Introduction
Atherosclerosis is a major cardiovascular disease.
Sonodynamic therapy (SDT), which is a noninvasive technique and
uses ultrasonic energy to activate certain drugs (i.e.
sonosensitizer) to produce chemical reactions and reactive oxygen
species, has been shown to have potential to attenuate progression
of atherosclerotic plaque formation (1–5).
Currently, porphyrins and their analogs are the
predominant sonosensitizers used in the clinic (6). Among these porphyrin-related
sonosensitizers, protoporphyrin IX (PpIX) was found to be
accumulated specifically in atherosclerotic plaques at a much
higher concentration, i.e. 10 times higher, than in normal vessel
walls (7), indicating that PpIX is a
potential atherosclerosis-selective sonosensitizer.
Cell autophagy, the degradation process of cellular
proteins or organelles in the cell (8), is an important defense and protection
mechanism. Previous studies have shown that autophagy plays an
important role in atherosclerosis (9,10). For
instance, autophagy can selectively remove macrophages in the
artery atheromatous plaque and participate in degradation of
low-density lipoprotein (LDL) cholesterol (11). In addition, induced autophagy in
vascular smooth muscle cells (VSMCs) can prevent oxidized LDL
(oxLDL)-triggered VSMC foam cell formation (12). In contrast, deficient VSMC autophagy
affects blood vessel contractability by changing the
Ca2+ steady-state response (13). Therefore, VSMC differentiation and
autophagy play important roles in the initiation and development of
atherosclerosis, and have been therapeutic targets for treatment of
atherosclerosis.
SDT has been shown to promote macrophage apoptosis
and maintain stability of atherosclerotic plaques (4); however, the role of VSMCs in the
SDT-treated plaques remains unclear. In the present study, we aimed
to investigate the PpIX-mediated sonodynamic effects on VSMCs in
order to provide molecular mechanisms by which SDT was clinically
employed to treat atherosclerosis.
Materials and methods
Reagents
PpIX was purchased from Sigma-Aldrich, reconstituted
in 100% DMSO (Sigma-Aldrich, St. Louis, MO, USA) to 0.1 g/ml and
stored at room temperature. The VSMC line A7r5 (ATCC) was derived
from murine thoracic vascular aorta. JC-1 probe was provided by
Beyotime Institute of Biotechnology (Haimen, China). DAPI and
propidium iodide (PI) were obtained from Sigma-Aldrich. Light chain
3B (LC3B) antibody and anti-rabbit secondary antibodies were
obtained from Cell Signaling Technology (Danvers, MA, USA).
Antibodies against microtubule-associated protein 1 LC3 were
purchased from Sigma Chemical Co. Fetal bovine serum (FBS) and
Dulbecco's modified Eagle's medium (DMEM) were obtained from
Hyclone Laboratories, Inc. (HyClone, Logan, UT, USA). All other
reagents were obtained from Sigma Chemical Co. Ltd.
Cell preparation and SDT
procedure
A7r5 cell line was maintained in DMEM supplemented
with 10% FBS, penicillin, and streptomycin. Cells were grown at
37°C in a humidified 5% CO2/95% air atmosphere. During
the SDT treatment procedure, VSMCs were exposed to ultrasound
generated by the ultrasonic generator and power amplifier (Harbin
Institute of Technology, Harbin, China) at 1.0 MHz with an
intensity of 1.0 W/cm2 in the presence or absence of
PpIX, which was maintained at a concentration of 2 µg/ml. During
the sonication procedure, the temperature of the solution inside
the Petri dishes increased less than 0.5°C, as monitored with a
thermometer.
Flow cytometry analysis
Cells were seeded into 35 mm Petri dishes and
divided into 4 groups: Control group, ultrasound group, PpIX group,
and SDT group. Each group consisted of 8 dishes. Five min after
ultrasound treatment, cells were placed in a 37°C and 5%
CO2 incubator for 6 h, followed by sequential incubation
with 5 µl Annexin V-FITC for 15 min and 10 µl PI for 5 min in the
dark room. Apoptosis rates were analyzed by flow cytometry (Epics
Altra II; Beckman Coulter, Brea, CA, USA). Experiments were
repeated three times for each group under the same conditions.
Laser scanning confocal
microscopy
Six h after SDT, cells were incubated with 5 µl
Annexin V in the dark for 15 min. Laser scanning confocal
microscopy was used to observe apoptosis with excitation at 488 nm
and emission at 525 nm wavelengths (SP8; Leica, Mannheim, Germany).
Experiments were repeated three times for each group under the same
conditions.
Mitochondrial membrane potential (MMP)
assessment
MMP was assessed using the probe JC-1, a sensitive
fluorescent dye used to detect changes in MMP (1). Briefly, 6 h after SDT, VSMCs were
incubated with 10 mg/ml JC-1 for 10 min at 37°C in the dark and
monitored with a fluorescence microscope. Red-orange fluorescence
was attributable to a potential-dependent aggregation in the
mitochondria. Green fluorescence, reflecting the monomeric form of
JC-1, appeared in the cytosol after mitochondrial membrane
depolarization.
