Introduction
Grapes are among the most commonly consumed fruits
worldwide. Grape seed extract contains lipids, proteins,
carbohydrates and polyphenols. Polyphenols have various biological
functions and are largely contained in the seeds (60–70%) and skin
(30%) of grapes (1). Among the
phenolic compounds, proanthocyanidins are the dominant fraction in
grape seeds and are high molecular weight polymers comprised of
dimers or polymers of catechin and epicatechin (2). Grape seed proanthocyanidin (GSP) has a
higher antioxidative activity compared with other well-known
antioxidants, including vitamin C, vitamin E and gallic acid
(3). GSP has been shown to exhibit a
wide range of biological activities, including antioxidant,
cardioprotective and anti-inflammatory effects (4–6).
Furthermore, it was reported that GSP had chemopreventive and
antineoplastic effects on breast, prostate, skin and colorectal
cancer cells (7–10).
Autophagy is an intracellular degradation system in
which proteins and organelles are sequestered, degraded and
recycled (11). Autophagy is an
important housekeeping process used throughout the body and is
involved in the regulation of pathogenesis, including in
neurodegenerative and muscular diseases and cancer (12). Under physiological conditions,
autophagy controls intracellular homeostasis and is therefore
considered a basal cellular mechanism. In addition, autophagy has
been described as an alternative route to cell death (termed
autophagic or type II programmed cell death) (13) and an adaptation mechanism to numerous
physical stresses, including protein aggregation, genotoxic
substances and nutrient loss (14,15).
Specifically, if a cell's primary mechanism of defense using
non-enzymatic molecules, such as flavonoids and vitamins A, C and
E, and enzyme-based scavengers, such as catalase, ascorbate
peroxidase and superoxide dismutase, fails to achieve the desired
outcome under oxidative stress, autophagy acts to remove oxidized
materials resulting in protection of the cell (16). However, oxidative stress is not always
overcome by these defense mechanisms, and this results in cell
death by autophagy (12).
Although apoptotic activation in various type of
cancer by GSP has been reported, the precise mechanisms of
cancer-associated cell death remain unknown. Therefore, the present
study aimed to investigate the effect of GSP on a squamous cell
carcinoma (SCC) cell line in order to elucidate the potential
mechanism of GSP-induced cell death in SCC following GSP treatment.
In addition, the role of reactive oxygen species (ROS) in
GSP-induced apoptosis and autophagy in SCC was analyzed. Focus was
placed on SCC, as it is a lethal disease with a poor prognosis as a
result of the ineffectiveness of therapy. The results of the
present study indicated a novel function of GSP as an inducer of
autophagy and provided evidence for the combined use of autophagy
inducers as potentiators of anticancer drugs.
Materials and methods
Reagents
GSP was provided by Hanlim Pharmaceutical Co., Ltd.
(Seoul, Korea). 3-methyladenine (3-MA) was purchased from
Sigma-Aldrich (Merck Millipore, Darmstadt, Germany).
Cell culture and treatment
The SCC12 cell line was donated by Dr James
Rheinwald (Brigham and Women's Hospital, Harvard Medical School,
Boston, USA) and was maintained in growth medium containing a 3:1
ratio of Dulbecco's modified Eagle's medium (DMEM) and Ham's F-12
Nutrient Mixture (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% fetal bovine serum (FBS), 0.5 mg/ml
hydrocortisone, 5 mg/ml insulin and 10 ng/ml epidermal growth
factor. The cells were treated with various concentrations of GSP
dissolved in PBS. Control cells received the same volume of the
vehicle solution (PBS).
Cell viability analysis
SCC12 cells (5×104 cells/well) were
seeded into 24-well plates and incubated at 37°C for 24 h, after
which the cells were treated with increasing concentrations of GSP
(10, 50, 100 and 200 µg/ml) for 24 h. The cell viability was
assessed using Cell Counting kit-8 (CCK-8) assays (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan). Briefly, CCK-8
solution (10 µl) was added to each well and incubated for 1 h at
37°C in a humidified atmosphere containing 5% CO2.
Absorbance was then measured at 450 nm using a microplate reader
(Molecular Devices, LLC, Sunnyvale, CA, USA). The effect of 3-MA on
cell death was determined after the cells had been treated for 24
h. Cells were pre-incubated with 3-MA (10 mM) for 1 h prior to the
addition of GSP.
Flow cytometric DNA analysis
The collected cells were washed twice with cold PBS,
fixed with 70% ethanol for 1 h at 4°C, treated with 1 mg/ml RNase A
(Sigma-Aldrich; Merck Millipore) and then stained with 50 µg/ml
propidium iodide (Sigma-Aldrich; Merck Millipore). The relative DNA
content per cell was determined using a FACSCalibur™ flow cytometer
(BD Biosciences, Franklin Lakes, NJ, USA). Data were analyzed with
CellQuest Pro software (version 4.0; BD Biosciences). The
percentage of cells in each phase of the cell cycle was calculated
using ModFit LT Software (version 3.0; Verity Software House, Inc.,
Topsham, ME, USA).
Cell migration assay
SCC12 cells were seeded at a density of
2×105 cells/well onto 24-well plates and incubated at
37°C overnight. Subsequent to the cell monolayer being formed,
horizontal lines were scraped onto the bottom of each well using a
200-µl yellow pipette tip. The cells were then washed with PBS and
incubated for 6 h with serum-free medium containing 10 or 50 µg/ml
GSP. Images of the plates were captured using an inverted
microscope equipped with an image capture system (Nikon
Corporation, Tokyo, Japan).
Invasion assay
Invasion assays were performed on 24-well Transwell
inserts (Costar; Sigma-Aldrich; Merck Millipore) with polycarbonate
filters (8-µm pore size). The Transwell inserts were coated with a
uniform layer of BD Matrigel™ Basement Membrane Matrix (BD
Biosciences). Stable cell lines were resuspended in DMEM/F12
containing 10% FBS and seeded into the upper wells
(1×105 cells/well) and incubated at 37°C for 24 h.
Invaded cells were fixed in 4% paraformaldehyde, stained with DAPI
and counted under a fluorescent microscope at ×100 magnification
for 5 random fields.
Gelatin zymography
The net gelatinase [matrix metalloproteinase (MMP)-2
and −9] activity of cell culture media supernatants was determined
using SDS-containing gels prepared by copolymerizing acrylamide and
gelatin at a final concentration of 0.1% (w/v). Samples were
dispersed for 10 min at room temperature in Laemmli solubilizing
solution from which dithiothreitol had been omitted.
Electrophoresis was performed at 10 mA and 4°C for 3 h. Subsequent
to electrophoresis, the gels were incubated for 60 min at room
temperature in the zymogram renaturing buffer containing 2.5% (v/v)
Triton X-100, followed by 16 h incubation at 37°C in 50 mM Tris-HCl
(pH 7.6) containing 5 mM CaCl2. The gels were stained
with 0.5% Coomassie Brilliant Blue G-250, according to a standard
protocol.
