Introduction
Resveratrol, an anti-fungal phytochemical that
occurs in grapes, blueberries, mulberries, cranberries and red
wine, is rapidly metabolized by the intestine and liver following
oral consumption (1–6). The major phase II metabolites of
resveratrol include glucuronidated, sulfated and methylated
products (2–6). Intestinal bacteria have been reported to
break down resveratrol to metabolic products including benzoic,
phenylacetic and propionic acids (7,8).
Dihydroresveratrol, another metabolic product of gut bacteria, has
been identified in the form of glucuronidated and sulfated products
in plasma and urine following the consumption of resveratrol
(9,10). Gut microbiota produce other conversion
products of resveratrol subsequent to ingestion, including
3,4′-dihydroxy-trans-stilbene and 3,4′-dihydroxybibenzyl (11–13). A
previous human study with oral doses of ≤5 g of resveratrol per day
demonstrated that the peak plasma levels of resveratrol,
resveratrol-3-O-glucuronide, resveratrol-4′-O-glucuronide and
resveratrol-3-O-sulfate were 4.2, 17.1, 10.2 and 18.3 µM,
respectively (2). These data indicate
that high concentrations of resveratrol glucuronide and sulfate
metabolites are achieved in the plasma following the dietary intake
of resveratrol. Pharmacokinetic analysis of resveratrol and its
metabolites in that study revealed that the phytochemical products
remain in the plasma for 5–8 h (2).
Camptothecin is a topoisomerase I inhibitor that
induces cytotoxicity by the generation of DNA strand breaks
(14). Topotecan is a water-soluble
derivative of camptothecin. Camptothecin compounds act primarily by
binding to and stabilizing the topoisomerase I-DNA complex, which
then collides with the replication fork during S phase of the cell
cycle. This collision results in topoisomerase I-linked DNA breaks,
the formation of double-strand DNA breaks and the irreversible
arrest of DNA replication. Camptothecin compounds inhibit
transcription by a similar mechanism: By binding to the
topoisomerase I-DNA complex and colliding with the RNA polymerase
complex, leading to the arrest of RNA synthesis and the generation
of single-strand DNA breaks (14).
DNA strand breaks result in the activation of the
DNA repair machinery and, if repair is not possible, apoptosis. DNA
strand breaks can be quantified by several methods, including by
the antibody-mediated detection of phosphorylated histone subunit
2AX (H2AX) and labeling the ends of broken strands with the
terminal deoxynucleotidyl transferase dUTP nick end-labeling
(TUNEL) assay (15,16). H2AX is a histone 2A isoform that is
present at levels of 2–25% in the histone core of the DNA complex.
It is phosphorylated, in response to DNA strand breaks, by the
phosphatidylinositol-3 kinase-like family of kinases, including
ataxia telangiectasia mutated (ATM) and ATM-and Rad3-related (ATR),
and by DNA-dependent protein kinase (DNA-PK) (17,18).
Resveratrol aglycone has been studied extensively
in vitro to determine its mechanisms of action; however, the
majority of these studies have utilized high concentrations of
resveratrol aglycone (50–100 µM) that are not yet achievable in
vivo, particularly in the plasma. Considering that resveratrol
aglycone reached a peak concentration of 4.2 µM in the blood
following the ingestion of 5 g per day (2), the data from the majority of in
vitro studies are not likely to be reflective of the actual
activity once resveratrol is absorbed and metabolized. There is
also limited information on the activity of resveratrol
metabolites.
In the present study, using Jurkat T cells as a
model representing a cell type that occurs in the blood,
camptothecin and topotecan were applied in order to determine
whether or not glucuronidated and sulfated metabolites of
resveratrol were able to increase or inhibit the DNA damage induced
by these drugs. DNA damage was measured by determining the extent
of H2AX phosphorylation and TUNEL staining. Apoptosis in the cells
was determined by measuring the extent of the cleavage of poly
ADP-ribose polymerase (PARP). The cells were pretreated with
physiological levels of resveratrol-3-O-glucuronide,
resveratrol-4′-O-glucuronide or resveratrol-3-O-sulfate prior to
the induction of DNA damage by camptothecin and topotecan, or
co-treated with metabolites and drugs. Flow cytometry was used to
measure the extent of DNA damage and apoptosis, and the activities
of the resveratrol metabolites were compared with equivalent
amounts of the resveratrol aglycone.
Materials and methods
Cell culture and chemicals
Jurkat acute lymphoblastic T leukemia cells
(American Type Culture Collection, Manassas, VA, USA) were cultured
at 37°C with 5% CO2 in RPMI-1640 medium (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10%
fetal bovine serum (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany),
50 IU/ml penicillin, 50 µg/ml streptomycin, 0.25 µg/ml amphotericin
B, 1 mM sodium pyruvate and 2 mM L-glutamine (Invitrogen; Thermo
Fisher Scientific, Inc.). Trans-resveratrol (≥99% pure), dimethyl
sulfoxide (DMSO; vehicle) and topotecan hydrochloride hydrate
(≥98%) were purchased from Sigma-Aldrich (Merck KGaA).
Trans-resveratrol 3-O-D-glucuronide (≥95%), trans-resveratrol
4′-O-D-glucuronide (≥95%) and trans-resveratrol-3-O-sulfate (≥98%)
were purchased from Cayman Chemical Company (Ann Arbor, MI, USA).
Camptothecin (≥98%) was purchased from MP Biomedicals, LLC (Santa
Ana, CA, USA).
