Introduction
Lung cancer is the most commonly diagnosed type of
cancer, with 1.82 million new cases diagnosed in 2012 and 1.6
million associated mortalities annually, which makes it the most
common cause of cancer-associated mortality worldwide (1). Lung cancer has two primary types,
small-cell lung carcinoma and non-small cell lung carcinoma
(NSCLC), with NSCLC being more prevalent (2). The 5-year survival rate following
initial diagnoses of patients with NSCLC is only 5–15%, which means
it is the most lethal type of cancer (3). The treatment of NSCLC was linked with
targeting the epidermal growth factor receptor (EGFR) by Wrann
et al (4) over four decades
ago. Inhibition of the EGFR signaling pathway by kinase inhibitors
and monoclonal antibodies is a viable option for therapy of NSCLC
(5–7).
The kinase inhibitor response to patients with NSCLC
is linked to mutations in the EGFR protein, and the mutations of
EGFR are classified into activating mutations and second mutations;
the latter are associated with lung cancer and the former mutations
cause drug resistance (8–10). The efficacy of kinase inhibitors is
associated with the mutational change from threonine to methionine
at amino acid position 790 (T790M); this mutation is associated
with disease resistance by sterically blocking kinase inhibitors,
including gefitinib and erlotinib (11–13). The
location of this threonine at position 790 acts as a gatekeeper, as
it is located at the entrance of a hydrophobic groove at the back
of the adenosine triphosphate binding pocket (14).
Atomic insight into altered architecture due to
mutations using molecular dynamic simulation (MDS) is a practice
currently in use (15,16). The present study used MDS to
investigate the anomalies in the gatekeeper region due to mutation
from threonine to methionine. The X-ray crystallographic structure
of the wild-type EGFR kinase domain with PDB ID no. 2GS2 (17) was selected for the MDS analysis. This
structure and the architecture of its mutated forms were analyzed
using Gromacs inbuilt tools. In order to understand the effect of
mutation on the flexibility of the two structures, principle
component analysis and free energy landscape analysis were
performed.
Materials and methods
Protein preparation
The crystallographic structure of the tyrosine
kinase domain of EGFR was retrieved from the Research Collaboratory
for Structural Bioinformatics protein data bank (http://www.rcsb.org/pdb/home/home.do)
and the structure with PDB ID 2GS2 (17) was used in the present study. The
structure was energy minimized prior to and following insertion of
mutations using the Swiss Protein Data Bank viewer (18). A total of two structures were
generated; the first was the wild-type EGFR tyrosine kinase domain
and the second was the tyrosine kinase domain with T790M
drug-resistant mutation.
Molecular dynamics simulation
Gromacs version 4.6.6 package (19) was developed for analysis of the
bimolecular systems of proteins, DNA and lipids in order to
investigate the architecture of the tyrosine kinase domain of EGFR.
All three systems were analyzed under a GROMOS96 43a1 force field
(20). The EGFR tyrosine kinase
domain was placed in a rectangular box of 15 Å marginal radius and
the protein domain under investigation was placed in the center.
Subsequently, the box was filled with water using the TIP3P model
(21) and the system was made neutral
using the Genion tool of the Gromacs package. The two systems
generated were subjected to a force of 100 kcal/mol for 5,000
steps, during which the solvent molecules were relaxed and the
solutes were restrained to their original position. In order to
regulate the temperature inside the system, the Berendsen
temperature coupling method (22) was
used and the system was maintained under 1 atm pressure with
allowed compressibility ranging from 4.5×10−5 atm. The
system was energy minimized twice prior to position restraint
simulation for 5 nsec. Following this step, the system was
subjected to a 50 nsec MDS run and the results were saved following
every 2 psec. In order to evaluate the alterations in architecture
of the tyrosine kinase domain of EGFR protein tools, g_rms, g_rmsf,
g_sas, g_hbond, g_gyrate, g_rama, g_rmsdist, g-sham and do_dssp
were used. The results were visualized using Pymol (Schrödinger,
Inc., New York, NY, USA) (23) and
VMD (University of Illinois at Urbana-Champaign, Champaign, IL,
USA) (24), and the graphs were
plotted using the Grace GUI toolkit version 5.1.19 (Oregon Graduate
Institute of Science and Technology, Hillsboro, OR, USA) (25).
Results
General structural changes in Kinase
domain of EGFR by T790M
In order to investigate the effect of T790M
mutations on the tyrosine kinase domain, the present study used the
X-ray crystallographic method and the structures were subjected to
MDS. The results were analyzed to investigate the anomaly in the
architecture of the kinase domain. The protein structure and its
function are interlinked and any change in the protein structure
affects its function. In the case of EGFR, the mutations that were
analyzed in the present study were responsible for the drug
failure.