Western blot analysis
The cells were lysed by incubation for 30 min with
NP-40 lysis buffer (20 mM Tris, pH 7.5, 140 mM NaCl, 1 mM EDTA)
containing 1% (v/v) Nonidet P-40, 5 µM AEBSF, 1.5 nM aprotinin, 10
nM E-64 and 10 nM Leupeptin. Cells were sonicated and centrifuged
at 12,000 × g for 10 min at 4°C to remove insoluble debris. Total
proteins (30 µg) were separated by 10% SDS-PAGE and transferred
onto a nitrocellulose membrane using semidry transfer apparatus
(Trans-Blot SD Semi-Dry Transfer Cell; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) at 15 V for 30 min. Membranes were blocked with
5% skimmed milk and incubated with specific antibodies against
MMP-2 (1:1,000 dilution; sc-10736; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) and microtubule-associated protein 1 light chain 3
(LC3) (1:1,000 dilution; AP1801a; Abgent, Inc., San Diego, CA, USA)
at 4°C overnight. After three washes with Tris-buffered saline
containing 0.1% Tween-20, membranes were incubated with horseradish
peroxidase-conjugated secondary antibody (1:3,000 dilution;
sc-2030; Santa Cruz Biotechnology, Inc.). Protein bands were
identified by the enhanced chemiluminescence detecting system
(Pierce; Thermo Fisher Scientific Inc.), according to the
manufacturer's protocol. β-actin was used as a loading control in
the stripped blot.
ROS measurement
Intracellular generation of ROS was determined using
2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA; Molecular
Probes, Thermo Fisher Scientific Inc.). The dye that integrated
into the cells was deacetylated by intracellular esterases. Upon
oxidation, DCF-DA is converted to highly fluorescent
2,7-dichlorofluorescein (17).
Briefly, cells were cultured at 37°C overnight in 6-well plates and
then treated with GSP in the presence or absence of N-acetyl
cysteine (NAC) for 4 h. The cells were stained with 5 µM DCF-DA in
serum-free medium for 15 min and removed from the plate with
trypsin-EDTA (Gibco, Thermo Fisher Scientific, Inc.). The
fluorescence intensity of the cells was determined by flow
cytometry with an excitation wavelength of 480 nm and an emission
wavelength of 525 nm (BD Biosciences). Data were analyzed using
CellQuest Pro software (version 4.0, BD Biosciences).
Recombinant adenovirus
An adenovirus encoding a green fluorescent protein
(GFP)-tagged LC3 (Ad-GFP-LC3) was created using the Virapower
adenovirus expression system (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Briefly, a DNA
construct encoding GFP fused to LC3 was subcloned into the pENTR
vector. Site-specific recombination between entry vectors
(pENTR-GFP-LC3) and the adenoviral destination vector
(pAd/CMV/V5-DEST) was performed with LR clonase II (Invitrogen;
Thermo Fisher Scientific, Inc.). All constructs were verified by
performing Sanger dideoxy sequencing analysis (Bioneer Inc., Seoul,
Korea). The verified clone (pAd-GFP-LC3) was linearized using Pac I
(New England Biolabs, Inc., Ipswich, MA, USA) and then transfected