H2AX phosphorylation and apoptosis
measurements
Jurkat T cells were cultured at a concentration of
0.5×106 cells/ml in 24-well plates (Sarstedt, Inc.,
Newton, NC, USA) with 0.1% DMSO (control) or 10 µM each of
resveratrol aglycone, resveratrol-3-O-glucuronide,
resveratrol-4′-O-glucuronide or resveratrol-3-O-sulfate for 24 h in
the previously described conditions. Cells were then washed with
RPMI-1640 and incubated with 5 µM camptothecin or 10 µM topotecan
for a further 4 h in the same conditions. The cells were fixed and
permeabilized for intracellular staining using the
Fixation/Permeabilization Solution kit (BD Biosciences, San Jose,
CA, USA) according to the manufacturer's protocol. For each
treatment group, 106 cells were stained with
phycoerythrin-conjugated anti-cleaved PARP (0.06 µg/20 µl test; cat
no. 552933) and Alexa Fluor-conjugated anti-phosphorylated H2AX
(0.125 µg/5 µl test; cat no. 56-447) (both from BD Biosciences)
antibodies. Following 30 min incubation on ice, the cells were
fixed in 1% paraformaldehyde prepared in phosphate-buffered saline
(Sigma-Aldrich; Merck KGaA). The cells were assessed with an
LSRFortessa flow cytometer and the results analyzed using FACSDiva
software v8.0.1 (both from BD Biosciences). A total of 30,000
events were collected per measurement, subsequent to gating to
exclude debris.
TUNEL assay
Cells were pretreated with resveratrol and its
metabolites for 24 h as previously described. Cells were then
washed with RPMI medium and treated with 5 µM camptothecin or 10 µM
topotecan for a further 4 h. In separate experiments, the cells
were co-treated with resveratrol or its metabolites, plus one of
the topoisomerase-inhibitor drugs at doses as previously described
for a total of 4 h. DNA strand breaks were labeled with a TUNEL
assay (Apo-Direct kit; BD Biosciences) with fluorescein
isothiocyanate (FITC)-labelled dUTP according to the manufacturer's
protocol, with the following modifications: The fixation and
membrane permeabilization step using 70% v/v ethanol was performed
for >18 h at −20°C and the end-labeling reaction with the
terminal transferase was 4 h at 37°C. Labeled cells were treated at
room temperature for 30 min with RNase and propidium iodide (PI) as
provided in the Apo-Direct kit at the concentrations recommended by
the manufacturer. A total of 30,000 events were collected on the
LSRFortessa flow cytometer for each treatment. The end-labeling of