Transition electron microscopy
(TEM)
Four groups of cells were collected by
centrifugation, washed with 1X phosphate buffered saline (PBS)
(0.01 M pH 7.2–7.4), embedded with agar, fixed with 2.5%
glutaraldehyde, post-fixed with osmium tetroxide, dehydrated in
gradient alcohol, and embedded in Epon812 (Yiwei Info Technology
Co. Ltd., Shanghai, China). The blocks were then cut into
ultra-thin sections, stained with uranium acetate, and observed and
photographed under a TEM (Hitachi, Tokyo, Japan).
Immunofluorescence staining and
fluorescence microscopy imaging
Six hours after different treatments, VSMCs were
washed with 1X PBS and fixed with 4% paraformaldehyde for 30 min.
VSMCs were then incubated with the primary antibody of interest at
4°C overnight. VSMCs were then washed with 1x PBS three times and
stained with fluorescent second antibody at 37°C for 30 min in the
dark. DAPI was used for nuclear staining. After fluorescence
quenching, images were acquired with the same exposure settings
using a fluorescence microscopy with standard excitation filters
(Olympus Corporation, Tokyo, Japan).
Western blot analysis
SDS-PAGE and immunoblotting were performed according
to standard procedures to detect the expression levels of autophagy
marker proteins, such as LC3. Briefly, cells were lysed with RIPA
buffer on ice, and supernatant samples were resolved in 10–15%
SDS-PAGE depending on the size of the target protein, followed by
transfer onto nitrocellulose membranes (Millipore, Billerica, MA,
USA). Thereafter, membranes were incubated in blocking buffer at
room temperature for 1 h, followed by incubation with anti-LC3
antibody at 4°C overnight. The protein-antibody complexes were then
tagged with IR Dye 680-labeled secondary antibodies at room
temperature for 1 h. The infrared fluorescence was detected with
the Odyssey infrared imaging system (Li-Cor Bioscience, Lincoln,
NE, USA). β-actin was used as a loading control. The ratios of
LC3-II/LC3-I to β-actin in each experiment were calculated by
Quantity One software (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Statistical analysis
All values are expressed as means ± SD of at least
three independent experiments, and one-way ANOVA was used to
analyze the statistical difference among the multiple groups. A
P-value less than 0.05 was considered statistically
significant.
Results
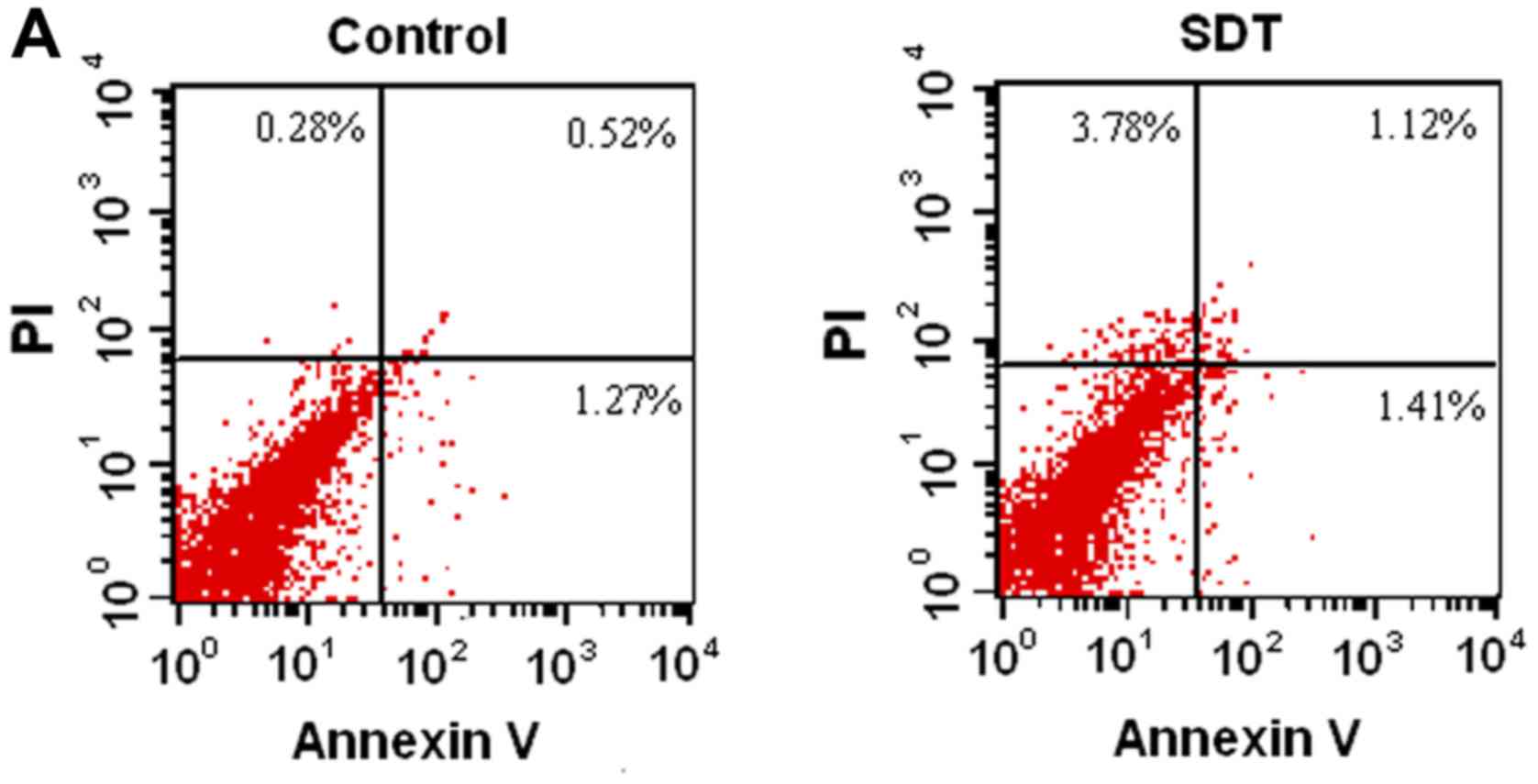
PpIX-SDT did not increase VSMC
apoptosis
First, we investigated whether PpIX-SDT had any
effect on VSMC apoptosis. VSMCs in SDT and control groups were
stained with Annexin V-FITC, followed by flow cytometric analysis.