into 293A cells with Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Subsequent to amplification, viruses from the
culture supernatants of 293 cells that showed cytopathogenic
effects were purified using the Adeno-X™ Virus Purification kit (BD
Biosciences) and viral titers were determined using a
plaque-forming assay with serial dilutions. Briefly, the cells were
plated onto 6-well plates at a density of 1×105 cells/ml
and infected with recombinant adenoviruses at a multiplicity of
infection of 10 on the following day.
Detection of autophagy
Cells were infected with Ad-GFP-LC3 adenovirus and
treated with different concentrations of GSP for 12 h. GFP-LC3
protein puncta-formation in the cells was observed under a confocal
microscope (Olympus FV-1000; Olympus Corporation, Tokyo,
Japan).
Statistical analysis
Data were analyzed using a two-tailed Student's
t-test. Statistical analyses were performed using SPSS software,
version 7.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
GSP induces cell cycle arrest and
apoptosis in SCC12 cells
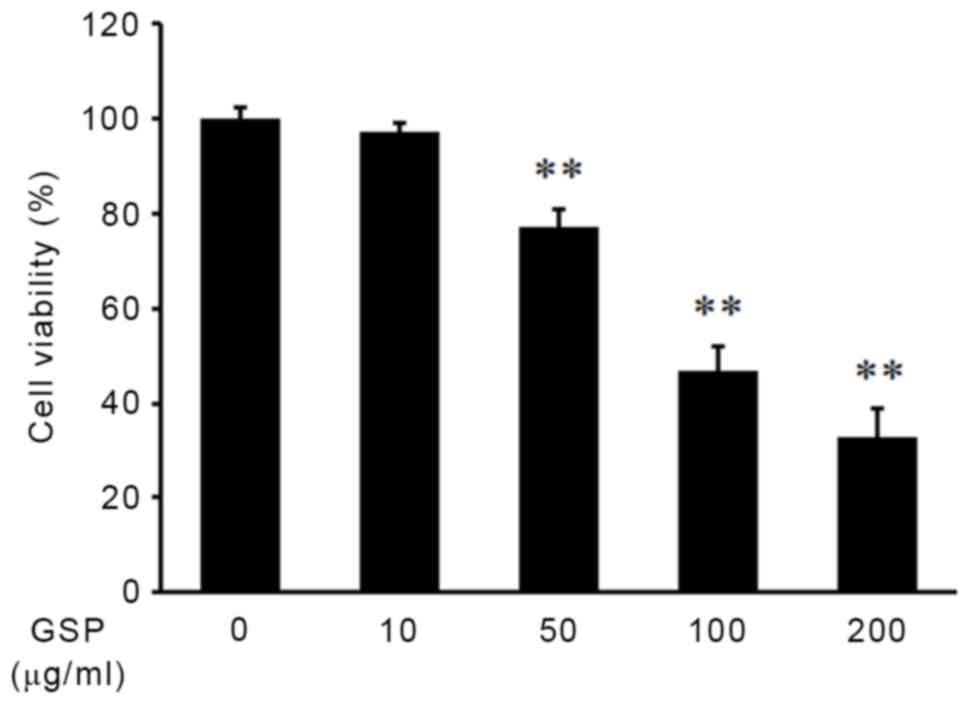
The effect of GSP on cell viability in human SCC12
cells was examined using CCK-8 assays. GSP caused a dose-dependent
reduction of SCC12 cell viability, and treatment with 100 µg/ml GSP
for 24 h resulted in an ~50% reduction in cell viability
(P<0.01; Fig. 1). Flow cytometry
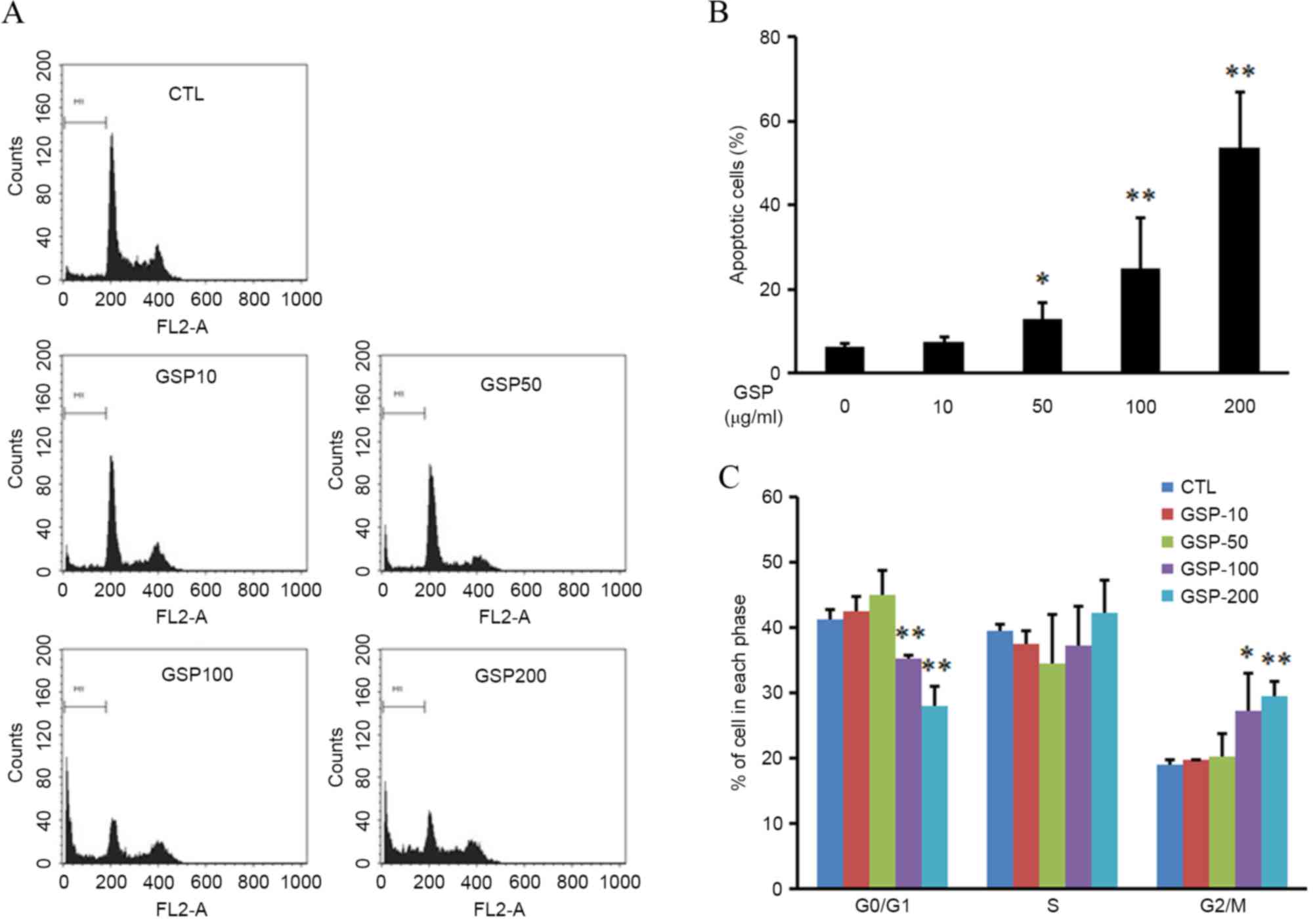
was used to investigate whether the reduction of cell viability by
GSP was due to apoptosis. Apoptosis was evaluated by the
measurement of the number of cells in the sub-G1 region. As shown
in Fig. 2A and B, GSP induced a
significant and dose-dependent increase in the percentage of cells
in the sub-G1 region (P<0.05 at 50 µg/ml GSP, P<0.01 at 100
and 200 µg/ml GSP). These data indicate that the anti-proliferative
effect of GSP on SCC12 cells may be associated with the induction
of apoptosis.
The present study next investigated the possible
effect of GSP on cell cycle progression. GSP treatment resulted in
an increase in the number of cells in the G2/M phase of
the cell cycle, while the number of cells in the
G0/G1 phase was decreased compared with the
control (P<0.05 at 100 µg/ml GSP, P<0.01 at 200 µg/ml GSP;
Fig. 2C). This result indicates that
GSP treatment induces the G2/M arrest in SCC12
cells.
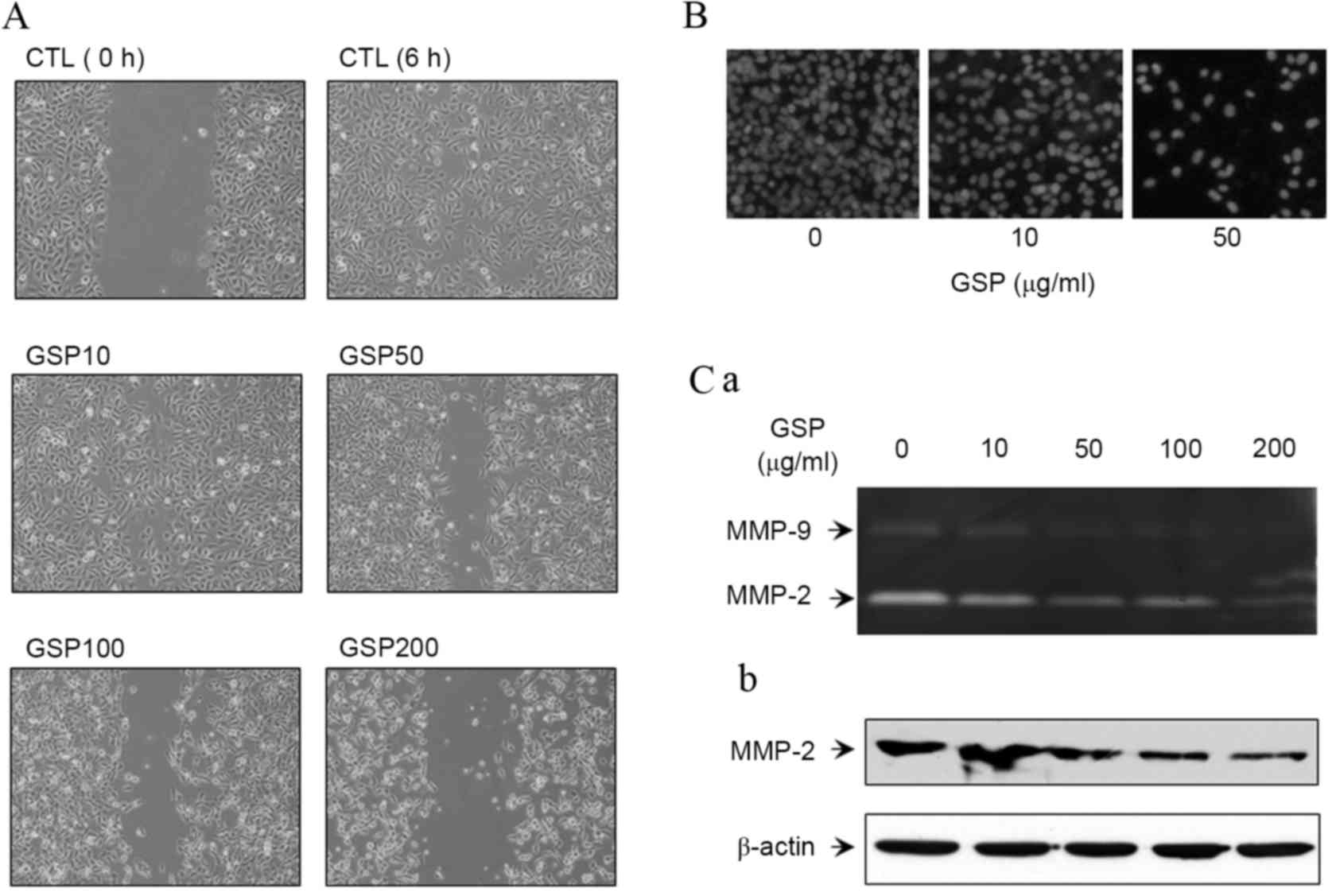
GSP inhibits the motility and
invasiveness of SCC12 cells through suppression of MMP-2/9
expression
SCC12 cells have previously been reported to have a
high metastatic potential and GSP has been shown to inhibit the
migration of cancer cells by disrupting the mitochondrial pathway
and increasing the activation of caspase-3 (18). However, the mechanism underlying the
inhibitory effect of GSP on the motility of SCC cells has yet to be
investigated. Therefore, the effect of GSP on the motility and
invasive abilities of SCC12 cells was analyzed in the present
study. Cell motility was investigated with a wound-healing assay.
