Introduction
Electroporation is a promising, minimally invasive
technique that is able to increase the permeability of cell
membranes and tissues located in the externally applied pulsed
electric fields (1). The consequence
of permeability varies as increasing voltage is applied. Reversible
electroporation (RE) occurs under a relatively low voltage and
causes the permeability of the cell membrane to increase
temporarily, meaning the treated cells survive (2). By contrast, irreversible electroporation
(IRE) happens when the pulsed electric fields exceed a certain
threshold and the treated cells are killed (3).
Neumann et al (1) have demonstrated a medical application of
electroporation by using pulsed electric fields to temporarily
permeabilize cell membranes and deliver foreign DNA into cells.
Strategies of employing a combination of pulsed electric fields and
chemotherapeutic drugs or DNA (small molecules compared to usual
plasmid sizes) emerged in the following decades, namely
electrochemotherapy (ECT) (4) and
gene electrotransfer (5). RE has been
mainly used in combination strategies to temporarily increase
permeability, whilst keeping the tissues and cells alive so the
transfected small molecules (e.g., chemotherapeutic drugs, short
hairpin RNA (shRNA) vector, or DNA vaccine) can bring about
therapeutic benefits (6,7).
Until 2005, Davalos et al (8) proposed the term IRE to distinguish
between cell destruction and RE by using electroporation as a
monotherapy without employing any small molecules to destroy
tissues. Subsequently in 2010, Pech et al (9) first reported a human clinical study
(kidney tumor; n=6) where electroporation was applied as a means of
soft tissue destruction. A number of pre-clinical tests have been
reported in various other types of tumor including liver (10,11), lung
(8), pancreatic (12) and prostate (13). Therefore, IRE has been considered as a
novel, physical cancer treatment. However, it is notable that the
tissue heterogeneity in structure affects electric conductivity and
electric field distribution, and thus cell survival upon IRE
treatment, due to the ‘electric field sinks’ effect (14), and the volume of a single time
ablation is <1 cm3 without repositioning the
electrodes (15,16).
In a previous study, Joyce et al (17) hypothesized that outside the central
zone of IRE ablation exists a peripheral zone of reversible
electroporation, where gene transfer may occur. This was
demonstrated by performing IRE in the liver of a Yorkshire pig
model, and by administrating a green fluorescent protein
(GFP)-labeled plasmid by bolus or primed infusion through the
hepatic artery or portal vein. It is notable that this study used a
high concentration of plasmid, delivered through the blood vessels
and the study was conducted in the liver of a healthy pig model
(17). Therefore, in the present
study, the feasibility of using IRE to mediate human papillomavirus
(HPV)18 E6 shRNA plasmid transfection into cervical cancer cells
in vitro and in vivo was investigated, and the effect
of this combined treatment on tumor growth was observed.
Materials and methods
shRNA plasmids
The enhanced (E)GFP labeled pGenesil-1 plasmid
(Shanghai GeneChem Co., Ltd., Shanghai, China) was used to
construct the shRNA plasmid targeting the HPV18 E6 gene, as
previously described (18). shRNA
targeted the HPV18 E6 coding region at nucleotides 391–411 in
intron 1 of the HPV18 bicistronic transcripts. A total of two pairs
of DNA oligonucleotides (Beijing Dingguo Changsheng Biotechnology
Co., Ltd., Beijing, China) were cloned into the
BamHI/HindIII restriction site of the pGenesil-1
plasmid. The sequences for the sense and antisense strands were
5′-GATCCCTGGGTTATACAATTTATTAATTCAAGAGATTAATAAATTGTATAACCCAGTGA-3′
and
3′-GGACCCAATATGTTAAATAATTAAGTTCTCTAATTATTTAACATATTGGGTCACTTCGA-5′,
respectively. The negative control shRNA (Beijing Dingguo
Changsheng Biotechnology Co., Ltd.) had limited homology to any
known sequences in the human genome. The sequences for the sense
and antisense strands were
5′-GATCCGGAGTACCCTGATGAGATCTTCAAGAGAGATCTCATCAGGGTACTCCTGA-3′ and
3′-GCCTCATGGGACTACTCTAGAAGTTCTCTCTAGAGTAGTCCCATGAGGACTTCGA-5′,
respectively. The constructed plasmids were amplified by polymerase
chain reaction (PCR) and verified by DNA sequencing with forward
primer (5′-AGGCGATTAAGTTGGGTA-3′) and reverse primer
(5′-CGGTAGGCGTGTACGGTG-3′). PCR amplification was performed using 3
µl 10X rTaq polymerase buffer (Dingguo Changsheng Biotechnology
Co., Ltd, Beijing, China), dNTP 2 µl, forward primer 1 µl, reverse
primer 1 µl, DNA 2 µl, 0.2 µl rTaq polymerase (Dingguo Changsheng
Biotechnology Co., Ltd.), and H2O 20.8 µl. PCR
amplification conditions included 30 cycles, each at 95°C for 5
min, 95°C for 30 sec, 55°C for 30 sec, 72°C for 45 sec and 72°C for
5 min. The PCR kits and reagents were purchased from Beijing
Dingguo Changsheng Biotechnology Co., Ltd. The constructed positive
control is named as E6 shRNA plasmid and the negative control named
as CTL shRNA plasmid.