As shown in Fig. 1A, no significant
difference in apoptosis between SDT and control group was observed,
which was further confirmed by confocal microscopy (Fig. 1B).
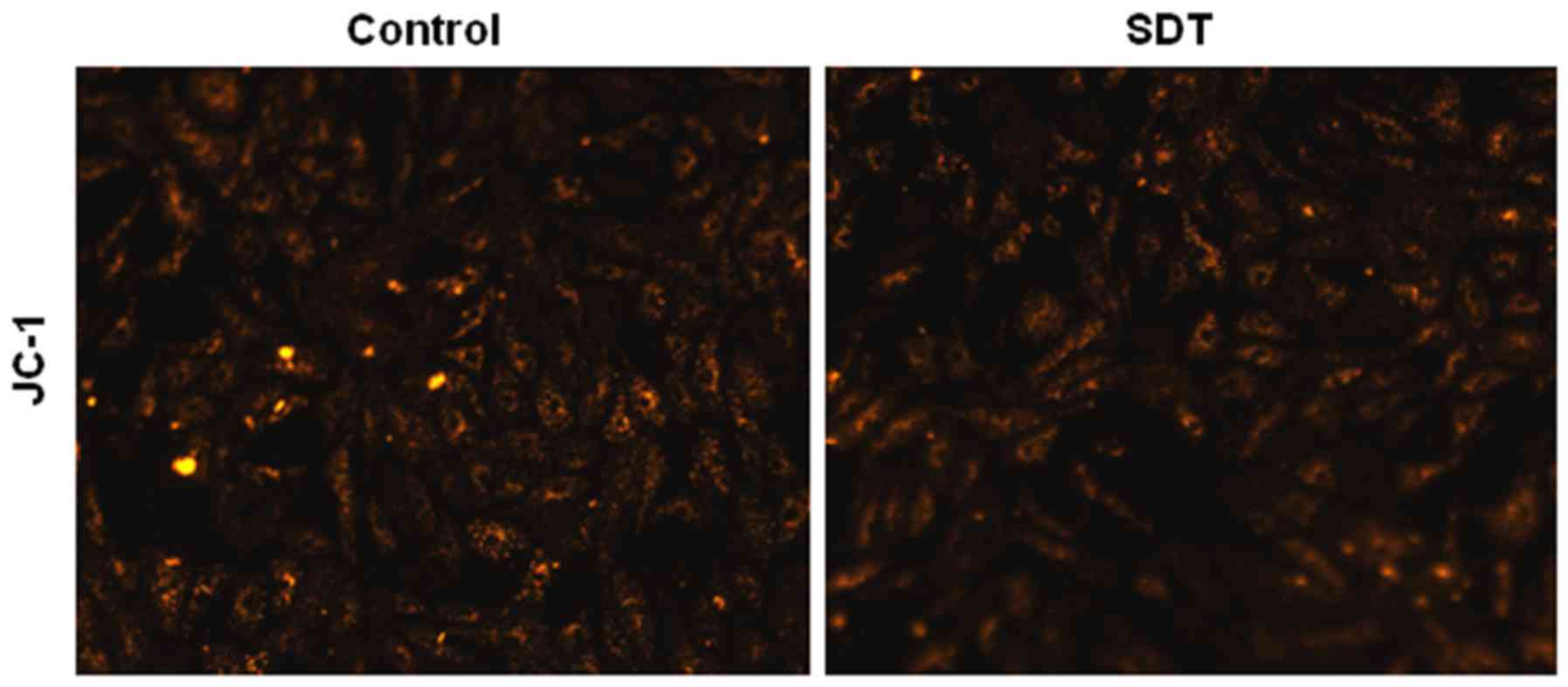
PpIX-SDT did not induce loss of MMP in
VSMCs
As shown in Fig. 2,
normal VSMCs with JC-1 staining exhibited orange fluorescence, and
neither light nor PpIX alone induced any changes in MMP. However,
SDT treatment induced a diffuse orange staining pattern in VSMCs,
which was representative of no significant decline in MMP.