GSP-treated SCC12 cells showed a marked reduction of cell motility
compared with the control (Fig. 3A).
Using the Matrigel invasion assay, a decrease in cell invasion was
observed in GSP-treated SCC12 cells, as compared with the control
(Fig. 3B). Subsequently, the effect
of GSP on the activities of MMP-2 and −9 was evaluated using a
gelatin zymogram analysis, and it was shown that GSP inhibited the
activities of MMP-2 and −9 in SCC12 cells in a dose-dependent
manner (Fig. 3C-a). Since the
inhibition of MMP-2 and −9 activities is associated with the
expression level of MMP-2 and −9, the present study evaluated the
expression levels of MMP-2. GSP downregulated the protein
expression of MMP-2 in SCC12 cells (Fig.
3C-b).
GSP induced-cytotoxicity of SCC12
cells involves autophagic cell death and apoptosis
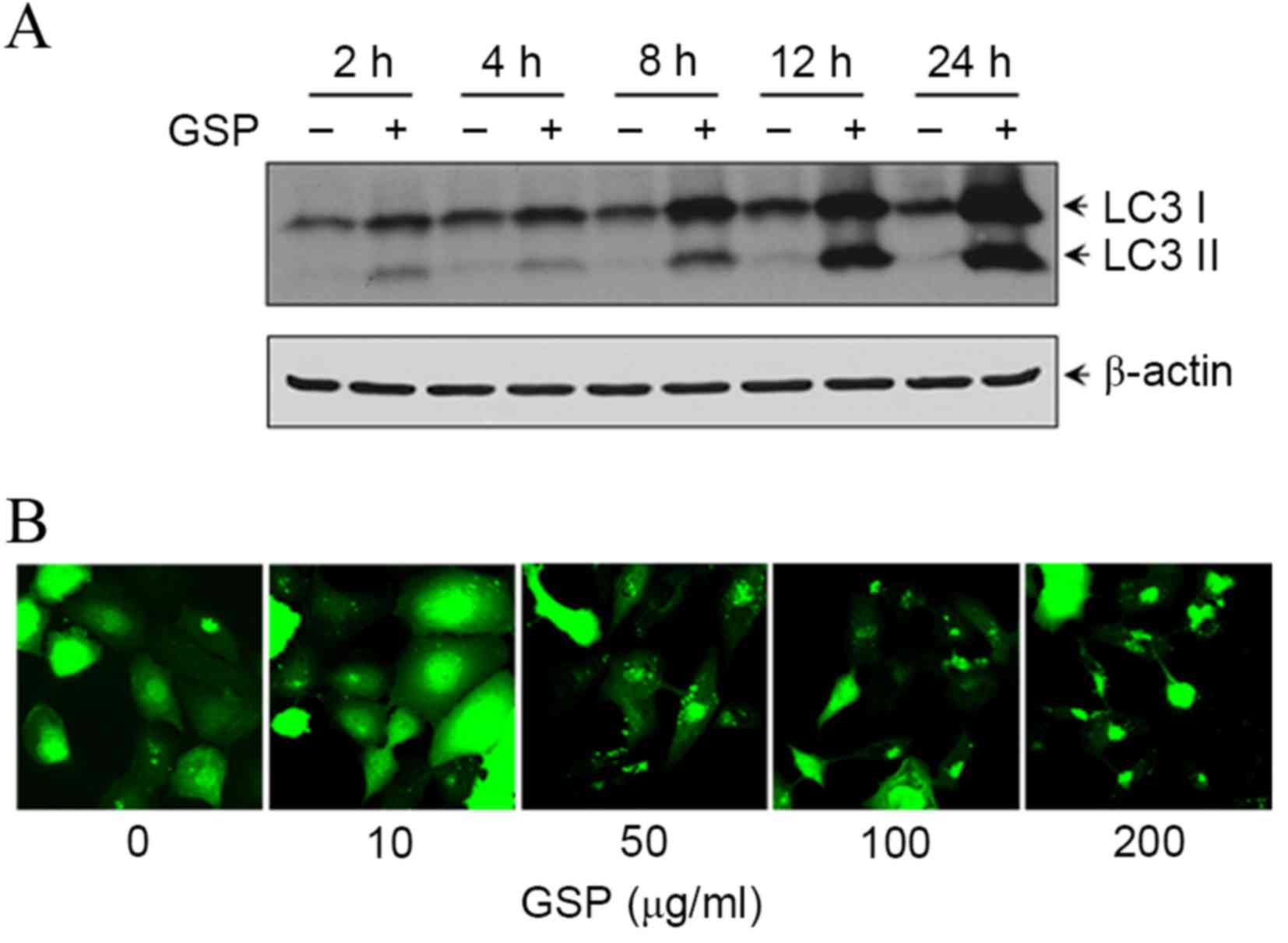
To assess the effect of GSP on autophagy induction,
the present study performed two independent LC3 analyses: LC3-II
production and formation of GFP-LC3 puncta. Upon induction of
autophagy, LC3 is lipidated and aggregates onto the membranes of
autophagic vacuoles (19). Following
treatment with 100 µg/ml GSP, the protein expression of LC3-I and
LC3-II was increased in a time-dependent manner (Fig. 4A). To further confirm GSP-induced
autophagy, a recombinant adenovirus expressing the GFP-LC3 fusion
protein was created and whether GSP was able to promote GFP-LC3
puncta formation, an indicator of autophagosome generation
(20), was investigated.
Ad-GFP-LC3-infected SCC12 cells were treated with GSP and the
GFP-LC3 fusion protein was visualized under a confocal microscope.