DNA strand breaks was evaluated, subsequent to gating singlet
populations, based on the fluorescence of the PI area vs.
width.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism v6.05 (GraphPad Software, Inc., La Jolla, CA, USA) and the
data were presented as the mean ± standard error of the mean.
P-values were obtained using one-way analysis of variance with
Tukey's multiple comparisons test to evaluate the significance of
differences between the means of the treatment groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
Phosphorylation of H2AX
The histone protein H2AX is phosphorylated in
response to DNA damage. The extent of phosphorylated H2AX was
assessed by flow cytometry to determine the effects of resveratrol
and its metabolites on DNA damage induced by camptothecin and
topotecan. The cells were pretreated with 10 µM of resveratrol, or
its metabolites, for 24 h prior to a 4-h treatment with the
DNA-damaging agents. Resveratrol aglycone was used at a
concentration of 10 µM as a comparative control for DNA damage and
apoptosis. The proportion of cells with phosphorylated H2AX and the
mean fluorescence intensity (MFI) of this population were measured
to determine alterations in DNA damage attributable to the
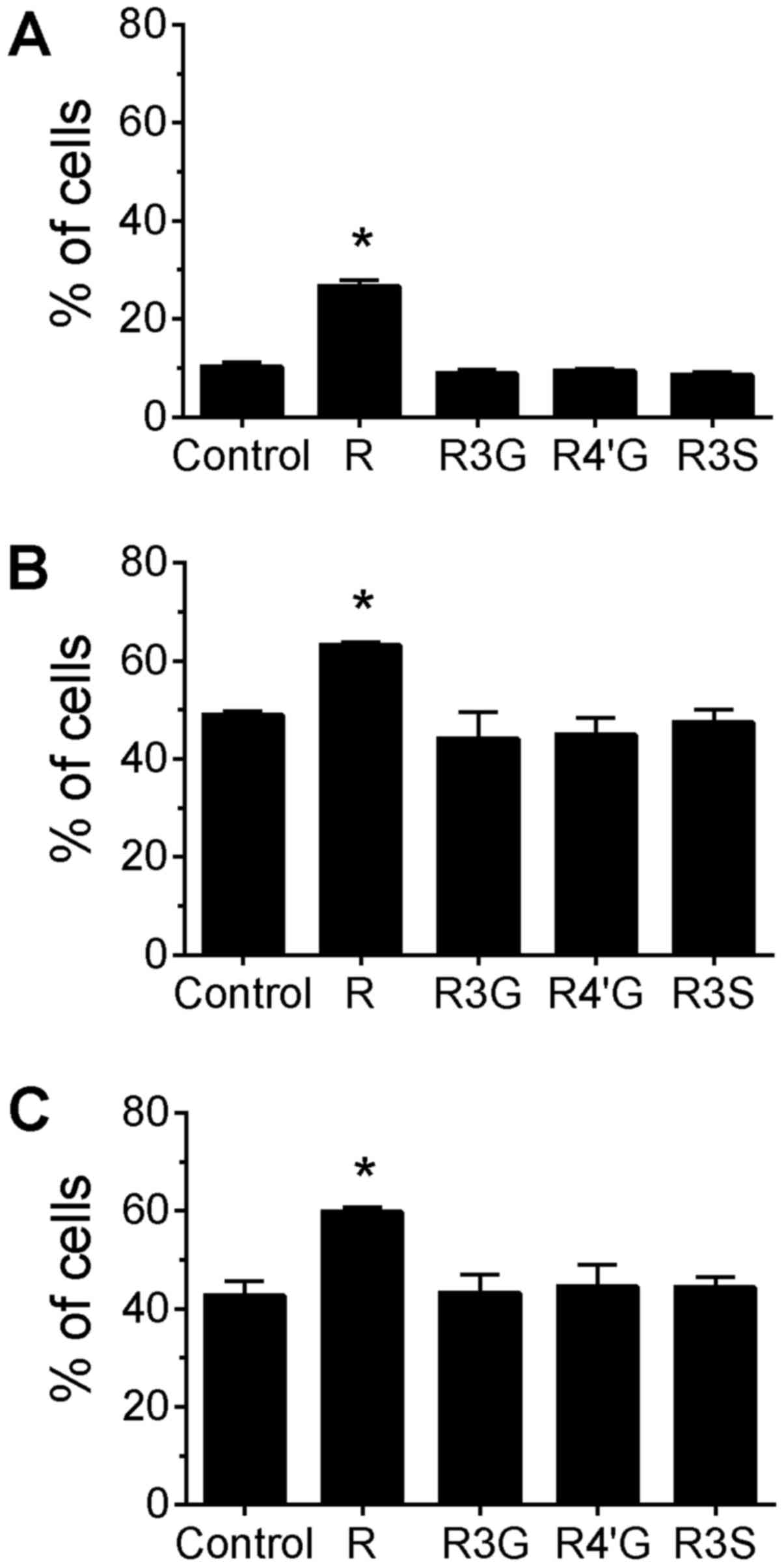
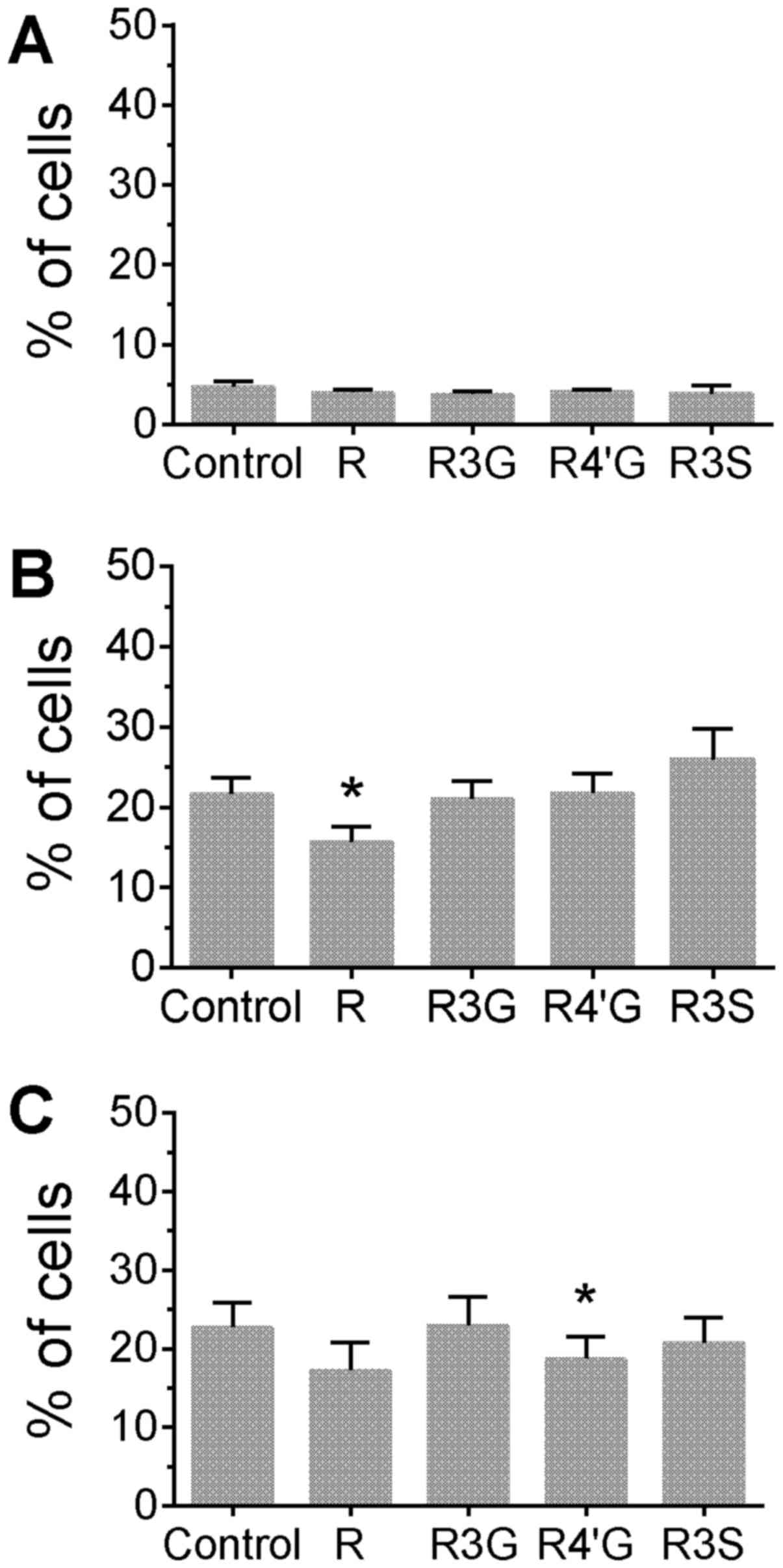
phytochemicals. Treatment of cells with resveratrol aglycone alone
for 24 h increased the percentage of cells with phosphorylated H2AX
compared with untreated control cells (Fig. 1A; P<0.05). Additionally,
resveratrol aglycone pretreatment with the subsequent addition of
camptothecin or topotecan was associated with an increased
percentage of cells with phosphorylated H2AX compared with cells
treated only with the topoisomerase inhibitors (Fig. 1B and C; P<0.05), suggesting an
additive effect. A corresponding increase in the MFI of H2AX
staining was observed following the pretreatment of Jurkat cells
with resveratrol aglycone followed by the DNA damaging agents (data
not shown). In camptothecin-treated cells, resveratrol aglycone
increased the MFI from 5,332±156 in the control group to 7,000±198;
and in topotecan-treated cells, resveratrol aglycone increased the
MFI from 5,105±115 (control) to 6,747±209 (P<0.05), indicating
an increase in DNA damage. The resveratrol glucuronide and sulfate
metabolites did not significantly alter the percentage of
H2AX+ cells or the MFI of H2AX staining following drug
treatments.
