leukemia cells. J Biol Chem. 280:18517–18524. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vikesaa J, Hansen TV, Jønson L, Borup R,
Wewer UM, Christiansen J and Nielsen FC: RNA-binding IMPs promote
cell adhesion and invadopodia formation. EMBO J. 25:1456–1468.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoffmann NE, Sheinin Y, Lohse CM, Parker
AS, Leibovich BC, Jiang Z and Kwon ED: External validation of IMP3
expression as an independent prognostic marker for metastatic
progression and death for patients with clear cell renal cell
carcinoma. Cancer. 112:1471–1479. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang Z, Lohse CM, Chu PG, Wu CL, Woda BA,
Rock KL and Kwon ED: Oncofetal protein IMP3: A novel molecular
marker that predicts metastasis of papillary and chromophobe renal
cell carcinomas. Cancer. 112:2676–2682. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li C, Rock KL, Woda BA, Jiang Z, Fraire AE
and Dresser K: IMP3 is a novel biomarker for adenocarcinoma in situ
of the uterine cervix: An immunohistochemical study in comparison
with p16(INK4a) expression. Mod Pathol. 20:242–247. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li C, Zota V, Woda BA, Rock KL, Fraire AE,
Jiang Z, Lu D, Xu B, Dresser K, Lutman CV and Fischer AH:
Expression of a novel oncofetal mRNA-binding protein IMP3 in
endometrial carcinomas: Diagnostic significance and
clinicopathologic correlations. Mod Pathol. 20:1263–1268. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu D, Vohra P, Chu PG, Woda B, Rock KL and
Jiang Z: An oncofetal protein IMP3: A new molecular marker for the
detection of esophageal adenocarcinoma and high-grade dysplasia. Am
J Surg Pathol. 33:521–525. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pryor JG, Bourne PA, Yang Q, Spaulding BO,
Scott GA and Xu H: IMP-3 is a novel progression marker in malignant
melanoma. Mod Pathol. 21:431–437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pryor JG, Simon RA, Bourne PA, Spaulding
BO, Scott GA and Xu H: Merkel cell carcinoma expresses K homology
domain-containing protein overexpressed in cancer similar to other
high-grade neuroendocrine carcinomas. Hum Pathol. 40:238–243. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sitnikova L, Mendese G, Liu Q, Woda BA, Lu
D, Dresser K, Mohanty S, Rock KL and Jiang Z: IMP3 predicts
aggressive superficial urothelial carcinoma of the bladder. Clin
Cancer Res. 14:1701–1706. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu H, Bourne PA, Spaulding BO and Wang HL:
High-grade neuroendocrine carcinomas of the lung express K homology
domain containing protein overexpressed in cancer but carcinoid
tumors do not. Hum Pathol. 38:555–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Strehl JD, Hoegel J, Hornicek I, Hartmann
A and Riener MO: Immunohistochemical expression of IMP3 and p53 in
inflammatory lesions and neoplastic lesions of the gastric mucosa.
Int J Clin Exp Pathol. 7:2091–2101. 2014.PubMed/NCBI
|
|
23
|
Wachter DL, Kristiansen G, Soll C,
Hellerbrand C, Breuhahn K, Fritzsche F, Agaimy A, Hartmann A and
Riener MO: Insulin-like growth factor II mRNA-binding protein 3
(IMP3) expression in hepatocellular carcinoma. A
clinicopathological analysis with emphasis on diagnostic value.
Histopathology. 60:278–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wachter DL, Schlabrakowski A, Hoegel J,
Kristiansen G, Hartmann A and Riener MO: Diagnostic value of
immunohistochemical IMP3 expression in core needle biopsies of
pancreatic ductal adenocarcinoma. Am J Surg Pathol. 35:873–877.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yantiss RK, Woda BA, Fanger GR, Kalos M,
Whalen GF, Tada H, Andersen DK, Rock KL and Dresser K: KOC (K
homology domain containing protein overexpressed in cancer): A
novel molecular marker that distinguishes between benign and
malignant lesions of the pancreas. Am J Surg Pathol. 29:188–195.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao H, Mandich D, Cartun RW and Ligato S:
Expression of K homology domain containing protein overexpressed in
cancer in pancreatic FNA for diagnosing adenocarcinoma of pancreas.