GFP-LC3 was observed to form punctate structures and the number of
GFP-LC3 puncta was increased in a dose-dependent manner (Fig. 4B). The GFP-LC3 exhibited a diffuse
nuclear and cytosolic distribution in control cells.
Autophagy has also been implicated in a type of
programmed cell death type II termed autophagic cell death
(13). The present study next
examined whether, besides apoptosis, GSP was able to induce
autophagic cell death. Cell viability assays were conducted using
cells exposed to GSP in the presence or absence of 3-methyladenine
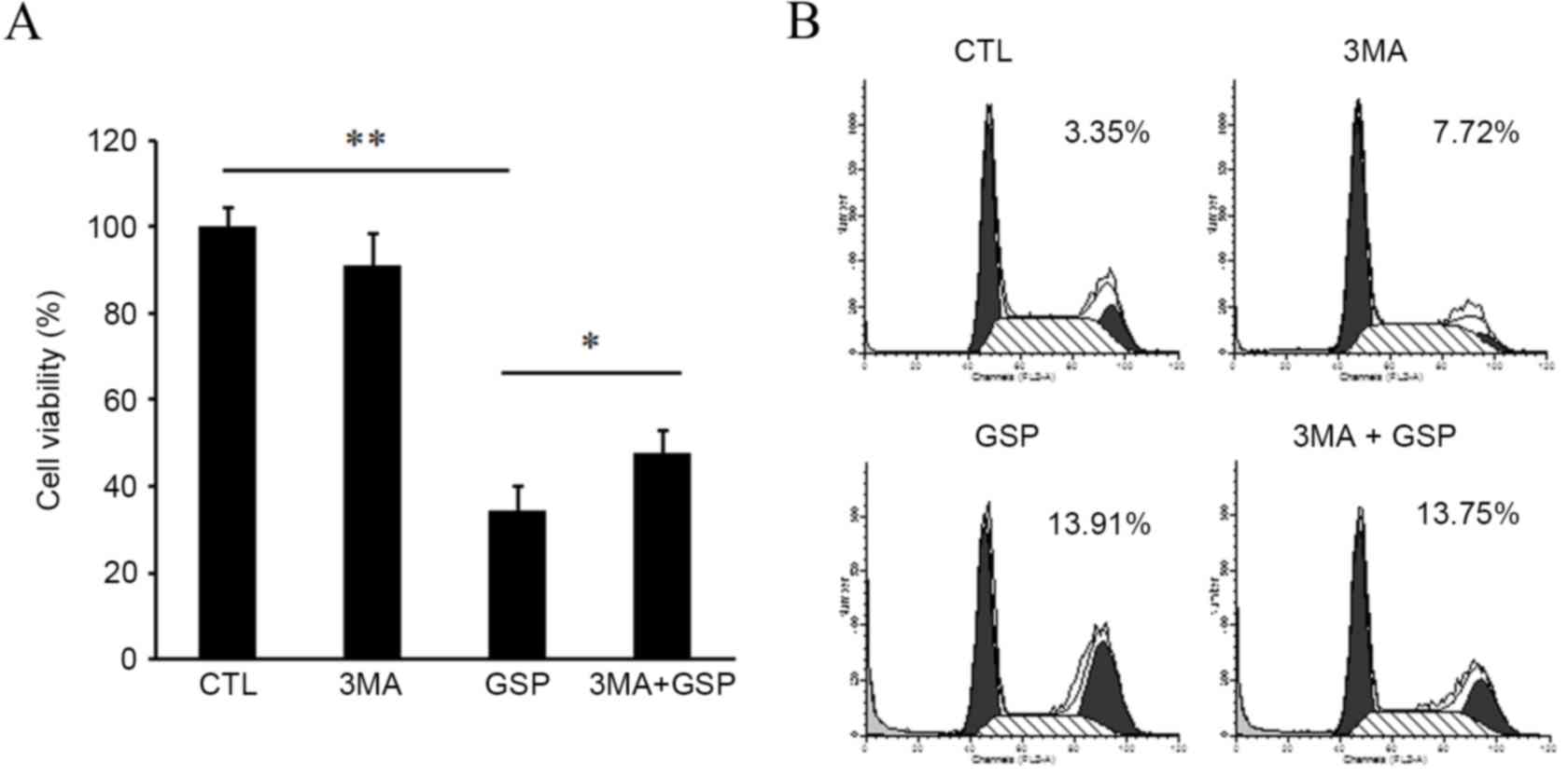
(3-MA), an autophagy inhibitor. Pre-treatment of the cells with
3-MA significantly reduced GSP-induced cytotoxicity (GSP vs.
3-MA+GSP, P<0.05; Fig. 5A), but
failed to block GSP-induced apoptosis (Fig. 5B). These results suggest that the
anti-proliferative activity of GSP may result, at least in part,
from autophagy-mediated cell death.
ROS modulation is involved in
GSP-induced apoptosis and autophagy
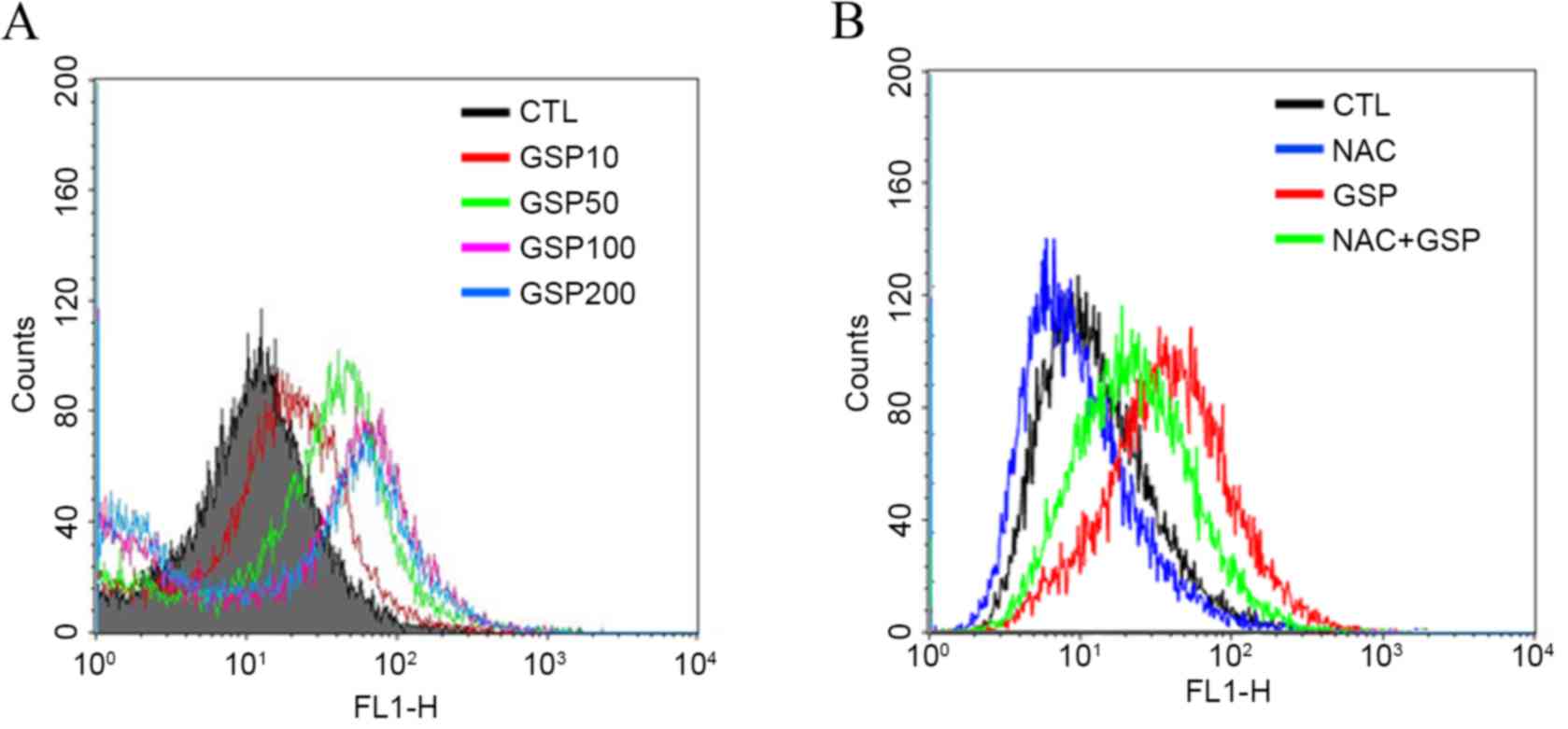
The intracellular ROS level was determined using the
ROS-sensitive fluorescent dye DCF-DA. ROS accumulation increased in
a dose-dependent manner in GSP-treated SCC12 cells (Fig. 6A). However, GSP-induced ROS generation
was significantly blocked by pretreatment of the cells with the
antioxidant agent, NAC (Fig. 6B).