To verify the presence and function of the plasmid,
cells were observed for expression of GFP under fluorescence
microscopy following IRE treatment or transfection with
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
Cell culture
HPV18-positive HeLa cervical carcinoma cells
(Shanghai Cell Bank, Type Culture Collection Committee, Chinese
Academy of Sciences, Shanghai, China) were maintained in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% fetal bovine
serum (FBS) (both from Hyclone; GE Healthcare Life Sciences, Logan,
UT, USA) at 37°C in a 5% CO2 humidified incubator.
Treatment of cells with IRE and
plasmid transfection
Exponentially growing HeLa cells were collected and
resuspended in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) (without FBS), with a final concentration of
2×106/ml. Cells were divided into 6 groups and subjected
to the following treatments: Group A, CTL (untreated control);
group B, IRE; group C, transfected with 6 µg CTL shRNA plasmid;
group D, IRE + CTL shRNA; group E, E6 shRNA plasmid; and group F,
IRE + E6 shRNA. Groups D and F were subjected to IRE treatment
after 10 µg of the appropriate plasmids were added in cell
suspension, as described previously (19). Briefly, IRE was performed on each 500
µl aliquot of HeLa cell suspension. Samples were placed in a
parallel aluminum plated Gene Pulser Cuvette (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) with an electric pulses therapeutic system
(State Key Laboratory of Power Transmission Equipment and System
Security and New Technology, Chongqing University, Chongqing,
China) at a pulse parameter of 1 Hz and 800 V, and for 10 pulses at
a duration of 100 µs for each pulse. Groups C and E were
transfected with 6 µg of the appropriate plasmid using
Lipofectamine 2000 reagent in OPTI-MEM medium (Invitrogen, Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions for 6 h. Group B was treated with IRE alone.
Total RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from cultured HeLa cells
using the RNAiso kit (Takara Biotechnology Co., Ltd., Dalian,
China) according to the manufacturer's protocol. HPV18 E6
transcripts were detected using primers 5′-AGGCGATTAAGTTGGGTA-3′
and 5′-CGGTAGGCGTGTACGGTG-3′. The housekeeping gene GAPDH was used
as a reference gene for normalization. Gene expression relative to
GAPDH was determined using the 2−ΔΔCq method (20). qPCR was performed using a 2X Brilliant
SYBR-Green QPCR Master Mix (Stratagene; Agilent Technologies, Inc.,
Santa Clara, CA, USA) as described previously (21). The qPCR cycling conditions included
pre-incubation for 5 min at 94°C, followed by 30 cycles of
denaturation for 30 sec at 94°C and annealing for 30 sec at 50°C,
prior to an extension step for 30 sec at 72°C and a final extension
step for 10 min at 72°C. PCR products were resolved and analyzed on
1% agarose gels containing 0.5% ethidium bromide (Beyotime
Institute of Biotechnology, Haimen, China).
Western blotting
Protein extracts were prepared 48 h following
transfection or IRE treatment and subsequently subjected to western
blot analysis for HPV18 E6, p53 and proliferating cell nuclear
antigen (PCNA). A total of 50 µg protein was loaded into each lane
and separated by SDS-PAGE (12.5% gel), transferred to
polyvinylidene difluoride membranes, and immunoblotted with primary
antibodies followed by incubation with a goat anti-mouse-HRP
conjugated secondary antibody (dilution, 1:5,000; BIOSS, Beijing,
China). Finally, detection procedures were performed using
Immobilon Western Chemiluminescent HRP substrate (EMD Millipore,
Billerica, MA, USA). Primary antibodies against the following
proteins were used: HPV18 E6 (catalog no. sc-460; dilution, 1:500),
p53 (catalog no. sc-47698; dilution, 1:1,000), PCNA (catalog no.
sc-25280; dilution, 1:1,000) (all from Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) and GAPDH (catalog no. bs-10900R; dilution,
1:1,000; BIOSS). Proteins were visualized with enhanced
chemiluminescent reagent (ECL; Thermo Fisher Scientific, Inc.) and
bands quantified using Quantity One software (version 4.4; Bio-Rad
Laboratories, Inc.).
Cell proliferation by cell-counting
kit-8 (CCK-8 assay)
To assay the growth of HeLa cells, cells suspended
in DMEM (100 µl of 2×104 cells/ml) were seeded into each
well of 96-well culture plates and cultured for 24, 48, 72 and 96 h
at 37°C (in a humidified incubator with a 5% CO2
atmosphere). Subsequently, 10 µl of CCK-8 solution (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) dissolved in DMEM
was added to each well, followed by incubation at 37°C for 2 h. The
number of viable cells was assessed by measurement of absorbance at
450 nm using a microplate reader (Bio-Rad Laboratories, Inc.). The
relative cell number of each group was calculated as OD value at
different time points/OD value when the cells just adhere to the
wall (~6 h following seeding). All samples were tested in
triplicate, and the differences between the controls and the test
groups were analyzed.
Animal experiments
Ethical approval of animal care and experiments was
obtained from the Second Affiliated Hospital of Chongqing Medical
University (Chongqing, China). Specific pathogen-free athymic
(T-cell deficient) nude mice (BALB/c nude; 4–6 weeks old; female)
were obtained from the Animal Experiment Center of Chongqing
Medical University (Chongqing, China). Suspensions of
2×106 cells in 0.2 ml PBS were injected into the dorsal
subcutis of the mice (n=21) by sterile syringes. In order to verify
that plasmids could be transfected by irreversible electroporation
and expressed in vivo when the diameter of the subcutaneous
tumors reached 8–10 mm (~30 days following subcutaneous injection
of HeLa cells suspension), one tumor was collected as a pre-test.