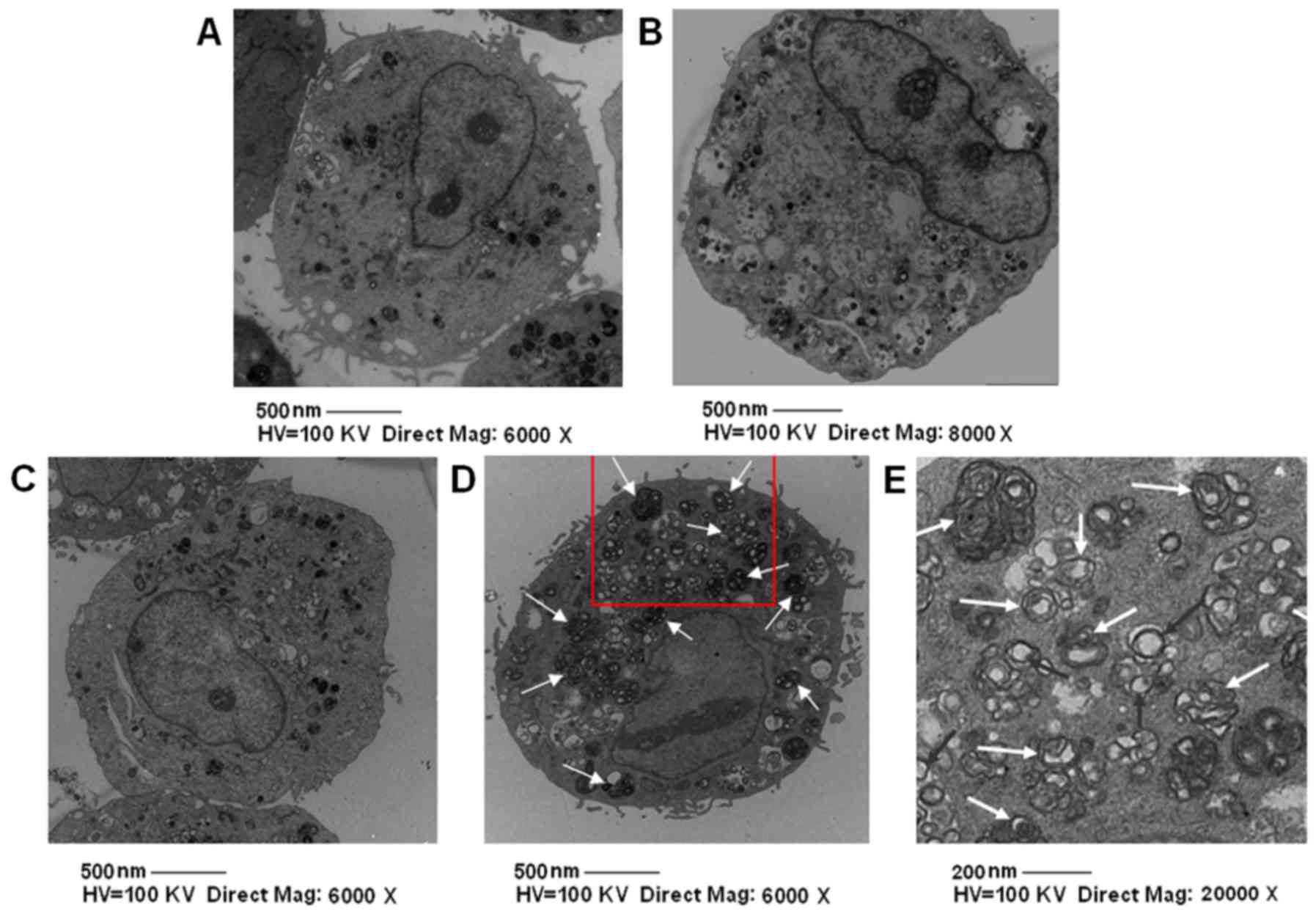
PpIX-SDT induces VSMC autophagy
Next, we explored VSMC autophagic activity in the
SDT and other groups with electron microscopy. The number of
autophagy bodies, including nuclear pyknosis, karyorrhexis, cell
shrinkage, without the formation of apoptotic body, in the SDT
group were significantly higher compared to the other groups
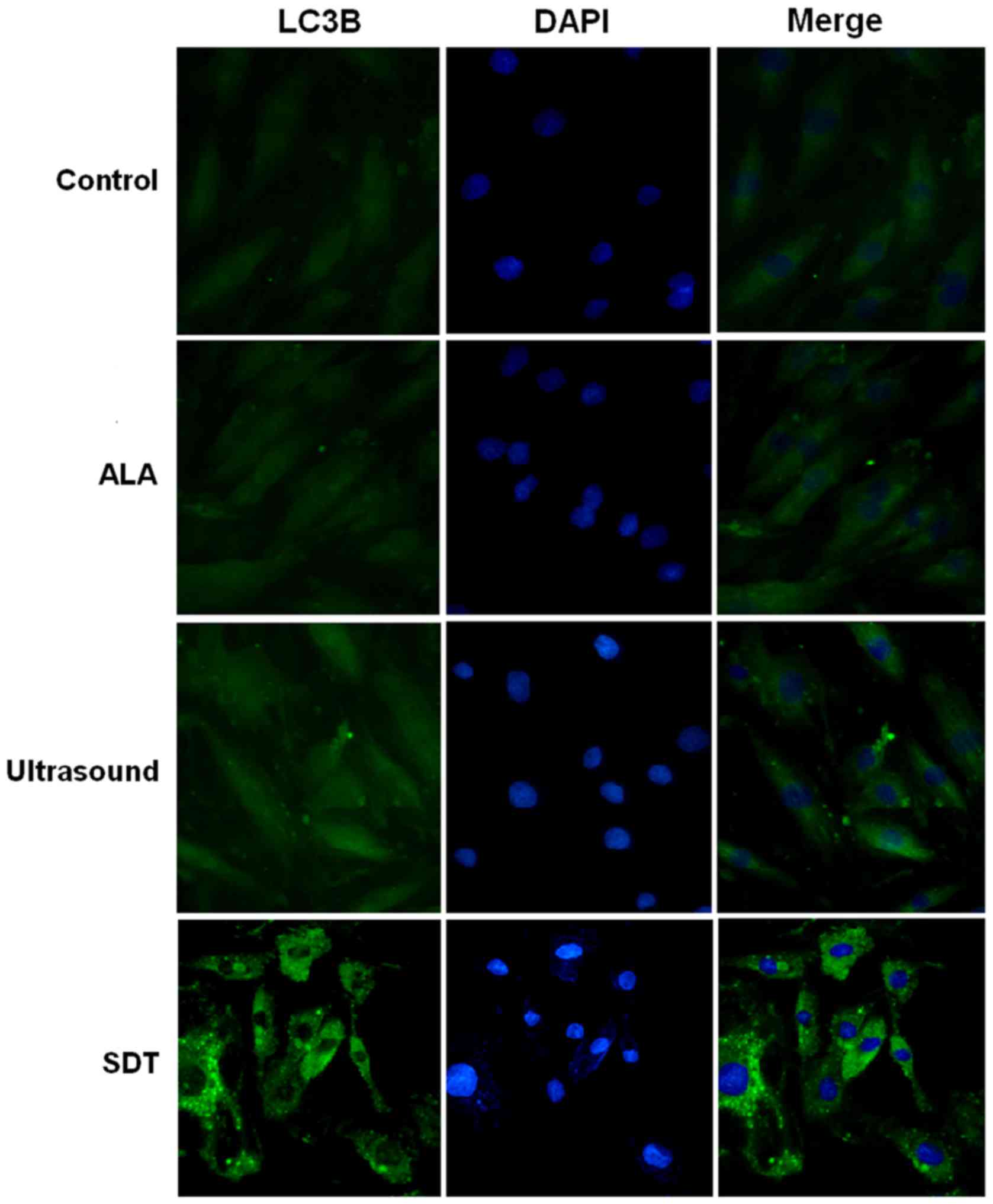
(Fig. 3). To further confirm that SDT
induced autophagy in VSMCs, we measured the levels of LC3-II, a
widely used indicator of autophagy (13), in VSMCs of different groups. As shown
in Fig. 4, a significant increase in
fluorescence was observed in the SDT treatment group compared to
the control group. We also measured the expression levels of LC3-I
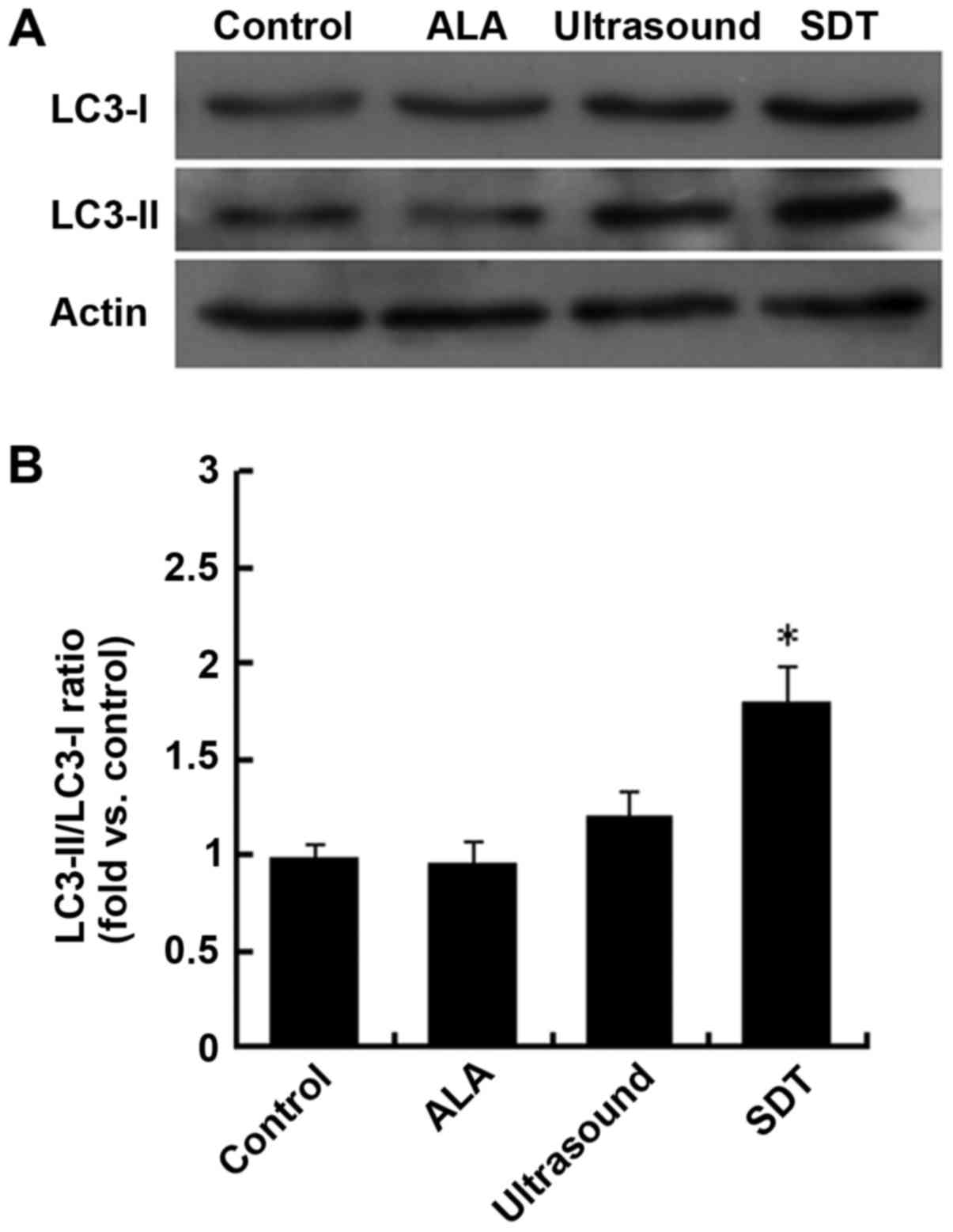
and LC3-II in VSMCs in different groups by western blot analysis.
As shown in Fig. 5, the LC3-II/LC3-I
ratio was significantly increased in the SDT group compared to the
control group. Thus, we conclude that SDT promotes VSMC autophagic
activity.
Discussion
Recently, Michiels et al, reported that
PpIX-SDT promoted VSMC phenotype transformation from a
dedifferentiated to a differentiated status (14), which partially explained why SDT
inhibited in-stent restenosis in animal models (4). PpIX has low toxicity and a short dark
period in the cells, as compared with other sonosensitizers
(15,16). In addition, Michiels et al
(14) showed that PpIX-SDT had no
effect on VSMC viability. Consistent with the above findings, we
did not observe that PpIX-SDT altered the viability of VSMCs,
despite having increased the ultrasound intensity to 1.0