To determine whether elevated ROS levels mediate the
cytotoxic effects of GSP, the effects of antioxidant agents on cell
viability was examined. Cells were pretreated with 10 mM NAC and
then treated with 100 µg/ml GSP for an additional 24 h. As shown in
Fig. 7A, GSP-induced cell death was
significantly prevented by pretreatment with NAC (GSP vs. NAC+GSP,
P<0.01). These results suggest that GSP-induced apoptosis and
autophagy may be associated with its activity in enhancing the
intracellular levels of ROS. To investigate this possibility, it
was first determined whether preincubation with NAC was able to
inhibit GSP-induced apoptosis. Notably, pretreatment with NAC
significantly suppressed GSP-induced apoptosis of SCC12 cells (GSP
vs. NAC+GSP, P<0.01; Fig. 7B). To
further analyze the involvement of ROS in autophagy, the effect of
GSP on autophagic activation in the presence of NAC was assessed.
GFP-LC3 puncta formation by GSP was markedly impeded in the
presence of NAC (Fig. 7C). These
observations suggest that GSP-induced oxidative stress may activate
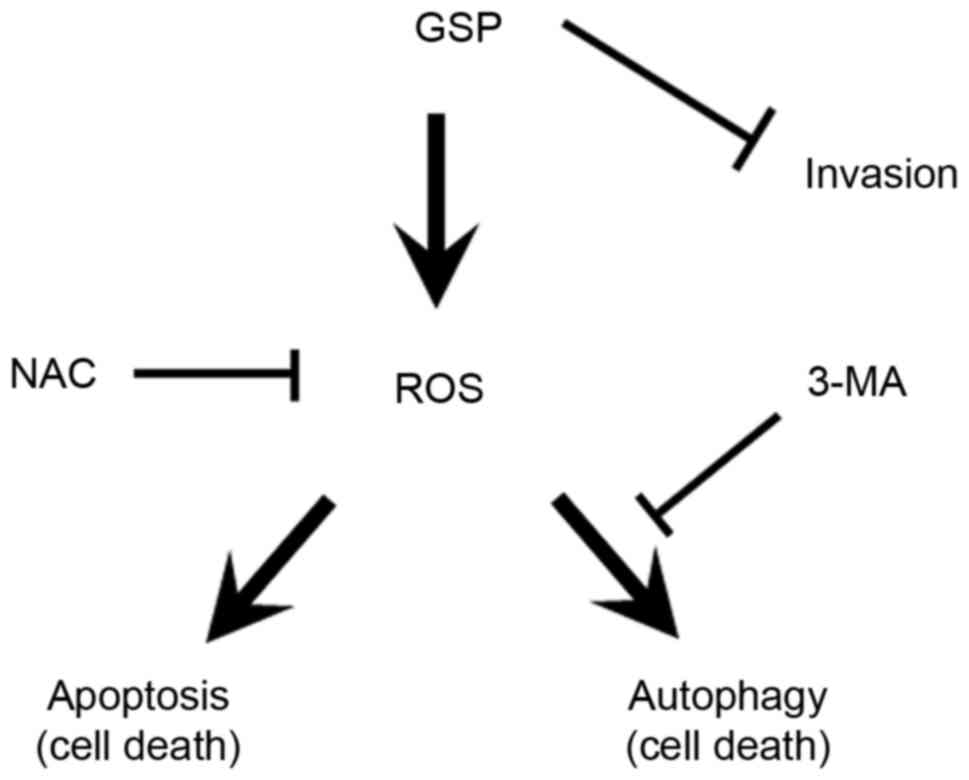
apoptosis and autophagy pathways in SCC12 cells. Based on the
results obtained, the present study has proposed a mechanism for
GPS-induced cell death (Fig. 8) that
involved ROS-mediated activation of autophagy and apoptosis.
