Introduction
Ovarian cancer is regarded as the most lethal
gynecologic malignancy and a serious threat to womens health
worldwide (1). The treatment for
ovarian cancer patients at an early stage (stages I or II) is
effective, with 5-year survival of up to 90%. However, cases
diagnosed at an end-stage are approximately 70% (stages III or IV)
with survive for 5 years of ≤30% (2).
The lacks of effective screening strategies for early detection
contribute to the delay in diagnosis and the high mortality rate of
ovarian carcinogenesis. Thus, deeper research and better
understanding of the mechanisms of ovarian cancer will facilitate
the identification of reliable diagnostic markers and the
development of effective treatment.
Tripartite motif-containing (TRIM)11 is a kind of
TRIM protein. TRIM proteins have a tripartite motif consisting of
gRING-finger, B-Box and coiled-coil domains (3). TRIM11 is identified as an E3 ubiquitin
ligase in humans (4), PHOX2B
(paired-like homeobox 2b) (5) and
PAX6 (paired-box gene 6) (6), thus
participate in the development of the nervous system. Moreover,
TRIM11 interacts with and destabilizes ARC105, which suppresses
ARC105-mediated transcriptional activation (7). Recently, it has been reported that
TRIM11 is upregulated and play an oncogenic function in glioma
(8), lung cancer (9) and colon cancer (10). Several members of TRIM proteins,
including TRIM25 (11), TRIM27
(12) and TRIM29 (13), were elevated in ovarian cancer, while
TRIM16 expression (14) decreased in
ovarian cancer. However, the expression, and functions of TRIM11 in
ovarian cancer have been insufficiently characterized.
The present study concluded that the level of TRIM11
expression is higher in ovarian cancer tissues than in matched
adjacent non-cancerous tissues. We then investigated the biological
functions of TRIM11 by knocking down its expression in ovarian
cancer cell lines. The TRIM11 knockdown significantly suppressed
proliferation and inhibited invasion of cells, and induced
apoptosis. Additionally, depletion of TRIM11 inhibited the
activities of AKT and ERK. TRIM11 is a potent oncogene in ovarian
cancer.
Patients and methods
Specimen collection and cell
culture
A total of 40 patients (median age, 53 years; range,
30–68 years) with ovarian cancer who underwent surgical resection
at Clinical Laboratory, the Womens Hospital, Zhejiang University
School of Medicine were registered in this project. Ovarian cancer
tissues and matched non-cancerous tissues were obtained from the
above 40 patients after written informed consent was obtained.
Tissue samples were stored in liquid nitrogen at −80°C before used.
This study was reviewed and supported by the Ethics Committee of
the Womens Hospital, Zhejiang University School of Medicine.
The human ovarian A2780 and SK-OV-3 cell lines were
provided by the Cell Bank of the Shanghai Institute for Biological
Sciences, Chinese Academy of Sciences (Shanghai, China) and
cultured in Dulbeccos modified Eagles medium (DMEM) supplemented
with 10% fetal bovine serum (FBS) (both from Gibco, Los Angeles,
CA, USA) and 1% penicillin/streptomycin 2 mM L-glutamine. Both cell
lines were maintained at 37°C in a 5% CO2 incubator.
RNA extraction and real-time PCR
Total RNA was isolated from tissue samples or cell
lines using TRIzol (Invitrogen, Carlsbad, CA, USA) per the
manufacturers instructions. After removing the residual DNA with
DNase I (Roche Diagnostics, Indianapolis, IN, USA), cDNA was
synthesized by reverse transcription of 2 µg of total RNA by using
M-MLV Reverse Transcriptase kit (Thermo Fisher Scientific, Inc.,
Rockford, IL, USA). Real-time PCR was conducted in triplicate on an
ABI 7300 machine (Applied Biosystems, Foster City, CA, USA) with
SYBR® Green PCR Master mix (Thermo Fisher Scientific, Inc.). GAPDH
served as an internal control. The sequences of PCR primers are as
follows: TRIM11, 5-CACC TAAGCTGCACAGTTCC-3 and 5-GGCTGCCTCCTAAT
TCTTCC-3; GAPDH, 5-CACCCACTCCTCCACCTTTG-3 and
5-CCACCACCCTGTTGCTGTAG-3.