W/cm2. Together, these findings suggest that PpIX-SDT is
a relatively safe therapeutic approach for treating atherosclerotic
plaques.
Previously, Cheng et al (17,18)
reported that PpIX-SDT induced cell death in THP-1 macrophages via
a mitochondria-dependent pathway. However, our results showed that
PpIX-SDT did not significantly induce cell apoptosis compared to
the control group in VSMCs, which coincided with the finding that
MMP was not altered by PpIX-SDT treatment. The maintenance of MMP
observed in our study is probably attributed to the limited
ultrasonic energy and acoustic sensitivity agent concentration.
Cell autophagy is generally regarded as a cell
survival mechanism because cells degrade non-essential, aging or
non-functional proteins, and/or cytoplasmic organelles, and recycle
those degraded components to maintain normal cellular homeostasis
(19). Increasing evidence suggests
that autophagy is involved in a wide range of diseases, including
atherosclerosis (20,21). During the development of
atherosclerotic plaques, harmful material, such as a large number
of reactive oxygen species, cause oxidative stress, leading to the
maintenance of basal activity of cell autophagy, which can protect
cells from the oxidative stress and promote plaque stability
(22–24). Therefore, these cells were protected
from cell death, i.e. apoptosis, and atherosclerotic plaques can
form and steadily grow. Platelet derived growth factor (PDGF) was
shown to mediate autophagy and adjust the response of VSMCs to
phenotypic transformation induced by oxidative damage (25). However, excessive activation of cell