Diagn Cytopathol. 35:700–704. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Riener MO, Fritzsche FR, Clavien PA,
Pestalozzi BC, Probst-Hensch N, Jochum W and Kristiansen G: IMP3
expression in lesions of the biliary tract: A marker for high-grade
dysplasia and an independent prognostic factor in bile duct
carcinomas. Hum Pathol. 40:1377–1383. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Walter O, Prasad M, Lu S, Quinlan RM,
Edmiston KL and Khan A: IMP3 is a novel biomarker for triple
negative invasive mammary carcinoma associated with a more
aggressive phenotype. Hum Pathol. 40:1528–1533. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin CY, Chen ST, Jeng YM, Yeh CC, Chou HY,
Deng YT, Chang CC and Kuo MY: Insulin-like growth factor II
mRNA-binding protein 3 expression promotes tumor formation and
invasion and predicts poor prognosis in oral squamous cell
carcinoma. J Oral Pathol Med. 40:699–705. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li S, Cha J, Kim J, Kim KY, Kim HJ, Nam W
and Cha IH: Insulin-like growth factor II mRNA-binding protein 3: A
novel prognostic biomarker for oral squamous cell carcinoma. Head
Neck. 33:368–374. 2011.PubMed/NCBI
|
|
31
|
Kim KY, Li S, Cha JD, Zhang X and Cha IH:
Significance of molecular markers in survival prediction of oral
squamous cell carcinoma. Head Neck. 34:929–936. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li HG, Han JJ, Huang ZQ, Wang L, Chen WL
and Shen XM: IMP3 is a novel biomarker to predict metastasis and
prognosis of tongue squamous cell carcinoma. J Craniofac Surg.
22:2022–2025. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Clauditz TS, Wang CJ, Gontarewicz A,
Blessmann M, Tennstedt P, Borgmann K, Tribius S, Sauter G, Dalchow
C, Knecht R, et al: Expression of insulin-like growth factor II
mRNA-binding protein 3 in squamous cell carcinomas of the head and
neck. J Oral Pathol Med. 42:125–132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen K, Cornejo KM, Ye W, Wu Q, Liang J
and Jiang Z: Oncofetal protein IMP3: A new diagnostic biomarker for
laryngeal carcinoma. Hum Pathol. 44:2126–2131. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Knör M, Tziridis K, Agaimy A, Zenk J and
Wendler O: Human Papillomavirus (HPV) Prevalence in Nasal and
Antrochoanal Polyps and association with clinical data. PLoS One.
10:e01417222015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ismerim AB, Ferreira SV, Lessa AM, Júnior
AS Pereira, Gurgel CA, Coutinho-Camillo CM, Soares FA, Vilas-Bôas
DS, Vidal MT and Santos JN: Insulin-like growth factor II messenger
RNA-binding protein 3 in Salivary Gland tumors. Appl
Immunohistochem Mol Morphol. 24:422–426. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wei Q, Fu B, Liu J, Xu J and Zhao T:
Combined detection of p16(INK4a) and IMP3 increase the concordance
rate between cervical cytologic and histologic diagnosis. Int J
Clin Exp Pathol. 6:1549–1557. 2013.PubMed/NCBI
|
|
38
|
Del Gobbo A, Bonoldi E, Cribiù FM,
Franceschetti I, Matinato C, Fiori S, Gianelli U and Bosari S:
Insulin-like growth factor II mRNA binding protein 3 (IMP3)
expression in cervical intraepithelial neoplasia and its
relationship with HIV-infection status. Sex Health. 12:22–26. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yoshitake Y, Fukuma D, Yuno A, Hirayama M,
Nakayama H, Tanaka T, Nagata M, Takamune Y, Kawahara K, Nakagawa Y,
et al: Phase II clinical trial of multiple peptide vaccination for
advanced head and neck cancer patients revealed induction of immune
responses and improved OS. Clin Cancer Res. 21:312–321. 2015.
View Article : Google Scholar : PubMed/NCBI
|