Western blot analysis
Using RIPA lysis buffer, protein can be extracted
from cell lines (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA and
0.5% Nonidet P-40) with fresh-added protease inhibitor cocktail
(Roche Diagnostics). Protein concentrations depend on Bradford
assay. Then equal number of proteins was separated for each sample
by SDS-PAGE and blotted onto nitrocellulose filter membranes (EMD
Millipore, Bedford, MA, USA). The membranes were incubated in 5%
skim milk for 30 min, at room temperature, after that, with primary
antibodies at 4°C overnight. Rabbit polyclonal TRIM11 antibody
(dilution, 1:500; cat. no. ab111694), rabbit polyclonal MMP-9
antibody (dilution, 1:500; cat. no. ab73734); rabbit polyclonal
MMP-2 antibody (dilution, 1:500; cat. no. ab37150); rabbit
monoclonal Bcl-2 antibody (dilution, 1:500; cat. no. ab32124);
rabbit monoclonal Bax antibody (dilution, 1:500; cat. no. ab32503);
rabbit polyclonal Akt antibody (dilution, 1:500; cat. no. ab8805)
and rabbit monoclonal pAkt antibody (dilution, 1:500; cat. no.
ab81283); rabbit polyclonal ERK antibody (dilution, 1:500; cat. no.
ab196883) and rabbit polyclonal p-ERK antibody (dilution, 1:500;
cat. no. ab192591) and rabbit polyclonal GAPDH antiboody (dilution,
1:500; cat. no. ab37168) were all purchased from Abcam (Cambridge,
MA, USA). Subsequently, the membranes were applied with secondary
goat anti-rabbit (HRP) IgG antibody (dilution, 1:2,000; cat. no.
ab6721; Abcam) and detected with the enhanced chemiluminescence
system (Bio-Rad, Richmond, CA, USA). GAPDH was used as loading
control. Band intensities were assessed on ImageJ software version
1.6 (http://rsb.info.nih.gov/ij/; National
Institutes of Health, Bethesda, MD, USA).
RNA interference
TRIM11 small interfering RNA (siRNA) (siTRIM11,
5-CUAUUCAUCUUUCCCGAGA-3) and negative control siRNA (siNC) were
from GenePharma (Shanghai, China). A2780 and SK-OV-3 cells were
transfected with siRNA or siNC by using Lipofectamine 2000
(Invitrogen) following the manufacturers instructions. At 48 h
post-transfection, by real-time PCR and western blot analysis, the
knockdown efficiency was assessed.
Cell proliferation assay
The evaluation of cell proliferation was made by
using Cell Counting Kit-8 (CCK-8; Beyotime, Haimen, China)
following the manufacturers instructions. A2780 and SK-OV-3 cells
were seeded onto 96-well plates (2,000 cells/well) and transfected
with siTRIM11 or siNC. After incubation for 0, 24, 48 and 72 h,
CCK-8 reagent was added and incubated at 37°C for 1 h. At 450 nm,
the absorbance was measured on a microplate reader (Perlong,
Beijing, China) with a reference wave length at 655 nm.
Cell apoptosis analysis
The proportion of apoptotic cells were determined by
using Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide
(PI) apoptosis kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing,
China) following the manufacturers instructions. Cells cultured in
6-well plates were transfected with siTRIM11 or siNC. After
incubating for 48 h, cells were harvested, labeled with Annexin
V-FITC and PI, and analyzed using a FACScan flow cytometry (BD
Biosciences, San Jose, CA, USA) within 1 h.
Transwell invasion assays
The Transwell assay of cell invasion was with
8-µm-pore filters pre-coated with Matrigel (Corning, New York, NY,
USA). Cells were treated with siTRIM11 or siNC. At 24 h after
treatment, cells were collected and plated in the upper chamber
(5×104 cells/well) with DMEM medium. The lower chamber
contained DMEM supplemented with 10% FBS. At 22 h, the
non-migrating cells were removed, and the filters fixed in 10%
formalin, stained by 0.5% crystal violet. Using an inverted
microscope, cells in 5-random fields were counted.