autophagy eventually resulted in plaque cell death, and tended to
be harmful. Because of the elastic strength of the plaque's fibrous
cap, which mostly depends on smooth muscle cells and their secreted
collagen (26), VSMC death usually
leads to unstable plaque (27), and
even plaque rupture. Hence, VSMC survival plays an important role
in plaque stability (26). During the
formation of atherosclerotic plaques, cell autophagy induced by
mild oxidative stress contributes to the removal of damaged
organelles. However, if induced autophagic activity is not
sufficient to eliminate all damaged cell components, excessive
oxidative stress and mitochondrial cytochrome C release can induce
cell apoptosis. Therefore, it is generally believed that low levels
of VSMC autophagy promote plaque stability, but high levels of cell
autophagy are not conducive to stable plaques (9,28).
In our study, we observed increased autophagic
activity following SDT treatment, which was supported by the
following evidence: i) increased autophagosome formation revealed
by electron microscopy, ii) increased protein levels of LC3B, an
autophagy molecular marker, revealed by immunofluorescence
staining, and iii) an increased LC3-II/LC3-I ratio, as revealed by
western blot analysis. LC3, originally named microtubule associated
protein 1A and 1B, referred to as MAP1LC3, plays an important role
in autophagy. The mammalian LC3 family has three members: LC3A,
LC3B and LC3C. Once synthesized, the C-terminus of LC3 is
immediately sheared by autophagy related protein 4 (Atg4) and
subsequently produces LC3-I, which is localized in the cytoplasm.