IRE was performed within 10 min after 10 µg E6 shRNA plasmid was
injected into the tumor tissue at multiple points. The tumor was
harvested to perform histopathology and fluorescence microscopy
using frozen sections 48 h later. Subsequently, the remaining mice
(n=20) were randomly divided into four groups: i) group 1
(control), received no treatment; ii) group 2, received only IRE
(800 V; 100 µs; 1 Hz; 10 pulses); iii) group 3, received
intratumoral injection of 10 µg isolated plasmid alone at multiple
points; iv) group 4, received both treatments (IRE was performed
within 10 min after 10 µg plasmid was injected into the tumor
tissue at multiple points). The tumors were measured every seven
days with a caliper until the animals were sacrificed by the
cervical dislocation method at day 28 following subcutaneous
injection of HeLa cells suspension, since a subcutaneous transplant
tumor with growth of >1 month or that is >1 cm in diameter is
prone to necrosis. Tumor volume was calculated by the following
formula: V=πabc/6, where V is the volume, a is the
maximum diameter, and b and c are the other two
perpendicular diameters.
Histology and microscopy
Initially, the tumor that received combined
treatment was harvested and cut in half to identify the IRE
ablation effect, the feasibility of IRE-mediated plasmid transfer
into tumor tissue and the expression of plasmid. One half of the
specimen was fixed in 10% neutral buffered formalin for
histopathology, and the other half was freshly frozen in optimal
cutting temperature compound (Tissue-Tek; Sakura Finetek USA, Inc.,
Torrance, CA, USA) for fluorescence microscopy. Formalin-fixed
tissues were processed routinely, sectioned at 4 µm thickness and
stained with hematoxylin and eosin. Frozen tissues were cut into 10
µm sections and stained with DAPI, and observed under a
fluorescence microscope (Nikon Eclipse TE300; Nikon Corporation,
Tokyo, Japan) equipped with a GFP emission filter to detect green
fluorescence as well as a TRIT-C filter to detect autofluorescence.
Images were acquired on NIS-Elements Basic Research software
(version 2.30; Nikon Corporation, Tokyo, Japan).
Statistical analysis
All experiments were performed in triplicate.
P<0.05 was considered to indicate a statistically significant
difference. Results were statistically analyzed with one-way
analysis of variance (ANOVA) and Post-hoc ANOVA Tukey's HSD test or
unpaired t-test at 5% level of significance. Statistical analysis
was performed using GraphPad Prism software (version 5.0; GraphPad
Software, Inc., La Jolla, CA, USA).
Results
IRE induces the transfection of E6
shRNA plasmid into HeLa cells and results in E6 mRNA knockdown
An EGFP labeled HPV18 E6 shRNA plasmid was
successfully constructed and identified by DNA sequencing. To
verify the feasibility of using IRE to transduce a plasmid into
HeLa cells, the expression of GFP was observed 48 h after IRE (800
V; 100 µs; 1 Hz; 8 pulses) treatment under an inverted fluorescence
microscope. Few HeLa cells survived 24 h after IRE combined with
plasmid treatment. Strong green fluorescence was observed in the
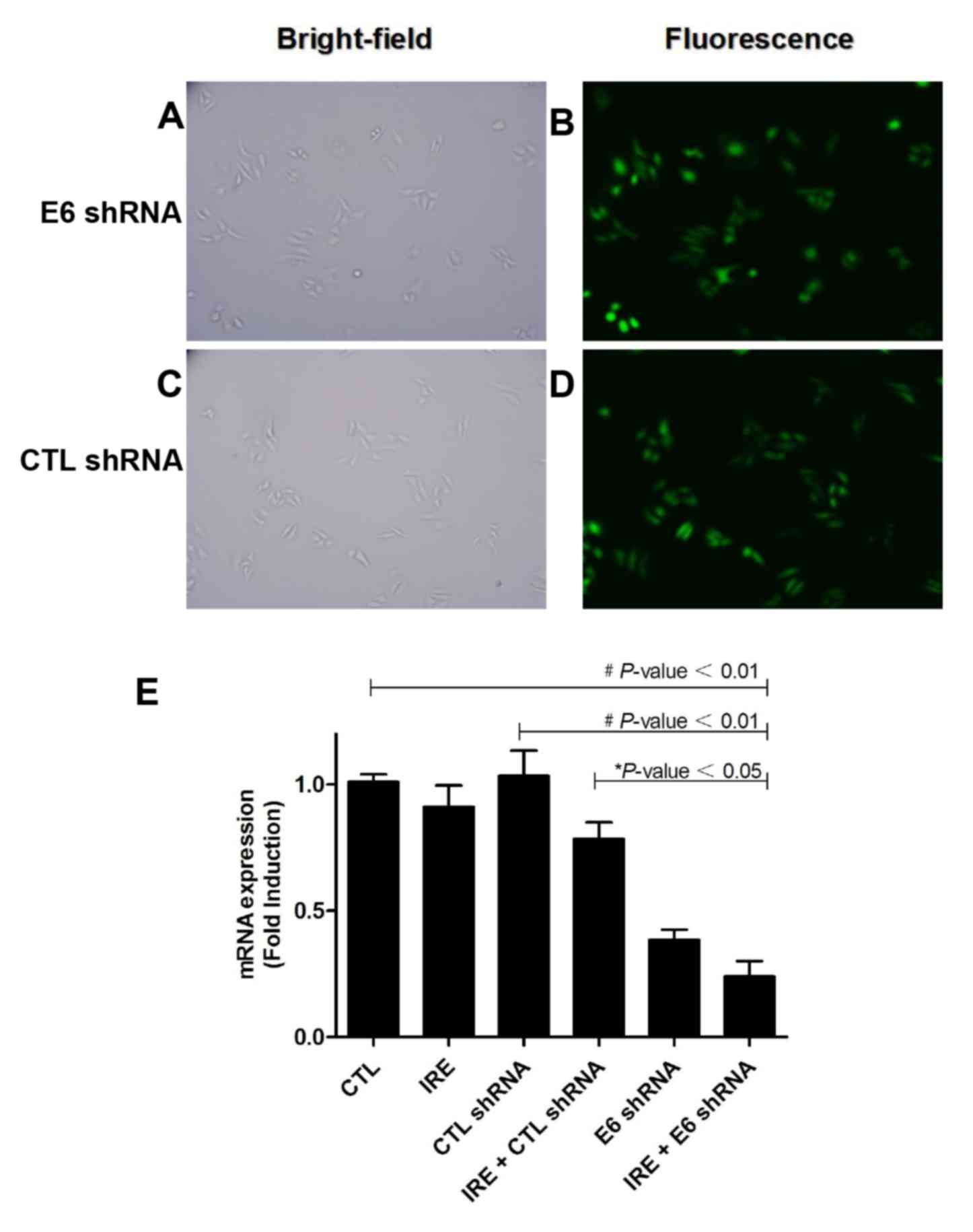
cells that survived (Fig. 1A-D).