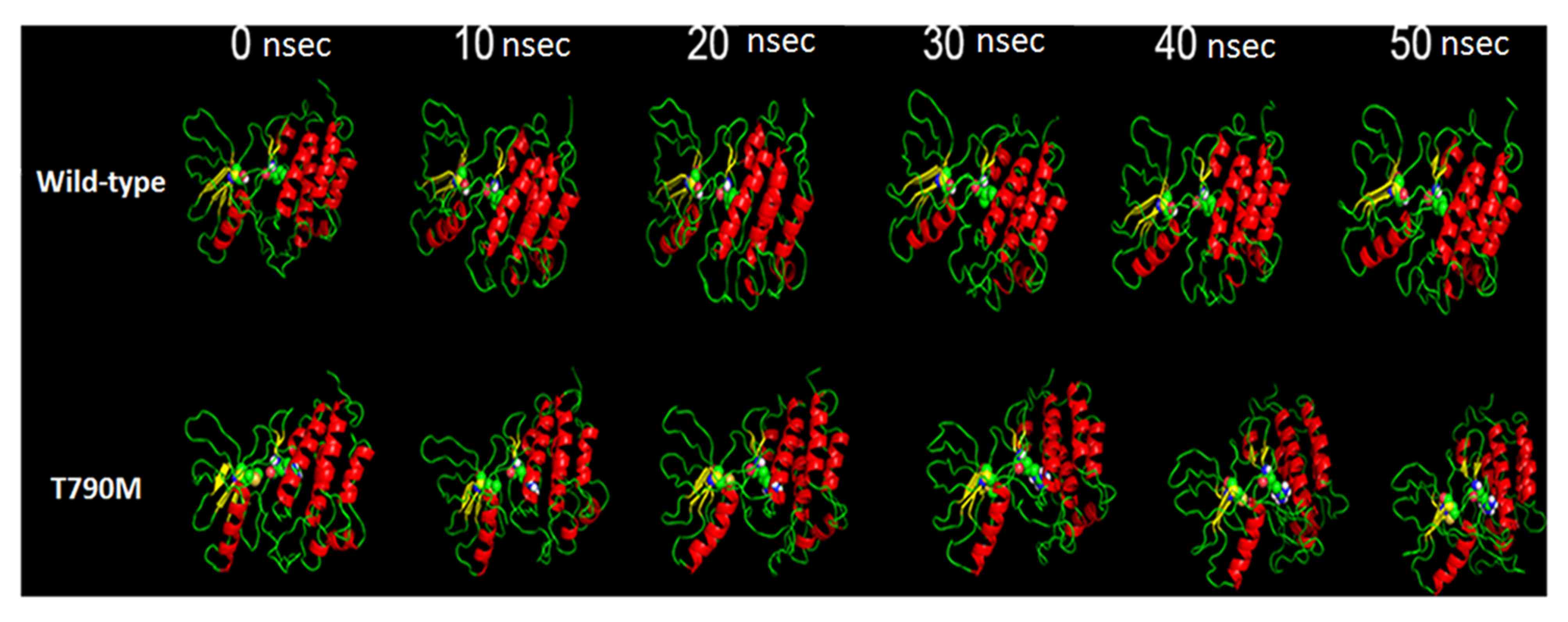
The pictorial representation of MDS of the two
structures under observation are presented in Fig. 1; the structures under observation were
stable throughout the process. The moviemaker from Pymol viewer
suite was used to make the movie of the three runs with each frame
of the movie retrieved following 100 psec (the movies are available
on request). The basic visualization demonstrated the change in the
area of the domain.