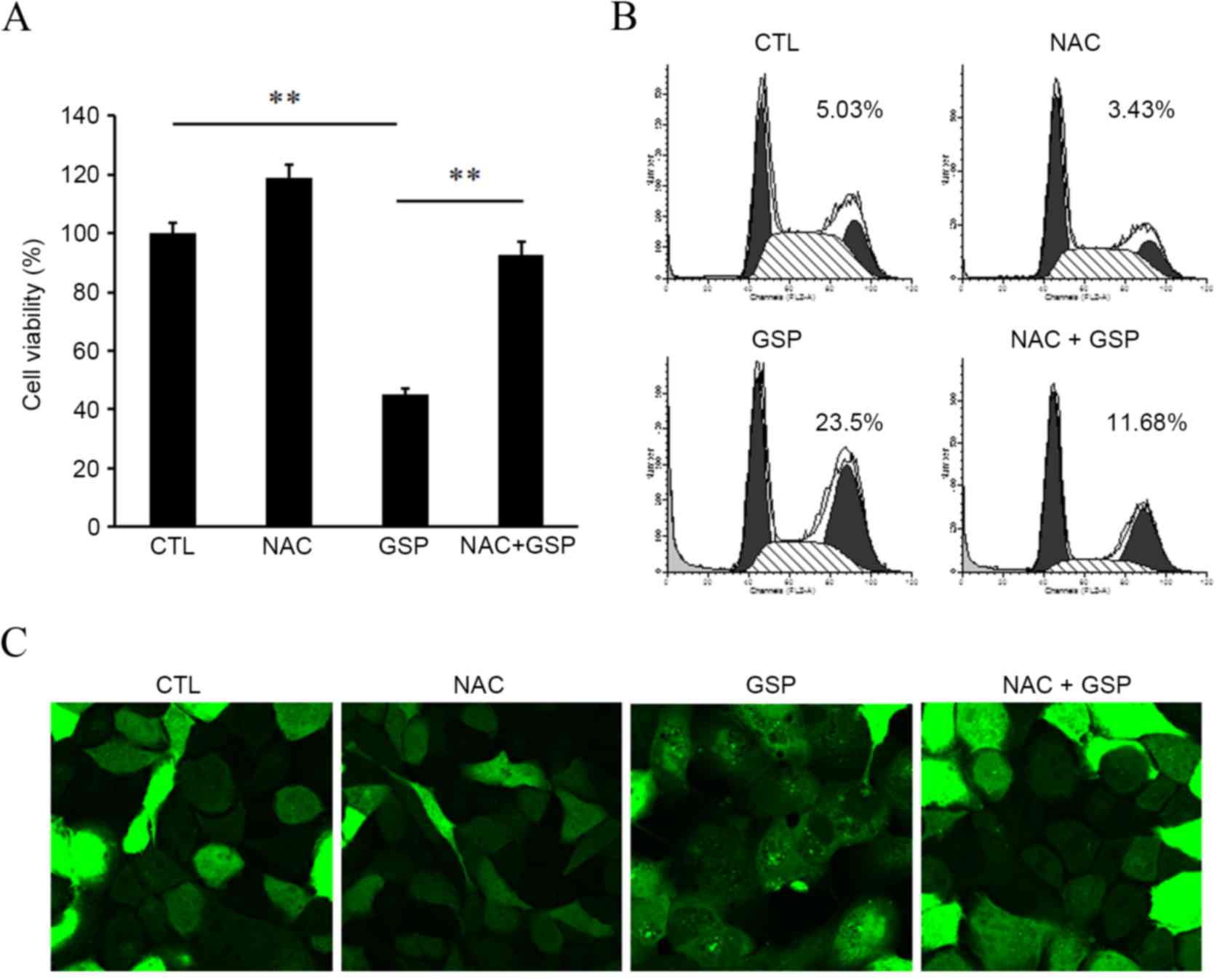 | Figure 7.Scavenging of ROS inhibits apoptosis
and autophagy. (A) SCC12 cells were pretreated with 10 mM NAC,
followed by co-treatment with 100 µg/ml GSP for an additional 24 h.
Cell viability was determined by Cell Counting kit-8 assays. Data
are presented as the mean ± standard deviation of at least three
independent experiments. **P<0.01. (B) SCC12 cells were treated
as in (A) and sub-G1 GSP-treated cells were determined by flow
cytometry. Original FACS plots are presented and the mean values of
three independent experiments are shown. (C) Ad-GFP-LC3-infected
SCC12 cells were pre-incubated with 10 mM NAC for 1 h prior to 6 h
incubation with 100 µg/ml GSP. Cells were fixed and examined under
a confocal microscope (magnification, ×200). ROS, reactive oxygen
species; NAC, N-acetyl cysteine; GSP, grape seed procyanidins;
FACS, fluorescence-activated cell sorting; Ad, adenovirus; GFP,
green fluorescent protein; LC3, microtubule-associated protein 1
light chain 3; CTL, control. |
Discussion
The present study aimed to investigate the effects
of GSP on SCC12 cells, and various responses were detected. The
proliferation of SCC12 cells was systemically inhibited by GSP in a
dose-dependent manner. In previous studies, apoptosis was thought
to be the cause of the GSP-mediated inhibition of cancer cell
proliferation (7–10,18). These
anti-proliferative effects may be attributed to alterations in
various intracellular mechanisms, including suppression of cellular
proliferation, growth arrest at cell cycle checkpoints and enhanced
apoptosis induction (21). Numerous
cancer cells have been shown to have defects in the cell cycle,
which allows them to proliferate uncontrollably. Conversely, in
normal cells, cell cycle progression is regulated by cell cycle
check points (22). The majority of
the chemopreventive agents induce either G1/S phase or
G2/M phase arrest, which prevents uncontrolled cell
division (23,24). In the present study, the cell growth
inhibition induced by GSP was associated with a moderate
accumulation in the G2/M phase of the cell cycle, with a
corresponding decrease in the percentage of G1 phase
cells. Additionally, a noticeable sub-G1 apoptotic
population was evident in the histogram of SCC12 cells treated with
various concentrations of GSP, and the size of this population
increased in a dose-dependent manner.
To the best of our knowledge, the present study is
the first to report a novel function of GSP: The induction of
autophagy, as shown by activation of the autophagosomal marker LC3.
Autophagy may have proapoptotic or antiapoptotic functions
depending on the cell type and stimulus (25). The present study provides evidence to
show the induction of autophagic and apoptotic machineries in SCC12
cells upon treatment with GSP, suggesting that GSP induces cell
death by apoptosis and autophagy. These results were not
unexpected, as numerous natural products are known to possess
antioxidant and anticancer properties (26–28). In a
previous study, GSP inhibited oxidative stress-induced apoptosis by
elevating the cellular antioxidant capacity (29). Conversely, other studies reported that
GSP exerted antiproliferative activity by inducing the apoptosis of
cancer cells (7–10,18).
Despite the antioxidant action of GSP, the present study
demonstrated that GSP exhibited pro-oxidant activity resulting in
selective cell death of SCC12 cells.
In certain situations, apoptosis and autophagy can
occur simultaneously in cells, such that their regulation can be
coordinated and the same proteins are involved in both processes.
One possible mechanism for simultaneous induction of both apoptosis
and autophagy is the stimulation of ROS production. ROS have been
shown to regulate apoptosis and autophagy (30,31). The
results of the present study suggested that GSP-induced ROS
generation caused apoptosis in SCC12 cells, as confirmed by the
progression of GSP-treated cells through the sub-G1 phase when also
pretreated with NAC. However, pre-treatment of SCC12 cells with
3-MA, an autophagy inhibitor, increased the cell viability,
suggesting the presence of an additional mechanism for cell death.
The present study hypothesized that GSP-generated ROS also
regulated autophagy. Accumulation of LC3-II and GFP-LC3 puncta in
SCC12 cells treated with GSP was also observed. However, this
autophagic process was diminished by NAC pretreatment of SCC12
cells. Autophagy is a well-established process that controls cell
survival and death (11). Autophagy
generally stimulates cell survival by sequestering and removing
damaged proteins or organelles from cells under conditions of
physiological stress (16). The
degradation of proteins and/or organelles by autophagy provides
essential nutrients necessary for cell survival under certain
extreme stress conditions such as starvation or hypoxia. However,
cell death may be triggered by the persistent autophagic
degradation of proteins or organelles (16).