HPV18 E6 mRNA expression was then measured by qPCR.
The knockdown efficiency of HPV18 E6 mRNA level was up to 90%.
There were statistically significant differences in HPV18 E6 mRNA
expression between CTL group and IRE+E6 shRNA group (P<0.01;
Fig. 1E), the difference also
appeared between IRE + CTL shRNA group and IRE+E6 shRNA group
(P<0.05, Fig. 1E).
Combination of HPV18 E6 shRNA plasmid
transfection and IRE inhibits HeLa cell proliferation in vitro
A previous study demonstrated that tumor cell
proliferation is positively dependent on E6 levels and HPV DNA load
(21). The present study confirmed
that IRE induced the transfection of interference plasmid into HeLa
cells, and the proliferation of which was inhibited; however, there
were two interfering factors (plasmid and IRE), so one factor may
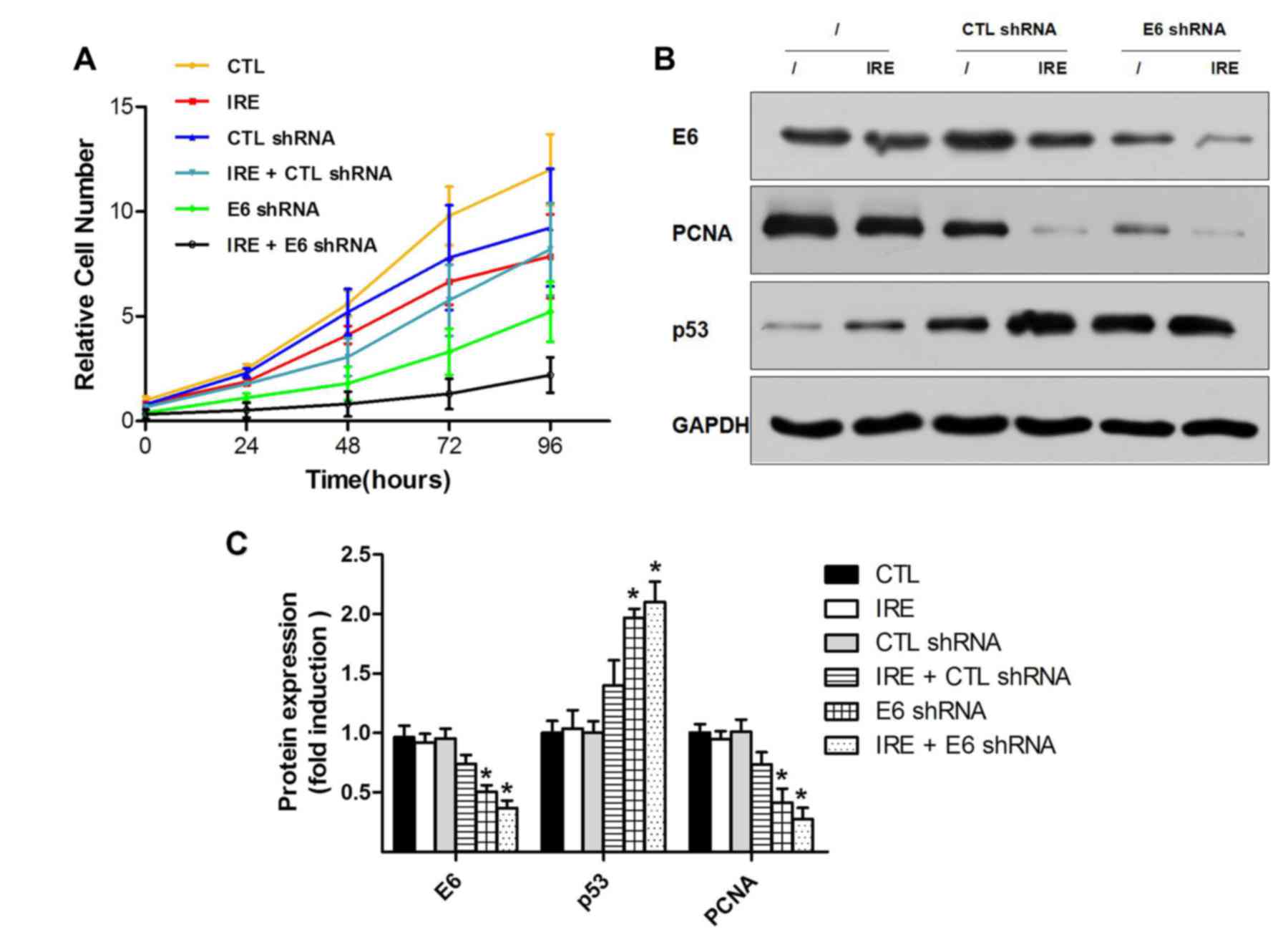
have worked independently. CCK-8 assay was performed to investigate
the proliferation of HeLa cells. The growth curves (P<0.01;
Fig. 2A) of the six groups showed
that the combination treatment with IRE and E6 shRNA significantly
inhibited HeLa cell proliferation vs. the CTL group, and lower than
the E6 shRNA plasmid group (pGenesil-E transfected by Lipofectamine