In order to investigate the intricate details of the
tyrosine kinase domain architecture alterations due to the
mutations analyzed, the present study used various Gromacs inbuilt
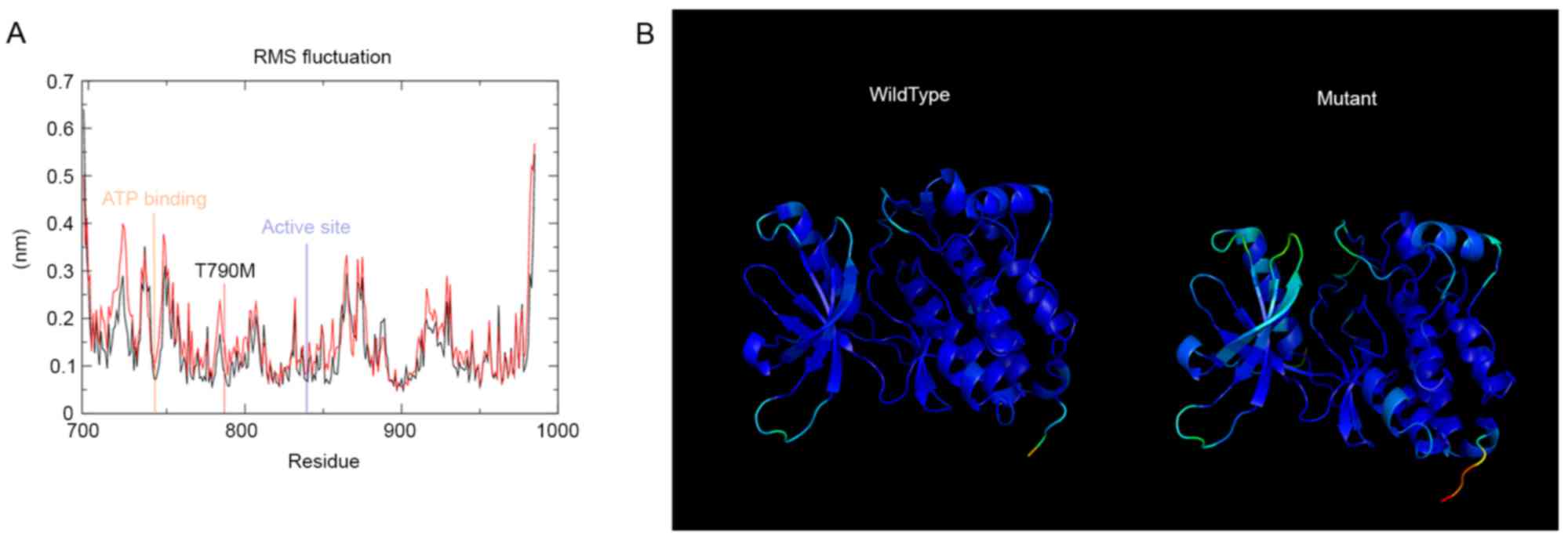
tools. g_rmsf was used to evaluate the root mean square fluctuation
of the residues of the tyrosine kinase domain. The results are
presented in Fig. 2A. T790M mutation
from rmsf analysis demonstrated a distinct pattern compared with
the wild-type, and the residues of importance are marked in
Fig. 2A. The g_rmsf tool was also
used to determine the b-factor to a file with the average
coordinates; the results are presented in Fig. 2B. The spectrum ranges from blue to
white to red, indicating the fluctuation in the region, with blue
being the least and red being the most flexible. The mutant domain
revealed the most fluctuation and the wild-type domain demonstrated