Statistical analysis
Statistical analysis were carried out by GraphPad
Prism 6 (GraphPad Software, San Diego, CA, USA). All values are
presented as the mean ± standard deviation. One-way analysis of
variance was used to test the statistical differences. A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
Upregulation of TRIM11 in ovarian
cancer tissues
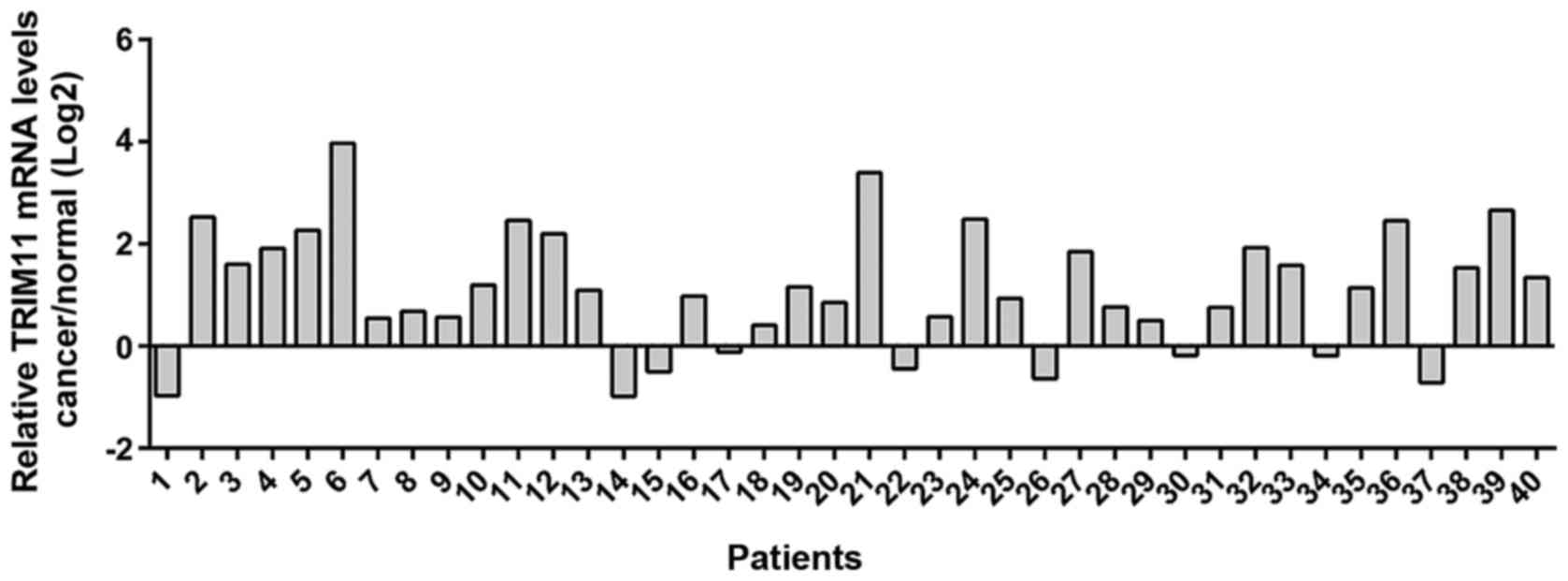
Real-time PCR analysis was performed in 40 pairs of
ovarian cancer and adjacent non-tumorous tissues and the log2
tumor/normal ratio of each cancer specimen was calculated. As shown
in Fig. 1, overexpression of TRIM11
was found in 77.5% (31/40) of the tested ovarian cancer
tissues.