2000) for 96 h, although this difference was not statistically
significant. Furthermore, western blotting was performed to compare
the relative protein level among the six groups. The western blot
showed similar results to the CCK-8 curve. HPV18 E6 oncoprotein was
significantly downregulated in pGenesil-E transfected groups: E6
shRNA plasmid and IRE+E6 shRNA vs. the CTL group (P<0.01;
Fig. 2B and C). The level of E6
downstream proliferation-associated proteins, including p53 and
PCNA; p53 was upregulated while PCNA was downregulated. When E6 was
downregulated in E6 shRNA plasmid and IRE+E6 ShRNA groups, p53 was
upregulated and PCNA was downregulated. Therefore, the
proliferation of these groups was lower than the control. Notably,
there was a small non-significant difference between the E6 shRNA
plasmid and IRE+E6 shRNA groups.
IRE induces plasmid transfection into
tumor tissue and suppresses tumor growth in cervical xenograft
model
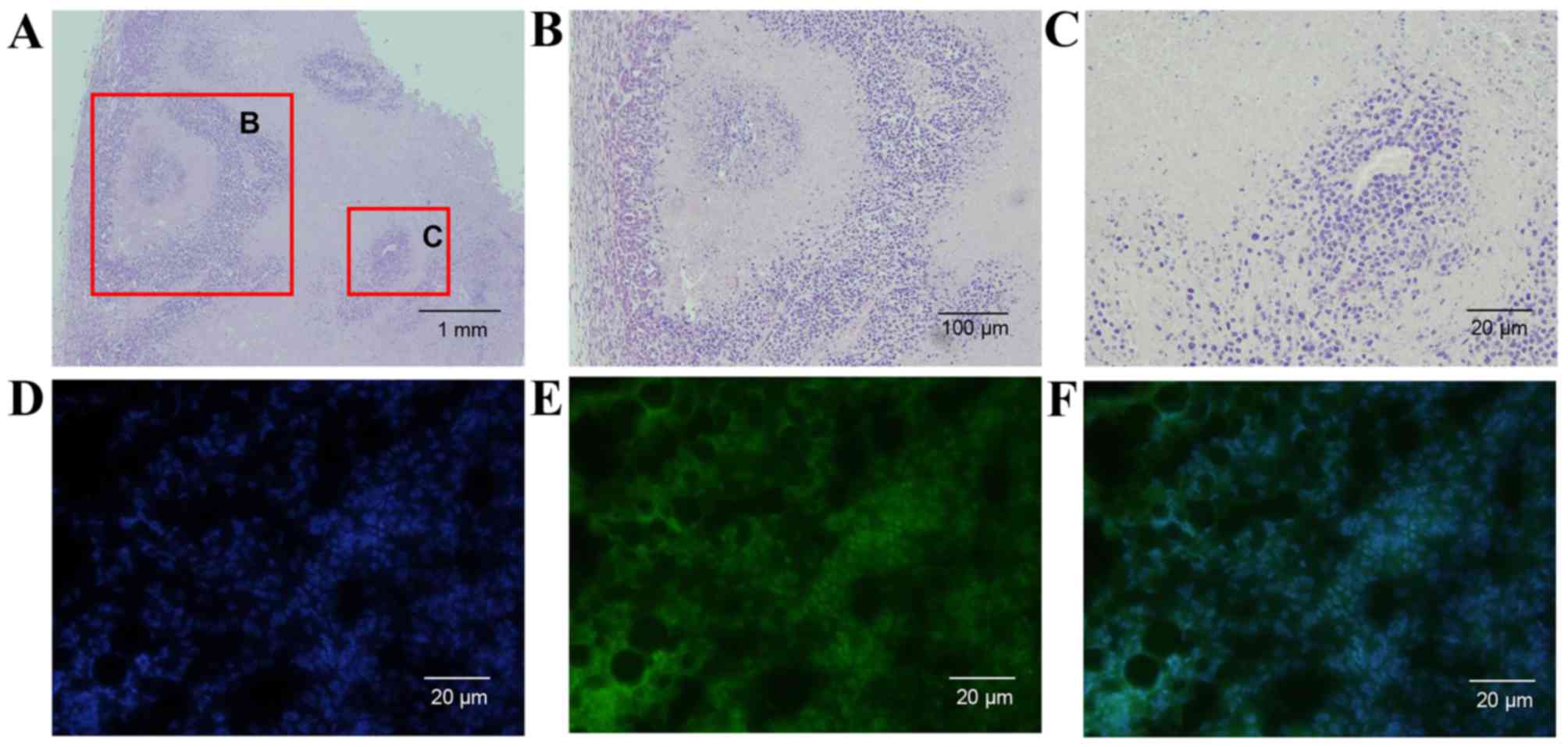
Histopathological results demonstrated that ablated
zones were well demarcated and part of the tumor remained intact
under the fixed IRE parameter (800 V; 100 µs; 1 Hz; 10 pulses).