the least.
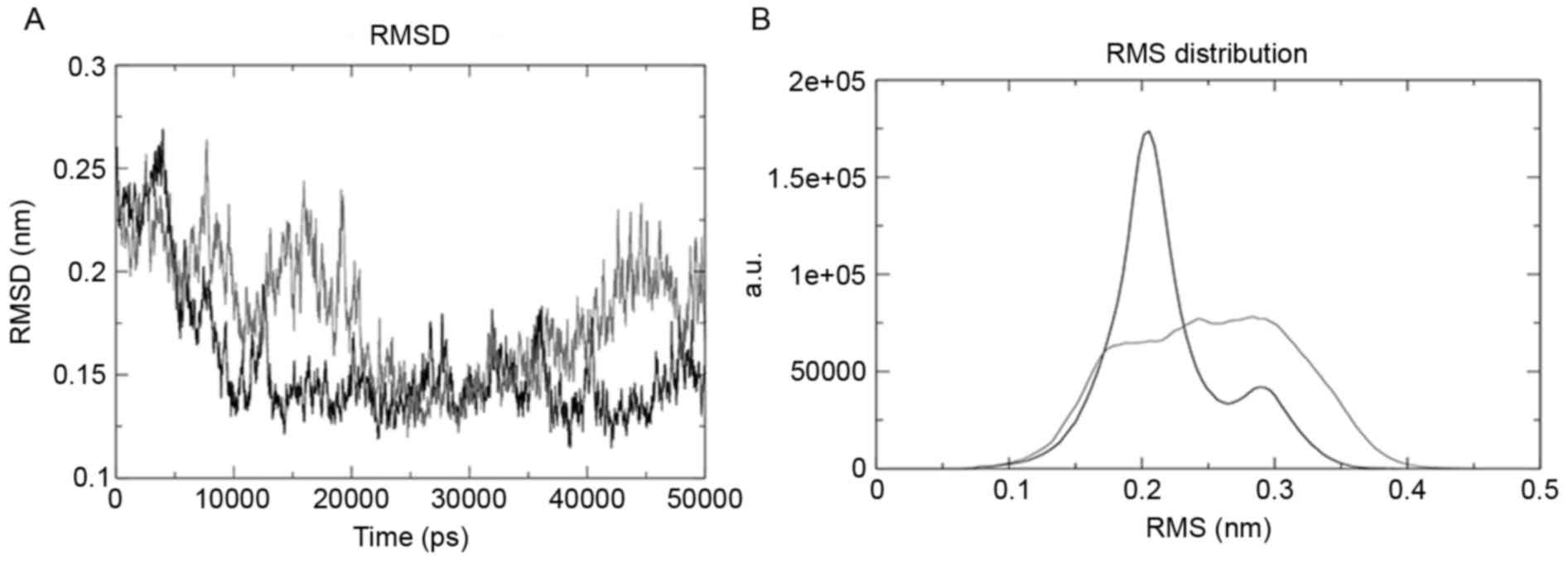
Root mean square deviation (RMSD) was evaluated
using the g_rms tool, and the tool was used to compare the
structures by evaluating their RMSD values. The mutant structure
revealed the maximum deviation, as presented in Fig. 3A. g_rmsdist determined the RMSD of
atom distances; the wild-type domain of the structure was the most
stable and the mutant was the most unstable, as presented in
Fig. 3B.
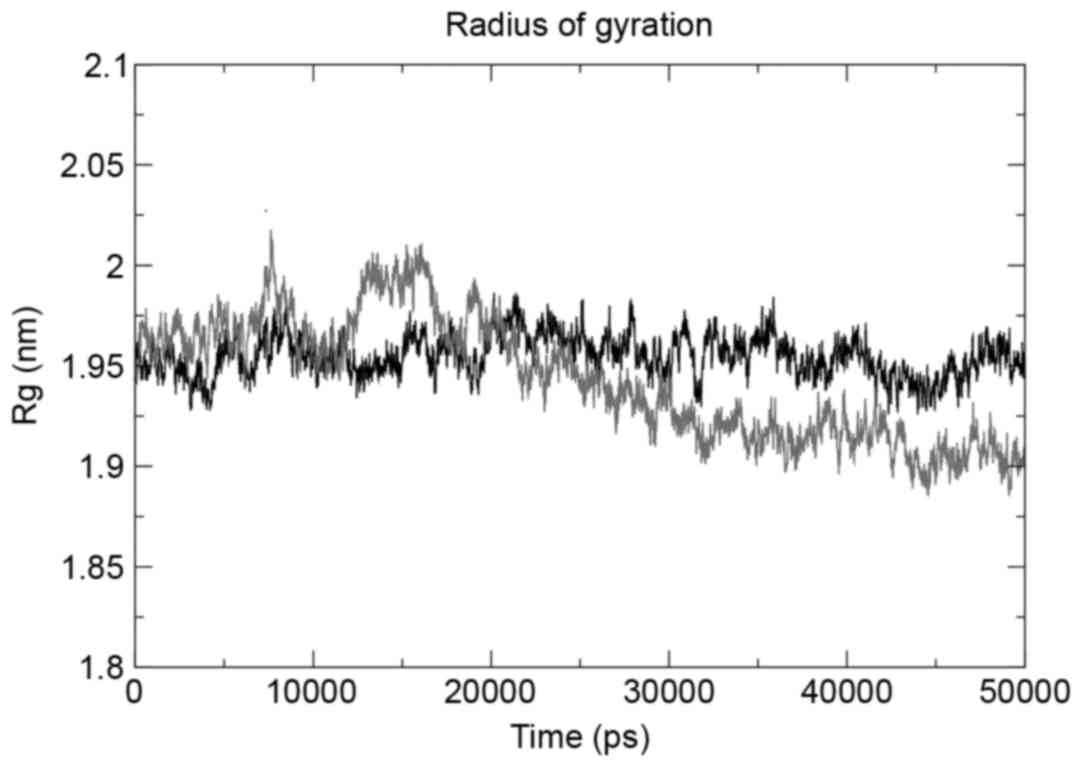
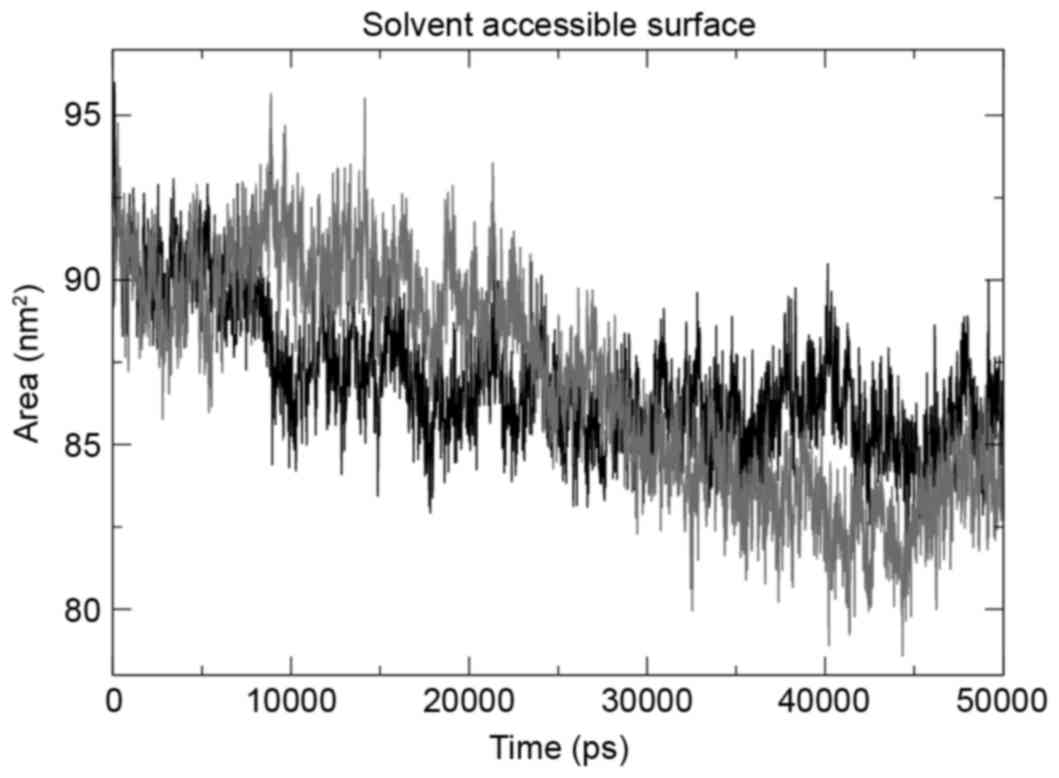
The g_gyrate tool was used to determine the radius
of gyration of the atoms in the three structures under observation,
and the mutant structure revealed the maximum deviation. The mutant
domain was demonstrated to decrease in the radius of gyration
following a 25-nsec run (Fig. 4),