In conclusion, GSP inhibited the growth and invasion
of SCC12 human cells, and induced ROS-mediated apoptotic and
autophagic cell death. These findings provide insights into the
association between apoptosis and autophagy induced by GSP, and
suggest that regulation of ROS generation and autophagy may be a
potential treatment option for SCC.
Acknowledgements
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
funded by the Ministry of Education (grant no.
NRF-2014R1A2A2A01005483).
References
|
1
|
Hassan HM: Protective effects of red grape
seed extracts on DNA, brain and erythrocytes against oxidative
damage. Glob J Pharmacol. 7:241–248. 2013.
|
|
2
|
Fine AM: Oligomeric proanthocyanidin
complexes: History, structure, and phytopharmaceutical
applications. Altern Med Rev. 5:144–151. 2000.PubMed/NCBI
|
|
3
|
Ariga T: The antioxidative function,
preventive action on disease and utilization of proanthocyanidins.
Biofactors. 21:197–201. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jang JK and Han JY: The antioxidant
ability of grape seed extracts. Korean J Food Sci Technol.
34:524–528. 2002.
|
|
5
|
Pataki T, Bak I, Kovacs P, Bagchi D, Das
DK and Tosaki A: Grape seed proanthocyanidins improved cardiac
recovery during reperfusion after ischemia in isolated rat hearts.
Am J Clin Nutr. 75:894–899. 2002.PubMed/NCBI
|
|
6
|
Li WG, Zhang XY, Wu YJ and Tian X:
Anti-inflammatory effect and mechanism of proanthocyanidins from
grape seeds. Acta Pharmacol Sin. 22:1117–1120. 2001.PubMed/NCBI
|
|
7
|
Eng ET, Ye J, Williams D, Phung S, Moore
RE, Young MK, Gruntmanis U, Braunstein G and Chen S: Suppression of
estrogen biosynthesis by procyanidin dimers in red wine and grape
seeds. Cancer Res. 63:8516–8522. 2003.PubMed/NCBI
|
|
8
|
Tyagi A, Agarwal R and Agarwal C: Grape
seed extract inhibits EGF-induced and constitutively active
mitogenic signaling but activates JNK in human prostate carcinoma
DU145 cells: Possible role in antiproliferation and apoptosis.
Oncogene. 22:1302–1316. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meeran SM and Katiyar SK: Grape seed
proanthocyanidins promote apoptosis in human epidermoid carcinoma
A431 cells through alterations in Cdki-Cdk-cyclin cascade, and
caspase-3 activation via loss of mitochondrial membrane potential.
Exp Dermatol. 16:405–415. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaur M, Singh RP, Gu M, Agarwal R and
Agarwal C: Grape seed extract inhibits in vitro and in vivo growth
of human colorectal carcinoma cells. Clin Cancer Res. 12:6194–6202.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baehrecke EH: Autophagy: Dual roles in
life and death? Nat Rev Mol Cell Biol. 6:505–510. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shintani T and Klionsky DJ: Autophagy in
health and disease: A double-edged sword. Science. 306:990–995.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kroemer G, Galluzzi L, Vandenabeele P,
Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS,
Golstein P, Green DR, et al: Classification of cell death:
Recommendations of the nomenclature committee on cell death 2009.
Cell Death Differ. 16:3–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kuma A, Hatano M, Matsui M, Yamamoto A,
Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T and Mizushima N: The
role of autophagy during the early neonatal starvation period.
Nature. 432:1032–1036. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mills KR, Reginato M, Debnath J, Queenan B
and Brugge JS: Tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL) is required for induction of autophagy during lumen
formation in vitro. Proc Natl Acad Sci USA. 101:pp. 3438–3443.
2004; View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lemasters JJ, Qian T, He L, Kim JS, Elmore
SP, Cascio WE and Brenner DA: Role of mitochondrial inner membrane
permeabilization in necrotic cell death, apoptosis, and autophagy.
Antioxid Redox Signal. 4:769–781. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Keston AS and Brandt R: The fluorometric
analysis of ultramicro quantities of hydrogen peroxide. Anal
Biochem. 11:1–5. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mantena SK, Baliga MS and Katiyar SK:
Grape seed proanthocyanidins induce apoptosis and inhibit
metastasis of highly metastatic breast carcinoma cells.
Carcinogenesis. 27:1682–1691. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kabeya Y, Mizushima N, Ueno T, Yamamoto A,
Kirisako T, Noda T, Kominami E, Ohsumi Y and Yoshimori T: LC3, a
mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mizushima N, Yoshimori T and Levine B:
Methods in mammalian autophagy research. Cell. 140:313–326. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schwartz GK and Shah MA: Targeting the
cell cycle: A new approach to cancer therapy. J Clin Oncol.
23:9408–9421. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lakin ND and Jackson SP: Regulation of p53
in response to DNA damage. Oncogene. 18:7644–7655. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi HJ, Lim DY and Park JH: Induction of
G1 and G2/M cell cycle arrests by the dietary compound
3,3′-diindolylmethane in HT-29 human colon cancer cells. BMC
Gastroenterol. 9:392009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fulda S and Debatin KM: Sensitization for
anticancer drug-induced apoptosis by the chemopreventive agent
resveratrol. Oncogene. 23:6702–6711. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cao Y and Klionsky DJ: Physiological
functions of Atg6/Beclin 1: A unique autophagy-related protein.
Cell Res. 17:839–849. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chang KH, Yan MD, Yao CJ, Lin PC and Lai
GM: Honokiol-induced apoptosis and autophagy in glioblastoma
multiforme cells. Oncol Lett. 6:1435–1438. 2013.PubMed/NCBI
|
|
27
|
Liu B, Cheng Y, Zhang B, Bian HJ and Bao
JK: Polygonatum cyrtonema lectin induces apoptosis and autophagy in
human melanoma A375 cells through a mitochondria-mediated
ROS-p38-p53 pathway. Cancer Lett. 275:54–60. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ooi KL, Muhammad TS and Sulaiman SF:
Growth arrest and induction of apoptotic and non-apoptotic
programmed cell death by, Physalis minima L. chloroform extract in
human ovarian carcinoma Caov-3 cells. J Ethnopharmacol. 128:92–99.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sharma SD, Meeran SM and Katiyar SK:
Dietary grape seed proanthocyanidins inhibit UVB-induced oxidative
stress and activation of mitogen-activated protein kinases and
nuclear factor-kappaB signaling in in vivo SKH-1 hairless mice. Mol
Cancer Ther. 6:995–1005. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Scherz-Shouval R, Shvets E, Fass E, Shorer
H, Gil L and Elazar Z: Reactive oxygen species are essential for
autophagy and specifically regulate the activity of Atg4. EMBO J.
26:1749–1760. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gao M, Yeh PY, Lu YS, Hsu CH, Chen KF, Lee
WC, Feng WC, Chen CS, Kuo ML and Cheng AL: OSU-03012, a novel
celecoxib derivative, induces reactive oxygen species-related
autophagy in hepatocellular carcinoma. Cancer Res. 68:9348–9357.
2008. View Article : Google Scholar : PubMed/NCBI
|