DNA strand breaks following
pretreatment of cells with phytochemicals
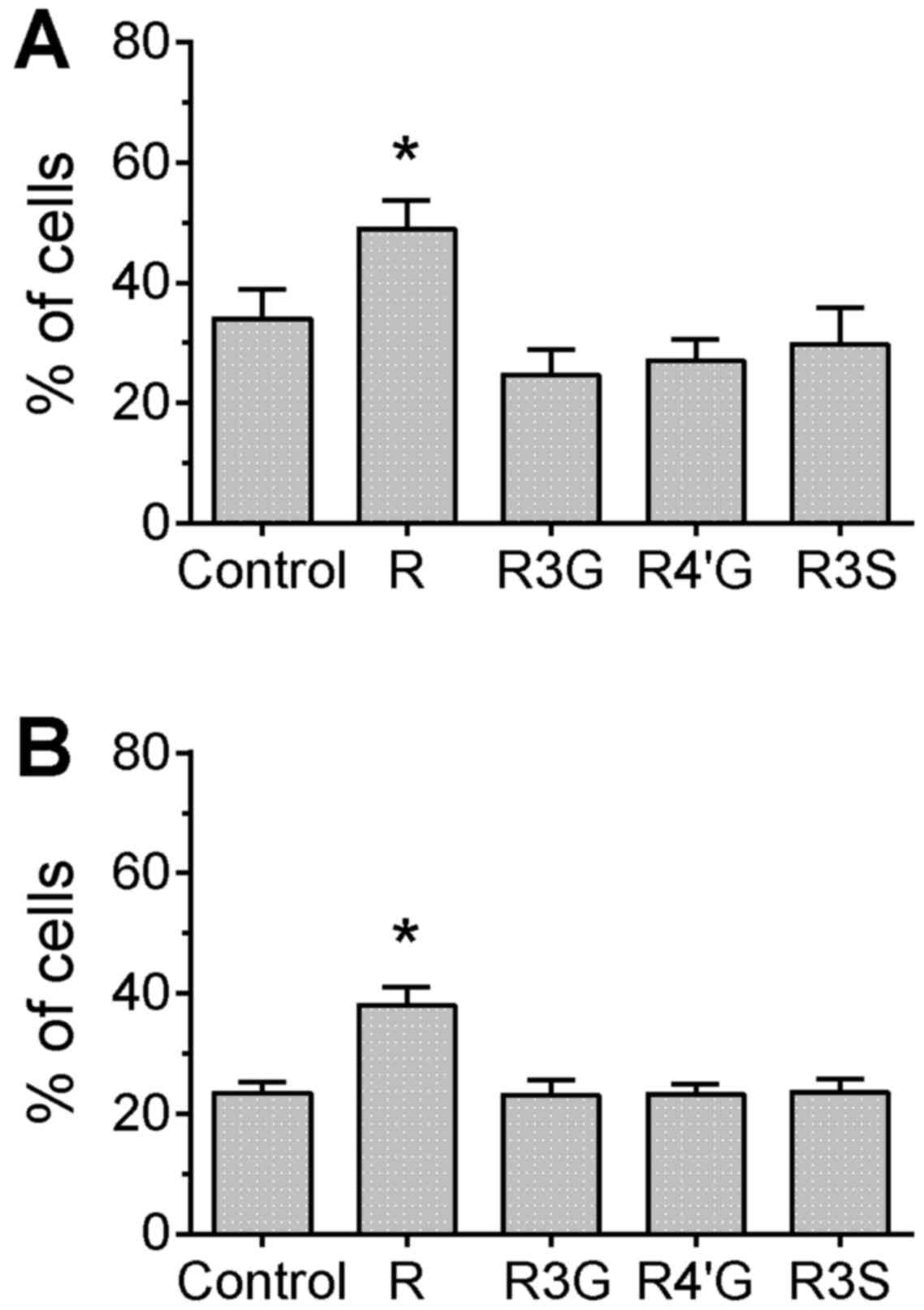
The TUNEL assay provides a direct measurement of DNA
damage by labeling the DNA break-point ends. In cells treated with
camptothecin or topotecan, only pretreatment with resveratrol
aglycone resulted in a significant increase of the percentage of
cells with detectable DNA breaks compared with the control group
(Fig. 2; P<0.05), which confirms
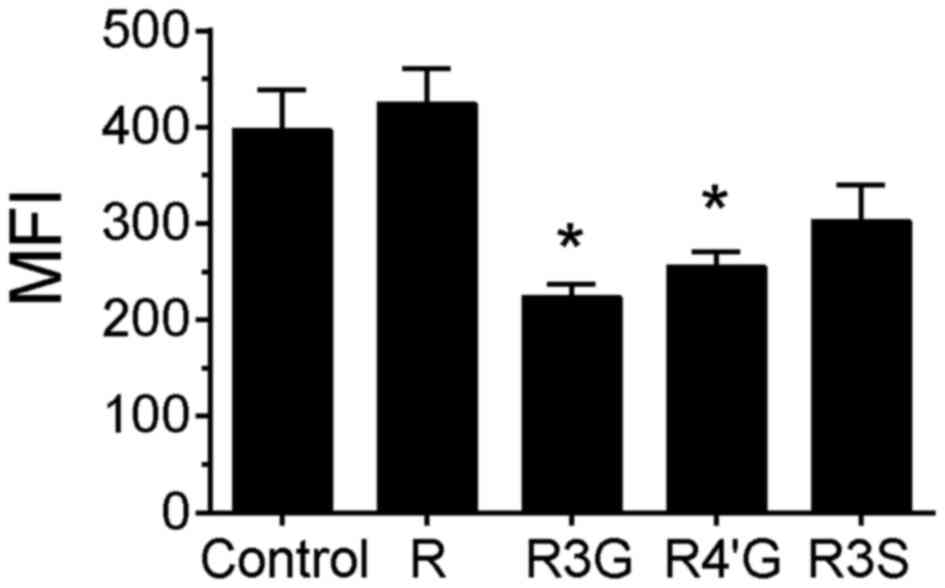
the result observed for H2AX phosphorylation. However, in
camptothecin-treated cells, pretreatment with
resveratrol-3-glucuronide or resveratrol-4′-glucuronide
significantly reduced the MFI compared with the control group,
suggesting that the extent of damage (or number of DNA strand
breaks) per cell was attenuated by pretreatment with these
metabolites (Fig. 3, P<0.05). In
the topotecan-treated cells, there were no differences in MFI
between any of the groups (data not shown).