In autophagy, LC3-I will be modified by Atg7 and Atg3 and
subsequently generate LC3-II, which is located in the
autophagosome. Thus, LC3 is widely recognized as an autophagy
molecular marker (29), and the
LC3-II/LC-I ratio may be used to evaluate the activity of
autophagic flux in cells/organs. Taken together, the findings
suggest that VSMC autophagy increases following PpIX-SDT
treatment.
Autophagy is crucial for normal VSMC function,
phenotype, and survival. Inhibition of autophagy as a therapeutic
strategy in the treatment of neointimal stenosis and
atherosclerosis would be unfavorable. Conversely, stimulation of
autophagy could be a valuable new strategy in the treatment of
arterial disease (30–33). Our study demonstrates that PpIX-SDT
induces autophagy in cultured VSMCs. Future studies are required to
elucidate the molecular mechanisms by which PpIX-SDT activates
autophagy, and to explore whether SDT promotes plaque stability in
in vivo atherosclerotic models.
In the present study, we demonstrate that PpIX-SDT
induces VSMC autophagy, characterized by increased autophagasome
formation. In addition, we show that PpIX-SDT does not trigger any
significant increase in apoptosis or decrease in MMP. These results
point to the possibility that induction of VSMC autophagy may be
one of the mechanisms whereby SDT promotes plaque stability in
vivo.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (8140027).
References
|
1
|
Perelman A, Wachtel C, Cohen M, Haupt S,
Shapiro H and Tzur A: JC-1: Alternative excitation wavelengths
facilitate mitochondrial membrane potential cytometry. Cell Death
Dis. 3:e4302012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen H, Gao W, Yang Y, Guo S, Wang H, Wang
W, Zhang S, Zhou Q, Xu H, Yao J, et al: Inhibition of VDAC1
prevents Ca2+-mediated oxidative stress and apoptosis
induced by 5-aminolevulinic acid mediated sonodynamic therapy in
THP-1 macrophages. Apoptosis. 19:1712–1726. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo S, Sun X, Cheng J, Xu H, Dan J, Shen
J, Zhou Q, Zhang Y, Meng L, Cao W and Tian Y: Apoptosis of THP-1
macrophages induced by protoporphyrin IX-mediated sonodynamic
therapy. Int J Nanomedicine. 8:2239–2246. 2013.PubMed/NCBI
|
|
4
|
Li X, Gao L, Zheng L, Kou J, Zhu X, Jiang
Y, Zhong Z, Dan J, Xu H, Yang Y, et al: The efficacy and mechanism
of apoptosis induction by hypericin-mediated sonodynamic therapy in
THP-1 macrophages. Int J nanomedicine. 10:821–838. 2015.PubMed/NCBI
|
|
5
|
Li Z, Sun X, Guo S, Wang L, Wang T, Peng
C, Wang W, Tian Z, Zhao R, Cao W and Tian Y: Rapid stabilisation of
atherosclerotic plaque with 5-aminolevulinic acid-mediated
sonodynamic therapy. Thromb Haemost. 4:793–803. 2015. View Article : Google Scholar
|
|
6
|
Sun X, Xu H, Shen J, Guo S, Shi S, Dan J,
Tian F and Tian Y and Tian Y: Real-time detection of intracellular
reactive oxygen species and mitochondrial membrane potential in
THP-1 macrophages during ultrasonic irradiation for optimal
sonodynamic therapy. Ultrason Sonochem. 22:7–14. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Krammer B and Plaetzer K: ALA and its
clinical impact, from bench to beside. Photochem Photobiol Sci.
7:283–289. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng C, Li Y, Liang H, Cheng J, Li Q, Sun
X, Li Z, Wang F, Guo Y, Tian Z, et al: Detection and photodynamic
therapy of inflamed atherosclerotic plaques in the carotid artery
of rabbits. J Photochem Photobiol B. 102:26–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mizushima N, Yoshimori T and Levine B:
Methods in mammalian autophagy research. Cell. 140:313–326. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martinet W and De Meyer GR: Autophagy in
atherosclerosis. Curr Atheroscler Rep. 10:216–223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lavandero S, Chiong M, Rothermel BA and
Hill JA: Autophagy in cardiovascular biology. J Clin Invest.
125:55–64. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ouimet M, Franklin V, Mak E, Liao X, Tabas
I and Marcel YL: Autophagy regulates cholesterol efflux from
macrophage foam cells via lysosomal acid lipase. Cell Metab.