Necrosis, cytoplasmic hypereosinophilia, nuclear pyknosis and
karyorrhexis can be observed in the ablated areas (Fig. 3A-C). Fluorescence microscopy revealed
that plasmid transfection was induced by IRE into the residual
tumor cell and the plasmids were expressed (Fig. 3D-F).
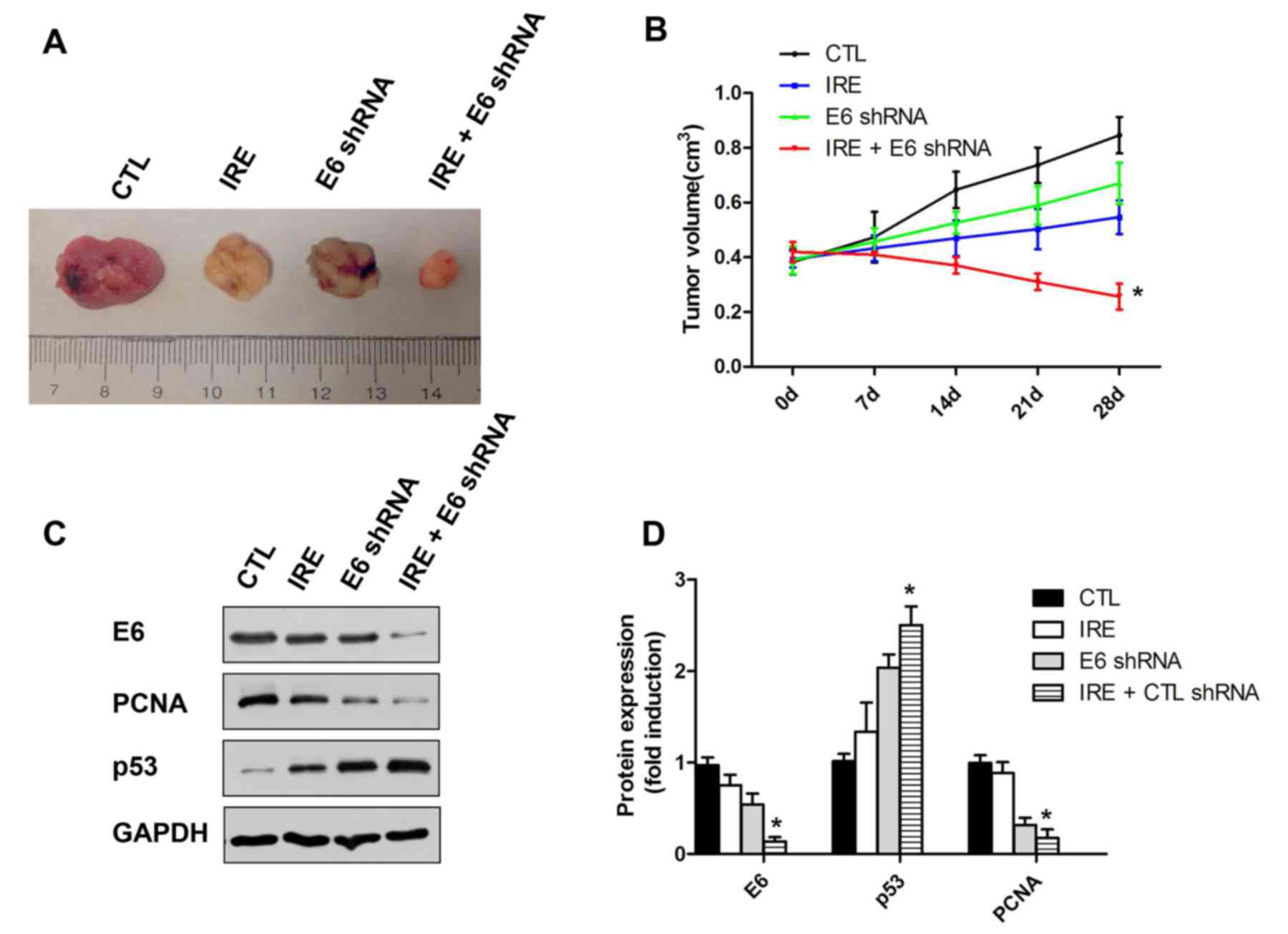
A total of 8 days following treatment, the tumor
size was significantly reduced in IRE+E6 shRNA group compared with
those in either the control group or single treatment (IRE or
plasmid) groups (P<0.05; Fig. 4A and
B). To investigate the effect of the combined treatment with
IRE and shRNA plasmid transfection, the tumors were harvested and
western blotting was performed. The HPV18 E6 oncoprotein level was
significantly decreased in the IRE + plasmid group. vs. the control
group (P<0.05; Fig. 4C and D). An
opposite trend was observed in the level of p53 protein, which is
consistent with the mechanism of p53 degradation by E6 (22).