indicating the decrease in size. The same trend was observed when
total solvent accessible surface area (SASA) was evaluated using
the g_sas tool. SASA results (Fig. 5)
revealed that the mutation had a contrasting effect on the tyrosine
kinase domain of EGFR, with mutation decreasing the size of the
domain, hence a smaller SASA.
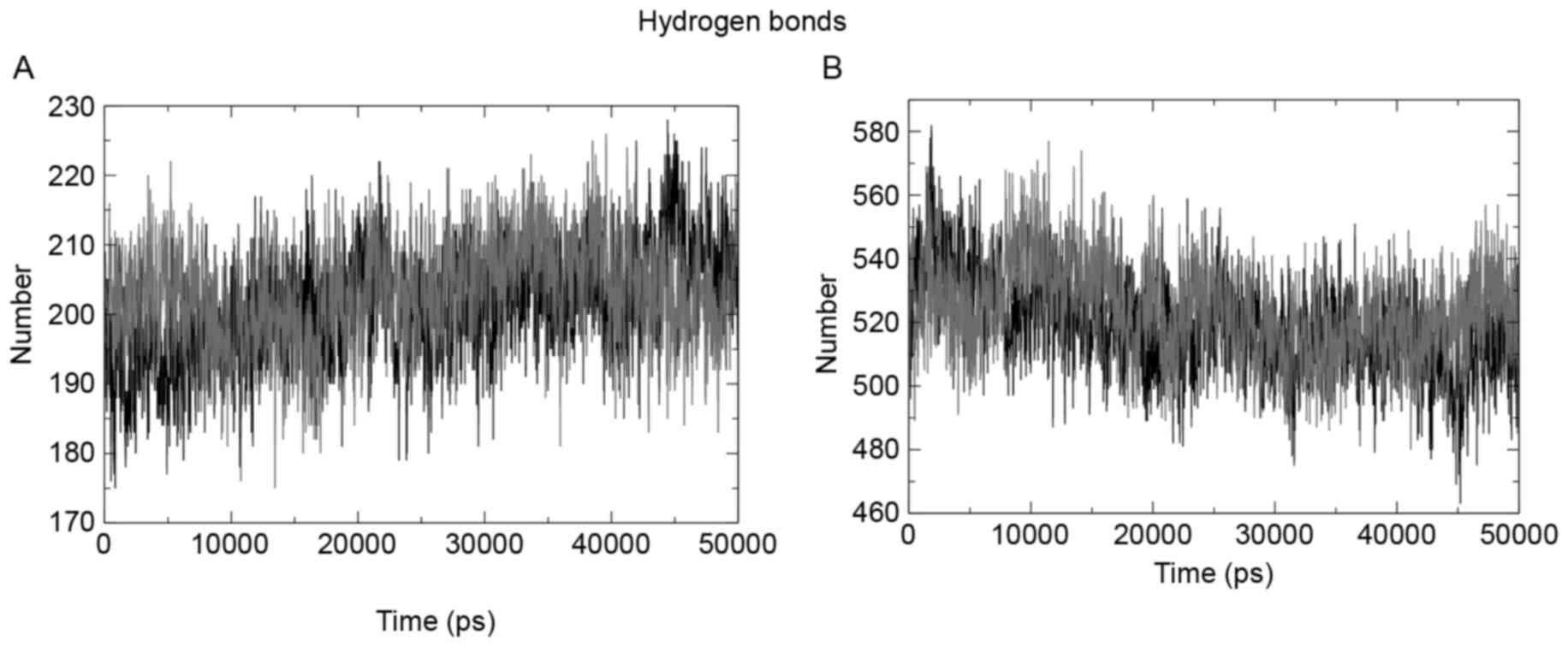
Intra- and interhydrogen bond pattern
analysis
The intrahydrogen bond pattern of the two structures
under observation was investigated using the g_hbond tool of the
Gromacs package. The wild-type structure formed a mean of 203
hydrogen bonds per time frame out of a possible 164,227, and the
mutant formed a mean of 201 hydrogen bonds per time frame out of
possible 166,173. Fig. 6A presents a
pictorial representation of the mean number of hydrogen bonds per
time frame within the domains. Fig.