13:655–667. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li BH, Yin YW, Liu Y, Pi Y, Guo L, Cao XJ,
Gao CY, Zhang LL and Li JC: TRPV1 activation impedes foam cell
formation by inducing autophagy in oxLDL-treated vascular smooth
muscle cells. Cell Death Dis. 5:e11822014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Michiels CF, Fransen P, De Munck DG, De
Meyer GR and Martinet W: Defective autophagy in vascular smooth
muscle cells alters contractility and Ca2+ homeostasis
in mice. Am J Physiol Heart Circ Physiol. 308:H557–H567. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dan J, Sun X, Li W, Zhang Y, Li X, Xu H,
Li Z, Tian Z, Guo S, Yao J, et al: 5-Aminolevulinic acid-mediated
sonodynamic therapy promotes phenotypic switching from
dedifferentiated to differentiated phenotype via reactive oxygen
species and p38 mitogen-activated protein kinase in vascular smooth
muscle cells. Ultrasound Med Biol. 41:1681–1689. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hagiya Y, Fukuhara H, Matsumoto K, Endo Y,
Nakajima M, Tanaka T, Okura I, Kurabayashi A, Furihata M, Inoue K,
et al: Expression levels of PEPT1 and ABCG2 play key roles in
5-aminolevulinic acid (ALA)-induced tumor-specific protoporphyrin
IX (PpIX) accumulation in bladder cancer. Photodiagnosis Photodyn
Ther. 10:288–295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Uto Y, Tamatani D, Mizuki Y, Endo Y,
Nakanishi I, Ohkubo K, Fukuzumi S, Ishizuka M, Tanaka T, Kuchiike
D, et al: Evaluation of the sonosensitizing activities of
5-aminolevulinic acid and Sn(IV) chlorin e6 in tumor-bearing chick
embryos. Anticancer Res. 34:4583–4587. 2014.PubMed/NCBI
|
|
18
|
Cheng J, Sun X, Guo S, Cao W, Chen H, Jin
Y, Li B, Li Q, Wang H, Wang Z, et al: Effects of 5-aminolevulinic
acid-mediated sonodynamic therapy on macrophages. Int J
Nanomedicine. 8:669–676. 2013.PubMed/NCBI
|
|
19
|
Li Y, Zhou Q, Hu Z, Yang B, Li Q, Wang J,
Zheng J and Cao W: 5-Aminolevulinic acid-based sonodynamic therapy
induces the apoptosis of osteosarcoma in mice. PLoS One.
10:e01320742015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kundu M and Thompson CB: Autophagy: Basic
principles and relevance to disease. Annu Rev Pathol. 3:427–455.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luo Y, Lu S, Zhou P, Ai QD, Sun GB and Sun
XB: Autophagy: An exposing therapeutic target in atherosclerosis. J
Cardiovasc Pharmacol. 67:266–274. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Perrotta I and Aquila S: The role of
oxidative stress and autophagy in atherosclerosis. Oxid Med Cell
Longev. 2015:1303152015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liao X, Sluimer JC, Wang Y, Subramanian M,
Brown K, Pattison JS, Robbins J, Martinez J and Tabas I: Macrophage
autophagy plays a protective role in advanced atherosclerosis. Cell
Metab. 15:545–553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mehta JL, Chen J, Hermonat PL, Romeo F and
Novelli G: Lectin-like, oxidized low-density lipoprotein receptor-1
(Lox-1): A critical player in the development of atherosclerosis
and related disorders. Cardiovasc Res. 69:36–45. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu K, Yang Y, Yan M, Zhan J, Fu X and
Zheng X: Autophagy plays a protective role in free cholesterol
overload-induced death of smooth muscle cells. J Lipid Res.
51:2581–2590. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Salabei JK, Cummins TD, Singh M, Jones SP,
Bhatnagar A and Hill BG: PDGF-mediated autophagy regulates vascular
smooth muscle cell phenotype and resistance to oxidative stress.
Biochem J. 451:375–388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mahmoudi MJ, Mahmoudi M, Siassi F, Shokri
F, Eshraghian MR, Zarnani AH, Chahardoli R, Hedayat M, Khoshnoodi
J, Nayeri H, et al: Lymphocyte cytotoxicity of oxLDL in patients
with atherosclerosis. Iran J Immunol. 8:27–33. 2011.PubMed/NCBI
|
|
28
|
Mehta JL, Chen J, Hermonat PL, Romeo F and
Novelli G: Lectin-like, oxidized low-density lipoprotein receptor-1
(LOX-1): A critical player in the development of atherosclerosis
and related disorders. Cardiovasc Res. 69:36–45. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martinet W and De Meyer GR: Autophagy in
atherosclerosis: A cell survival and death phenomenon with
therapeutic potential. Circ Res. 104:304–317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kabeya Y, Mizushima N, Ueno T, Yamamoto A,
Kirisako T, Noda T, Kominami E, Ohsumi Y and Yoshimori T: LC3, a
mammalian homogue of yeast Apg8p, is localizing in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Grootaert MO, da Costa Martins PA, Bitsch
N, Pintelon I, De Meyer GR, Martinet W and Schrijvers DM: Defective
autophagy in vascular smooth muscle cells accelerates senescence
and promotes neointima formation and atherogenesis. Autophagy.
11:2014–2032. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Klionsky DJ, Abeliovich H, Agostinis P,
Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA,
Ballabio A, et al: Guidelines for the use and interpretation of
assays for monitoring autophagy in higher eukaryotes. Autophagy.
4:151–175. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Salabei JK, Cummins TD, Singh M, Jones SP,
Bhatnagar A and Hill BG: PDGF-mediated autophagy regulates vascular
smooth muscle cell phenotype and resistance to oxidative stress.
Biochem J. 451:375–388. 2013. View Article : Google Scholar : PubMed/NCBI
|