Discussion
IRE is a type of physical therapy for cancer based
on electrical and biological effects of pulsed electric fields. The
electric biological effects of electric pulse are known. In the
1970s, cell electroporation or electropermeabilization (defined as
the permeabilization of the cell membrane induced by exposure to
short and intense electric pulses) were used to increase the
cellular uptake of normally non-permeable molecules (e.g., drugs,
dyes or DNA) (23). Recently,
electroporation-based treatments (based mainly on RE), including
ECT and electrogenetherapy (EGT) have been employed in clinical
settings (24). By modulating the
electric pulses and the parameters of electroporation, permanent
permeabilization may be observed under transmembrane potential
which eventually leads to cell death; this is termed IRE. IRE has
been used in the food industry for sterilization for decades
(25) until in 2005 Davalos et
al (8) proposed the concept of
using electroporation as a monotherapy which is distinct from ECT
or EGT.
IRE and RE co-exist and cannot be separated due to
the existence of dielectric impedance (24). Given the inherent dielectric
properties of the cell suspension or tumor tissue, the electric
field strength decreases as the distance from needle electrode
increases (24). A simplified example
of this co-existence of IRE and RE is a shooting target of
concentric circles with IRE in the middle and RE in the
periphery.
IRE was considered as a major side effect in
RE-based techniques (such as ECT or EGT). The cells in the target
area survived following RE exposure. Electric field parameters were
strictly controlled to avoid IRE, which could lead cell to death.
Conversely, during the treatment of IRE, appropriate electric field
parameters were selected to ensure that the target area was
completely covered by IRE. The target area was destroyed by IRE.
During IRE treatment, all RE activity in which reversible nanopores
in the surface of the cells are formed, and the treated cells are
not killed, needs to be minimized. In a Yorkshire pig model, Joyce
et al (17) demonstrated that
a peripheral zone of reversible electroporation, where gene
transfer can occur, exists outside the central zone of IRE
ablation. IRE was performed in the liver of a Yorkshire pig model
with the administration of 7 mg GFP-labeled plasmid via bolus or
primed infusion directly through the hepatic artery or portal vein.
This study showed that liver ablation by IRE was clearly demarcated
on histology, and 31/36 liver specimens treated with IRE and the
GFP plasmids demonstrated strong green fluorescence (17). In subsequent studies, it was observed
that IRE facilitated gene transfer of the granulocyte-macrophage
colony-stimulating factor plasmid and brought about a local and
systemic biologic response (26).
This demonstrated that the technique holds the potential for tumor
eradication and immunotherapy of residual cancer.
In the present study, by modulating the electric
pulse parameters of IRE, a situation of incomplete ablation was
simulated within a certain area (in a cuvette or a tumor). A
therapeutic dosage [≥IRE threshold of 667 V/cm (27)] of pulsed field was projected to the
subjects (cells or tumors), in which the co-existence of IRE and RE
following treatment was observable. IRE treatment killed the
majority of the HeLa cells in the cell suspension and ablated part
of the tumors. The cells that survived showed green fluorescence
under an inverted fluorescence microscope. Frozen sections of the
treated tumor showed that the peripheral margin was intact and
demonstrated strong green fluorescence. These results indicated a
therapeutic dose of IRE was able to mediate plasmid transfection
into the tumor in vivo and in vitro. Further results
confirmed that the plasmid was expressed in the surviving tumor
cells, and the effect of the combined treatment with IRE and shRNA
was greater than the single treatment with IRE or shRNA. Notably,
the resultant changes in protein levels and cell growth were more
significant in the combined IRE and shRNA treatment group compared
to the changes in the E6 shRNA transfected group, although no
statistically significant difference was observed. These results
may be due to a co-effect of IRE and shRNA plasmid transfection on
tumor cells. This notable observation may be explored in future
studies.
In conclusion, the present study verified the
feasibility of utilizing IRE to mediate HPV-18 E6 shRNA
transfection into cervical cancer HeLa cells in vitro and
in vivo. The shRNA plasmid was well expressed in HeLa cells
in vitro and in vivo, and the interference effect was
detected by PCR, western blotting and CCK-8 assay. This combined
treatment strategy has promising implications in cancer treatment
for the ablation of tumors and in eliminating microscopic residual
tumor tissue.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81201745 and
81301928), the Health and Family Planning Commission of Chongqing
(General Program) (grant nos. 2011-2-155 and 2012-2-068) and the
Scientific and Technological Research Program of Chongqing
Municipal Education Commission (grant no. KJ1400223).
References
|
1
|
Neumann E, Schaefer-Ridder M, Wang Y and
Hofschneider PH: Gene transfer into mouse lyoma cells by
electroporation in high electric fields. EMBO J. 1:841–845.
1982.PubMed/NCBI
|
|
2
|
Yarmush ML, Golberg A, Serša G, Kotnik T
and Miklavčič D: Electroporation-based technologies for medicine:
Principles, applications, and challenges. Annu Rev Biomed Eng.
16:295–320. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Golberg A and Yarmush ML: Nonthermal
irreversible electroporation: Fundamentals, applications, and
challenges. IEEE Trans Biomed Eng. 60:707–714. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mir LM, Belehradek M, Domenge C, Orlowski
S, Poddevin B, Belehradek J Jr, Schwaab G, Luboinski B and Paoletti
C: Electrochemotherapy, a new antitumor treatment: First clinical
trial. C R Acad Sci III. 313:613–618. 1991.(In French). PubMed/NCBI
|
|
5
|
Daud AI, DeConti RC, Andrews S, Urbas P,
Riker AI, Sondak VK, Munster PN, Sullivan DM, Ugen KE, Messina JL
and Heller R: Phase I trial of interleukin-12 plasmid
electroporation in patients with metastatic melanoma. J Clin Oncol.