6B presents the hydrogen bond pattern between the protein and
water; the results showed 519 and 520 bonds for the wild-type and
mutant structures, respectively.
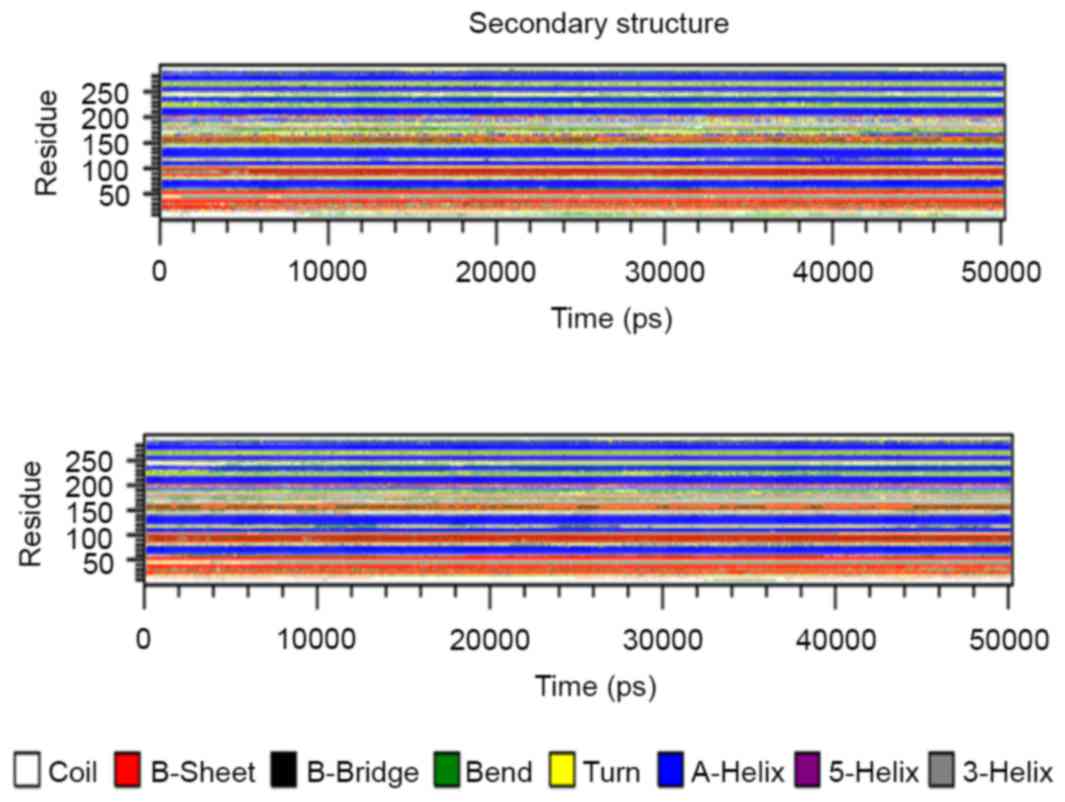
Secondary structure analysis
The secondary structure architecture of the protein
was investigated for alterations in the layout of the domain in
general. The average structure of the EGFR tyrosine kinase domain
was retrieved from the two simulations, and the structures were
analyzed for secondary structure architecture layout. Fig. 7 presents the three secondary structure
layouts, where clear alterations can be observed in the mutant
layouts when compared with the wild-type. To investigate the
secondary structure changes over time, the do_dssp tool was used
and the structures were retrieved following every 100 psec; the
results are presented in Fig. 8.