Induction of apoptosis following
pretreatment with resveratrol and metabolites
The topoisomerase I inhibitors camptothecin and
topotecan function to produce DNA damage, cell cycle arrest and
apoptosis. The extent of apoptosis was measured in the Jurkat cells
using an antibody against the cleaved form of PARP, as cleavage of
PARP occurs at a late stage in the apoptotic pathway. The cells
were pretreated with resveratrol or its metabolites for 24 h, and
DNA damage was induced by the topoisomerase I inhibitors. Cells
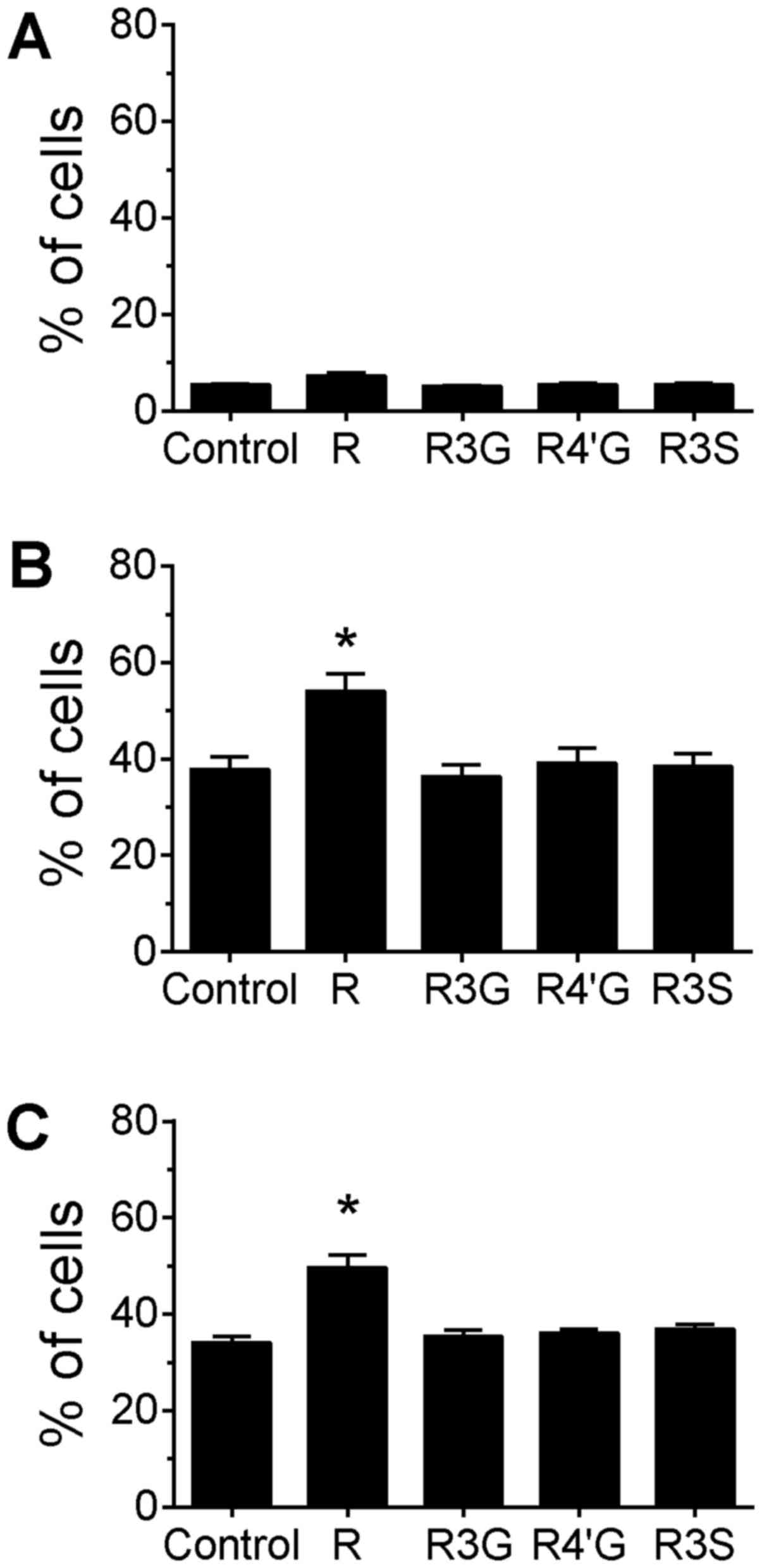
treated only with resveratrol or its metabolites for 24 h did not
induce PARP cleavage compared with untreated cells (Fig. 4A). In the topoisomerase I
inhibitor-treated cells, only pretreatment with 10 µM resveratrol
aglycone increased the percentage of cells with cleaved PARP
compared with the cells treated only with the drugs (Fig. 4B and C; P<0.05), whereas the
resveratrol metabolites had no effect on the level of apoptosis
induced by treatment with camptothecin and topotecan.