26:5896–5903. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Granot Y and Rubinsky B: Mass transfer
model for drug delivery in tissue cells with reversible
electroporation. Int J Heat Mass Transf. 51:5610–5616. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Otten G, Schaefer M, Doe B, Liu H,
Srivastava I, zur Megede J, O'Hagan D, Donnelly J, Widera G,
Rabussay D, et al: Enhancement of DNA vaccine potency in rhesus
macaques by electroporation. Vaccine. 22:2489–2493. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Davalos RV, Mir IL and Rubinsky B: Tissue
ablation with irreversible electroporation. Ann Biomed Eng.
33:223–231. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pech M, Janitzky A, Wendler JJ, Strang C,
Blaschke S, Dudeck O, Ricke J and Liehr UB: Irreversible
electroporation of renal cell carcinoma: A first-in-man phase I
clinical study. Cardiovasc Intervent Radiol. 34:132–138. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheung W, Kavnoudias H, Roberts S,
Szkandera B, Kemp W and Thomson KR: Irreversible electroporation
for unresectable hepatocellular carcinoma: Initial experience and
review of safety and outcomes. Technol Cancer Res Treat.
12:233–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Martin RC II, McFarland K, Ellis S and
Velanovich V: Irreversible electroporation therapy in the
management of locally advanced pancreatic adenocarcinoma. J Am Coll
Surg. 215:361–369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martin RC II, McFarland K, Ellis S and
Velanovich V: Irreversible electroporation in locally advanced
pancreatic cancer: Potential improved overall survival. Ann Surg
Oncol. 20 Suppl 3:S443–S449. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Neal RE II, Millar JL, Kavnoudias H, Royce
P, Rosenfeldt F, Pham A, Smith R, Davalos RV and Thomson KR: In
vivo characterization and numerical simulation of prostate
properties for non-thermal irreversible electroporation ablation.
Prostate. 74:458–468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Golberg A, Bruinsma BG, Uygun BE and
Yarmush ML: Tissue heterogeneity in structure and conductivity
contribute to cell survival during irreversible electroporation
ablation by ‘electric field sinks’. Sci Rep. 5:84852015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ellis TL, Garcia PA, Rossmeisl JH Jr,
Henao-Guerrero N, Robertson J and Davalos RV: Nonthermal
irreversible electroporation for intracranial surgical
applications. Laboratory investigation. J Neurosurg. 114:681–688.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Guo Y, Zhang Y, Klein R, Nijm GM, Sahakian
AV, Omary RA, Yang GY and Larson AC: Irreversible electroporation
therapy in the liver: Longitudinal efficacy studies in a rat model
of hepatocellular carcinoma. Cancer Res. 70:1555–1563. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Joyce TA, Wong J, Mittra A, Carpenter S,
Haddad D, Carson J, Jayaraman S, Monette S, Solomon SB, Ezell P and
Fong Y: Irreversible electroporation is a surgical ablation
technique that enhances gene transfer. Surgery. 150:474–479. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen L, Wu YY, Liu P, Wang J, Wang G, Qin
J, Zhou J and Zhu J: Down-regulation of HPV18 E6, E7, or VEGF
expression attenuates malignant biological behavior of human
cervical cancer cells. Med Oncol. 28 Suppl 1:S528–S539. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou W, Xiong Z, Liu Y, Yao C and Li C:
Low voltage irreversible electroporation induced apoptosis in HeLa
cells. J Cancer Res Ther. 8:80–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rosty C, Sheffer M, Tsafrir D, Stransky N,
Tsafrir I, Peter M, de Crémoux P, de La Rochefordière A, Salmon R,
Dorval T, et al: Identification of a proliferation gene cluster
associated with HPV E6/E7 expression level and viral DNA load in
invasive cervical carcinoma. Oncogene. 24:7094–7104. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Doorbar J: Molecular biology of human
papillomavirus infection and cervical cancer. Clin Sci (Lond).
110:525–541. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Breton M and Mir LM: Microsecond and
nanosecond electric pulses in cancer treatments.
Bioelectromagnetics. 33:106–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang C, Davalos RV and Bischof JC: A
review of basic to clinical studies of irreversible electroporation
therapy. IEEE Trans Biomed Eng. 62:4–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Beveridge JR, Wall K, MacGregor SJ,
Anderson JG and Rowan NJ: Pulsed electric field inactivation of
spoilage microorganisms in alcoholic beverages and the influence of
pulse profile. Proc IEEE. 92:pp. 1138–1143. 2004; View Article : Google Scholar
|
|
26
|
Joyce TA, Mittra A, Song TJ, Cavnar M, Jun
K, Carson J, Gholami S, Haddad D, Gaujoux S, Monette S, et al:
Irreversible electroporation facilitates gene transfer of a GM-CSF
plasmid with a local and systemic response. Surgery. 154:496–503.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rubinsky B, Onik G and Mikus P:
Irreversible electroporation: A new ablation modality-clinical
implications. Technol Cancer Res Treat. 6:37–48. 2007. View Article : Google Scholar : PubMed/NCBI
|