Discussion
Following mutation there is an alteration in amino
acids and their basic features of size, charge and hydrophobicity
value. These changes induce protein architectural alterations,
which in turn affect its function, as reported by Chikan et
al (15). In the present study,
the change from threonine to methionine also induced these
alterations; methionine is a larger residue with a greater
hydrophobic property compared with wild-type threonine. Due to
these changes, the mutant structure demonstrated considerable
alterations in the secondary structure architecture, which revealed
its effect on EGFR kinase domain size and its total polar
solubility. Each of these properties demonstrated a reduced value
compared with that of the wild-type structure.
The threonine at position 790 was conserved, but a
few other residue types were observed at this position, including
methionine. This means that homologous proteins exist with the same
residue type as the mutant at this position, and this mutation is
possibly not damaging to the protein. The threonine also forms
hydrogen bonds with arginine at position 776; with the alteration
in properties of the residue this intrahydrogen bond formation is
lost, which induces the variation of intrahydrogen bond pattern in
the core of the kinase domain, a change that may have an effect on
tyrosine kinase inhibitor (TKI) failure. Threonine 790, the
‘gatekeeper’ residue in EGFR, has previously been suggested to
cause resistance by sterically blocking the binding of known TKIs
and is sensitive to structurally similar irreversible inhibitors
(11). The data from the present
study suggested that the intrahydrogen bond pattern in the core of
the kinase domain can be a factor triggering selective inhibition
of EGFR by TKIs.
The structural insight obtained regarding the kinase
domain of EGFR due to T790M mutation may pave the way for novel TKI
developments. Previous studies have used computational approaches
to look into novel TKI development; the approach has been used to
propose lead compounds that exhibit more efficient binding with
mutated EGFR (26,27). Zhao et al (26) reported on T790M and L858R mutations in
EGFR and their results are in accordance with the findings from the
present study. The present study proposes that changing the
intrahydrogen bond pattern in the core of the kinase domain serves
as a base for structure-based drug sensitivity in EGFR. This may
provide a basis on which novel TKI development can be achieved,
where the stable mutant structure from the simulation trajectory
can act as a template and novel TKI can be screened over it. The
possible TKI developed using the structure-based drug designing
method may inhibit the kinase activity without any effect from drug
resistant mutations (26,27).
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Longo D, Fauci A, Kasper D, Hauser S,
Jameson J and Loscalzo J: Harrison's Principles of Internal
Medicine. 18th. McGraw-Hill Professional; New York, NY: 2011
|
|
3
|
Dacic S, Flanagan M, Cieply K, Ramalingam
S, Luketich J, Belani C and Yousem SA: Significance of EGFR protein
expression and gene amplification in non-small cell lung carcinoma.
Am J Clin Pathol. 125:860–865. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wrann MM and Fox CF: Identification of
epidermal growth factor receptors in a hyperproducing human
epidermoid carcinoma cell line. J Biol Chem. 254:8083–8086.
1979.PubMed/NCBI
|
|
5
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Prewett M, Rockwell P, Rockwell RF,
Giorgio NA, Mendelsohn J, Scher HI and Goldstein NI: The biologic
effects of C225, a chimeric monoclonal antibody to the EGFR, on
human prostate carcinoma. J Immunother Emphasis Tumor Immunol.
19:419–427. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kris MG, Natale RB, Herbst RS, Lynch TJ
Jr, Prager D, Belani CP, Schiller JH, Kelly K, Spiridonidis H,
Sandler A, et al: Efficacy of gefitinib, an inhibitor of the
epidermal growth factor receptor tyrosine kinase, in symptomatic
patients with non-small cell lung cancer: A randomized trial. JAMA.