Co-treatment with resveratrol
metabolites and topoisomerase I inhibitors
Jurkat cells were co-treated with resveratrol
aglycone or its metabolites plus a topoisomerase I inhibitor for 4
h, and DNA damage was analyzed using the TUNEL assay. Treatment
with only resveratrol or one of its metabolites for 4 h did not
increase the extent of DNA strand breakage detected by the assay
(Fig. 5A). Co-treatment of cells with
resveratrol aglycone and camptothecin for 4 h decreased the
percentage of cells with DNA damage compared with treatment with
camptothecin alone (Fig. 5B;
P<0.05). A similar reduction in the percentage of cells with DNA
damage was observed for cells co-treated with topotecan and
resveratrol aglycone, although this reduction did not reach
statistical significance. A reduction in DNA damage was observed
between topotecan treatment alone (control) and co-treatment with
topotecan plus resveratrol 4′-O-D-glucuronide (Fig. 5C; P<0.05). Co-treatment with
resveratrol 3-O-D-glucuronide or resveratrol-3-O-sulfate did not
alter DNA damage induced by camptothecin or topotecan, and no
differences in MFI were observed for any of the treatment groups
(data not shown).
Discussion
The topoisomerase I inhibitors camptothecin and
topotecan were utilized in the present study as a means to induce
DNA damage and test the ability of glucuronidated and sulfated
metabolites of resveratrol to prevent DNA strand breaks. The
pretreatment of Jurkat T cells with resveratrol-3-O-glucuronide and
resveratrol-4′-O-glucuronide significantly decreased the mean
fluorescence signal from the FITC-UTP used for end-labeling DNA
strand breaks induced by camptothecin, suggesting that these
resveratrol metabolites were able to reduce the number of strand
breaks per cell caused by this drug. No differences in
camptothecin- or topotecan-induced DNA damage, as measured by H2AX
phosphorylation, were observed between the control and any of the
pretreatment groups, with the exception of resveratrol aglycone.
DNA strand breaks induce a rapid response by the DNA
repair/apoptotic machinery. H2AX is phosphorylated following
single-strand DNA breaks and replication stress (by ATR),
double-strand DNA breaks (by ATM), and fragmentation of DNA during
apoptosis (by DNA-PK) (19,20). It has been reported that the region of
phosphorylated H2AX extends up to 1 megabase on either side of a
double-strand DNA break in mammalian cells; this post-translational
modification is the first step for the recruitment of DNA repair
complexes to the damaged sites (19–22). The
labeling of DNA ends by the TUNEL assay is a direct method of
determining DNA strand breakage and is a more sensitive method,
compared with phosphorylated H2AX, for determining the extent of
DNA damage on a per-cell basis.
Camptothecin is a lipophilic compound and topotecan
is its water-soluble analogue. It has been reported that lipophilic
camptothecin has a greater topoisomerase inhibitory effect and
cytotoxicity than its water-soluble counterparts (23,24).
However, the two compounds demonstrated a similar ability to induce
the phosphorylation of H2AX and DNA strand breaks in the present
study. Therefore, it remains unclear why pretreatment with the
glucuronide metabolites of resveratrol produced a reduction in DNA
strand breaks (as determined by the TUNEL assay) following
camptothecin, but not topotecan, treatment.
Resveratrol aglycone has been reported to have
anticancer and chemosensitizing activities against cancer cells;
mechanisms of action for this have previously been proposed
(1,25–28).
Anticancer mechanisms of action for resveratrol, which have been
described predominantly from in vitro studies, include the
inhibition of transcription factors, kinases and other molecules
involved in cell proliferation and survival. In animal models,
resveratrol has demonstrated efficacy against breast, esophageal,
lung and colon cancer, and was reported to decrease the extent of
metastasis of melanoma and lung or colon carcinoma (29–37).
However, resveratrol aglycone has been reported to protect certain
types of cells from DNA damage. For example, a previous study
demonstrated that resveratrol could inhibit DNA damage in the
kidneys of rats treated with the carcinogen KBrO3
(38). In addition, resveratrol
attenuated DNA damage induced by H2O2 in
glioma cells and peripheral blood lymphocytes, protected DNA from
damage by chromium, and reduced the number of DNA adducts induced
by the carcinogen dibenzo [a,l]pyrene (39–42). In
the present study, the pretreatment of Jurkat cells with 10 µM
resveratrol aglycone consistently increased the level of DNA damage
induced by camptothecin and topotecan and was used as a positive
control in the DNA damage and apoptosis assays. However, a decrease
in DNA strand breaks in cells co-treated for 4 h with resveratrol
and camptothecin was observed, as in cells co-treated with
resveratrol 4′-O-D-glucuronide and topotecan. As has been observed
for a number of chemotherapeutic drugs, camptothecins induce
oxidative stress in various tissues (43–45); the
short co-treatment period with resveratrol in the present study may
have provided protective effects against drug-induced oxidative
stress due to resveratrol's antioxidant activities (1). Furthermore, camptothecin and resveratrol
are lipophilic agents, which may have increased direct interactions
between the molecules during co-treatment.
There is limited data available concerning the
activity of resveratrol metabolites. Resveratrol-3-O-glucuronide
and resveratrol-4′O-glucuronide are two major metabolic products of
resveratrol subsequent to ingestion, and the concentrations used in
the present study were intended to be physiologically relevant
based on a previous study (2). It
will be important to understand the potential of these metabolites
to protect cells from DNA damage induced by chemotherapeutic drugs.
We hypothesize that the effects of these metabolites may be
particularly important for the protection of normal cells during
chemotherapy. Future research on the protective activities of the
metabolic products of resveratrol are necessary to elucidate their
interaction with DNA-damaging drugs used in the treatment of
cancer. Of note, camptothecins are used in the combinatorial
treatment of colorectal cancers (46–48). The
increased DNA damage and apoptosis of cells treated with
camptothecin and topotecan following pretreatment with resveratrol
aglycone in the present study suggests that dietary resveratrol may
be a useful addition for treating gastrointestinal cancers that
have direct contact with unmetabolized resveratrol.
Acknowledgements
This study was supported by the USDA CRIS Project
(grant no. 2032-53000-001-00D). USDA is an equal opportunity
provider and employer.