290:2149–2158. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shigematsu H and Gazdar AF: Somatic
mutations of epidermal growth factor receptor signaling pathway in
lung cancers. Int J Cancer. 118:257–262. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Johnson BE and Jänne PA: Epidermal growth
factor receptor mutations in patients with non-small cell lung
cancer. Cancer Res. 65:7525–7529. 2005.PubMed/NCBI
|
|
11
|
Yun CH, Mengwasser KE, Toms AV, Woo MS,
Greulich H, Wong KK, Meyerson M and Eck MJ: The T790M mutation in
EGFR kinase causes drug resistance by increasing the affinity for
ATP. Proc Natl Acad Sci USA. 105:pp. 2070–2075. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pao W, Miller VA, Politi KA, Riely GJ,
Somwar R, Zakowski MF, Kris MG and Varmus H: Acquired resistance of
lung adenocarcinomas to gefitinib or erlotinib is associated with a
second mutation in the EGFR kinase domain. PLoS Med. 2:e732005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kobayashi S, Boggon TJ, Dayaram T, Jänne
PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG and Halmos
B: EGFR mutation and resistance of non-small-cell lung cancer to
gefitinib. N Engl J Med. 352:786–792. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kwak EL, Sordella R, Bell DW,
Godin-Heymann N, Okimoto RA, Brannigan BW, Harris PL, Driscoll DR,
Fidias P, Lynch TJ, et al: Irreversible inhibitors of the EGF
receptor may circumvent acquired resistance to gefitinib. Proc Natl
Acad Sci USA. 102:pp. 7665–7670. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chikan N, Bukhari S, Shabir N, Amin A,
Shafi S, Qadri RA and Patel TN: Atomic insight into the altered
O6-Methylguanine-DNA methyltransferase protein architecture in
gastric cancer. PLoS One. 10:e01277412015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bukhari S, Mokhdomi TA, Chikan NA, Amin A,
Qazi H, Wani SH, Wafai AH, Tyub S, Mustafa F, Mir MS, et al:
Affinity proteomics led identification of vimentin as a potential
biomarker in colon cancers: Insights from serological screening and
computational modelling. Mol Biosyst. 11:159–169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang X, Gureasko J, Shen K, Cole PA and
Kuriyan J: An allosteric mechanism for activation of the kinase
domain of epidermal growth factor receptor. Cell. 125:1137–1149.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guex N and Peitsch MC: SWISS-MODEL and the
Swiss-Pdb Viewer: An environment for comparative protein modeling.
Electrophoresis. 18:2714–2723. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hess B, Kutzner C, van der Spoel D and
Lindahl E: GROMACS 4: Algorithms for highly efficient,
load-balanced, and scalable molecular simulation. J Chem Theory
Comput. 4:435–447. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schuler LD, Daura X and Van Gunsteren WF:
An improved GROMOS96 force field for aliphatic hydrocarbons in the
condensed phase. J Comput Chem. 22:1205–1218. 2001. View Article : Google Scholar
|
|
21
|
Mark P and Nilsson L: Structure and
dynamics of the TIP3P, SPC, and SPC/E water models at 298K. J Phy
Chem. 105:9954–9960. 2001. View Article : Google Scholar
|
|
22
|
Berendsen HJC, Postma JPM, van Gunsteren
WF, DiNola A and Haak JR: Molecular dynamics with coupling to an
external bath. J Chem phys. 81:36841984. View Article : Google Scholar
|
|
23
|
DeLano WL: The PyMOL molecular graphics
system. 2002, http://www.pymol.org/May 30–2017
|
|
24
|
Humphrey W, Dalke A and Schulten K: VMD:
Visual molecular dynamics. J Mol Graph. 14:33–38, 27-28. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Turner PJ: XMGRACE, Version 5.1.19. Center
for Coastal and Land-Margin Research. Oregon Graduate Institute of
Science and Technology; Beaverton, OR: 2005
|
|
26
|
Zhao FL, Yang GH, Xiang S, Gao DD and Zeng
C: In silico analysis of the effect of mutation on epidermal growth
factor receptor in non-small-cell lung carcinoma: From mutational
analysis to drug designing. J Biomol Struct Dyn. 35:427–434. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Doss GP, Rajith B, Chakraborty C,
NagaSundaram N, Ali SK and Zhu H: Structural signature of the
G719S-T790M double mutation in the EGFR kinase domain and its
response to inhibitors. Sci Rep. 4:58682014.PubMed/NCBI
|