References
|
1
|
Aggarwal BB, Bhardwaj A, Aggarwal RS,
Seeram NP, Shishodia S and Takada Y: Role of resveratrol in
prevention and therapy of cancer: Preclinical and clinical studies.
Anticancer Res. 24:2783–2840. 2004.PubMed/NCBI
|
|
2
|
Brown VA, Patel KR, Viskaduraki M, Crowell
JA, Perloff M, Booth TD, Vasilinin G, Sen A, Schinas AM, Piccirilli
G, et al: Repeat dose study of the cancer chemopreventive agent
resveratrol in healthy volunteers: Safety, pharmacokinetics, and
effect on the insulin-like growth factor axis. Cancer Res.
70:9003–9011. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patel KR, Scott E, Brown VA, Gescher AJ,
Steward WP and Brown K: Clinical trials of resveratrol. Ann NY Acad
Sci. 1215:161–169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Walle T, Hsieh F, DeLegge MH, Oatis JE Jr
and Walle UK: High absorption but very low bioavailability of oral
resveratrol in humans. Drug Metabol Dispos. 32:1377–1382. 2004.
View Article : Google Scholar
|
|
5
|
Vitrac X, Desmoulière A, Brouillaud B,
Krisa S, Deffieux G, Barthe N, Rosenbaum J and Mérillon JM:
Distribution of [14C]-trans-resveratrol, a cancer chemopreventive
polyphenol, in mouse tissues after oral administration. Life Sci.
72:2219–2233. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wenzel E, Soldo T, Erbersdobler H and
Somoza V: Bioactivity and metabolism of trans-resveratrol orally
administered to Wistar rats. Mol Nutr Food Res. 49:482–494. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Crozier A, Jaganath IB and Clifford MN:
Dietary phenolics: Chemistry, bioavailability and effects on
health. Nat Prod Rep. 26:1001–1043. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
van Duynhoven J, Vaughan EE, Jacobs DM,
Kemperman RA, van Velzen EJ, Gross G, Roger LC, Possemiers S,
Smilde AK, Doré J, et al: Metabolic fate of polyphenols in the
human superorganism. Proc Natl Acad Sci USA. 108 Suppl 1:pp.
4531–4538. 2011; View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rotches-Ribalta M, Andres-Lacueva C,
Estruch R, Escribano E and Urpi-Sarda M: Pharmacokinetics of
resveratrol metabolic profile in healthy humans after moderate
consumption of red wine and grape extract tablets. Pharmacol Res.
66:375–382. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Radko Y, Christensen KB and Christensen
LP: Semi-preparative isolation of
dihydroresveratrol-3-O-β-d-glucuronide and four resveratrol
conjugates from human urine after oral intake of a
resveratrol-containing dietary supplement. J Chromatogr B Analyt
Technol Biomed Life Sci. 930:54–61. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bode LM, Bunzel D, Huch M, Cho GS, Ruhland
D, Bunzel M, Bub A, Franz CM and Kulling SE: In vivo and in vitro
metabolism of trans-resveratrol by human gut microbiota. Am J Clin
Nutr. 97:295–309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jung CM, Heinze TM, Schnackenberg LK,
Mullis LB, Elkins SA, Elkins CA, Steele RS and Sutherland JB:
Interaction of dietary resveratrol with animal-associated bacteria.
FEMS Microbiol Lett. 297:266–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Azorín-Ortuño M, Yáñez-Gascón MJ, Vallejo
F, Pallarés FJ, Larrosa M, Lucas R, Morales JC, Tomás-Barberán FA,
García-Conesa MT and Espín JC: Metabolites and tissue distribution
of resveratrol in the pig. Mol Nutr Food Res. 55:1154–1168. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu LF, Desai SD, Li TK, Mao Y, Sun M and
Sim SP: Mechanism of action of campothecin. Ann N Y Acad Sci.
922:1–10. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuo LJ and Yang LX: Gamma-H2AX-a novel
biomarker for DNA double-strand breaks. In Vivo. 22:305–309.
2008.PubMed/NCBI
|
|
16
|
Gavrieli Y, Sherman Y and Ben-Sasson SA:
Identification of programmed cell death in situ via specific
labeling of nuclear DNA fragmentation. J Cell Biol. 119:493–501.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rogakou EP, Pilch DR, Orr AH, Ivanova VS
and Bonner WM: DNA double-stranded breaks induce histone H2AX
phosphorylation on serine 139. J Biol Chem. 273:5858–5868. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shiloh Y: ATM and related protein kinases:
Safeguarding genome integrity. Nat Rev Cancer. 3:155–168. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fernandez-Capetillo O, Lee A, Nussenzweig
M and Nussenzweig A: H2AX: The histone guardian of the genome. DNA
Repair (Amst). 3:959–967. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Srivastava N, Gochhait S, de Boer P and
Bamezai RN: Role of H2AX in DNA damage response and human cancers.
Mutat Res. 682:180–188. 2009. View Article : Google Scholar
|
|
21
|
Redon C, Pilch D, Rogakou E, Sedelnikova
O, Newrock K and Bonner W: Histone H2A variants H2AX and H2AZ. Curr
Opin Genet Dev. 12:162–169. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shroff R, Arbel-Eden A, Pilch D, Ira G,
Bonner WM, Petrini JH, Haber JE and Lichten M: Distribution and
dynamics of chromatin modification induced by a defined DNA
double-strand break. Curr Biol. 14:1703–1711. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bom D, Curran DP, Kruszewski S, Zimmer SG,
Strode J Thompson, Kohlhagen G, Du W, Chavan AJ, Fraley KA,
Bingcang AL, et al: The novel silatecan
7-tert-butyldimethylsilyl-10-hydroxycamptothecin displays high
lipophilicity, improved human blood stability, and potent
anticancer activity. J Med Chem. 43:3970–3980. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Van Hattum AH, Pinedo HM, Schlüper HM,
Hausheer FH and Boven E: New highly lipophilic camptothecin BNP1350
is an effective drug in experimental human cancer. Int J Cancer.
88:260–266. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Colin D, Limagne E, Jeanningros S, Jacquel
A, Lizard G, Athias A, Gambert P, Hichami A, Latruffe N, Solary E
and Delmas D: Endocytosis of resveratrol via lipid rafts and
activation of downstream signaling pathways in cancer cells. Cancer
Prev Res (Phila). 4:1095–1106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li G, He S, Chang L, Lu H, Zhang H, Zhang
H and Chiu J: GADD45α and Annexin A1 are involved in the apoptosis
of HL-60 induced by resveratrol. Phytomedicine. 18:704–709. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kartal M, Saydam G, Sahin F and Baran Y:
Resveratrol triggers apoptosis through ceramide metabolizing genes
in human K562 chronic myeloid leukemia cells. Nutr Cancer.
63:637–644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gupta SC, Kannappan R, Reuter S, Kim JH
and Aggarwal BB: Chemosensitization of tumors by resveratrol. Ann N
Y Acad Sci. 1215:150–160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Banerjee S, Bueso-Ramos C and Aggarwal BB:
Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary
carcinogenesis in rats by resveratrol: Role of nuclear
factor-kappaB, cyclooxygenase 2, and matrix metalloproteinase 9.
Cancer Res. 62:4945–4954. 2002.PubMed/NCBI
|
|
30
|
Li ZG, Hong T, Shimada Y, Komoto I, Kawabe
A, Ding Y, Kaganoi J, Hashimoto Y and Imamura M: Suppression of
N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumorigenesis
in F344 rats by resveratrol. Carcinogenesis. 23:1531–1536. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee EO, Lee HJ, Hwang HS, Ahn KS, Chae C,
Kang KS, Lu J and Kim SH: Potent inhibition of Lewis lung cancer
growth by heyneanol A from the roots of Vitis amurensis through
apoptotic and anti-angiogenic activities. Carcinogenesis.
27:2059–2069. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu HS, Pan CE, Yang W and Liu XM:
Antitumor and immunomodulatory activity of resveratrol on
experimentally implanted tumor of H22 in Balb/c mice. World J
Gastroenterol. 9:1474–1476. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tessitore L, Davit A, Sarotto I and
Caderni G: Resveratrol depresses the growth of colorectal aberrant
crypt foci by affecting bax and p21(CIP) expression.
Carcinogenesis. 21:1619–1622. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baur JA and Sinclair DA: Therapeutic
potential of resveratrol: The in vivo evidence. Nat Rev Drug
Discov. 5:493–506. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kimura Y and Okuda H: Resveratrol isolated
from Polygonum cuspidatum root prevents tumor growth and metastasis
to lung and tumor-induced neovascularization in lewis lung
carcinoma-bearing mice. J Nutr. 131:1844–1849. 2001.PubMed/NCBI
|
|
36
|
Busquets S, Ametller E, Fuster G, Olivan
M, Raab V, Argilés JM and López-Soriano FJ: Resveratrol, a natural
diphenol, reduces metastatic growth in an experimental cancer
model. Cancer Lett. 245:144–148. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Weng YL, Liao HF, Li AF, Chang JC and
Chiou RY: Oral administration of resveratrol in suppression of
pulmonary metastasis of BALB/c mice challenged with CT26 colorectal
adenocarcinoma cells. Mol Nutr Food Res. 54:259–267. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cadenas S and Barja G: Resveratrol,
melatonin, vitamin E, and PBN protect against renal oxidative DNA
damage induced by the kidney carcinogen KBrO3. Free Radic Biol Med.
26:1531–1537. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Quincozes-Santos A, Andreazza AC, Nardin
P, Funchal C, Gonçalves CA and Gottfried C: Resveratrol attenuates
oxidative-induced DNA damage in C6 glioma cells. Neurotoxicology.
28:886–891. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Burkhardt S, Reiter RJ, Tan DX, Hardeland
R, Cabrera J and Karbownik M: DNA oxidatively damaged by
chromium(III) and H(2)O(2) is protected by the antioxidants
melatonin, N(1)-acetyl-N(2)-formyl-5-methoxykynuramine, resveratrol
and uric acid. Int J Biochem Cell Biol. 33:775–783. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liu GA and Zheng RL: Protection against
damaged DNA in the single cell by polyphenols. Pharmazie.
57:852–854. 2002.PubMed/NCBI
|
|
42
|
Russell GK, Gupta RC and Vadhanam MV:
Effect of phytochemical intervention on dibenzo[a,l]pyrene-induced
DNA adduct formation. Mutat Res. 774:25–32. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Singh K, Bhori M and Marar T: α-Tocopherol
mediated amelioration of camptothecin-induced free radical damage
to avert cardiotoxicities. Hum Exp Toxicol. 34:380–389. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Singh KC, Kaur R and Marar T: Ameliorative
effect of vitamin E on chemotherapy induced side effects in rat
liver. J Pharmacol Toxicol. 6:481–492. 2011. View Article : Google Scholar
|
|
45
|
Singh KC and Marar T: Acute toxicity of
camptothecin and influence of α-tocopherol on hematological and
biochemical parameters. J Cell Tiss Res. 11:2833–2837. 2011.
|
|
46
|
Guo Y, Shi M, Shen X, Yang C, Yang L and
Zhang J: Capecitabine plus irinotecan versus 5-FU/leucovorin plus
irinotecan in the treatment of colorectal cancer: A meta-analysis.
Clin Colorectal Cancer. 13:110–118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Vanhoefer U, Harstrick A, Achterrath W,
Cao S, Seeber S and Rustum YM: Irinotecan in the treatment of
colorectal cancer: Clinical overview. J Clin Oncol. 19:1501–1518.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fujita K, Kubota Y, Ishida H and Sasaki Y:
Irinotecan, a key chemotherapeutic drug for metastatic colorectal
cancer. World J Gastroenterol. 21:12234–12248. 2015. View Article : Google Scholar : PubMed/NCBI
|