Knockdown of TRIM11 expression by
siRNA transfection
To investigate the biological functions of TRIM11 in
ovarian cancer progression, siRNA transfection was used to knock
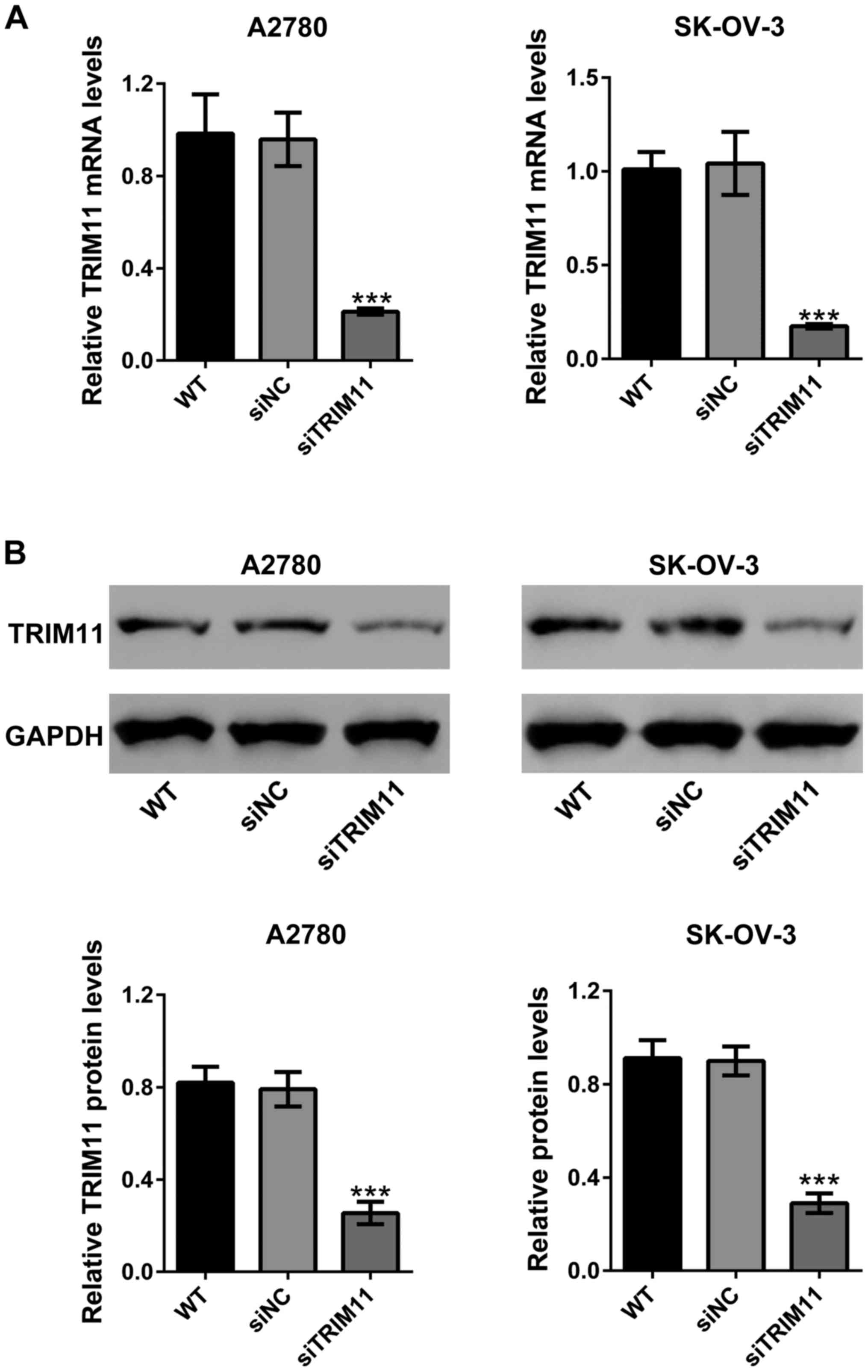
down TRIM11 expression in A2780 and SK-OV-3 cells. As shown in
Fig. 2A, siNC did not change the mRNA
expression of TRIM11 compared to wild-type cells. TRIM11 siRNA
(siTRIM11) significantly reduced TRIM11 mRNA expression compared to
cells transfected with siNC in both cell lines. Western blot
analysis revealed that siTRIM11 efficiently inhibited the protein
expression of TRIM11 (Fig. 2B).
Downregulation of TRIM11 expression
inhibits ovarian cancer cell proliferation
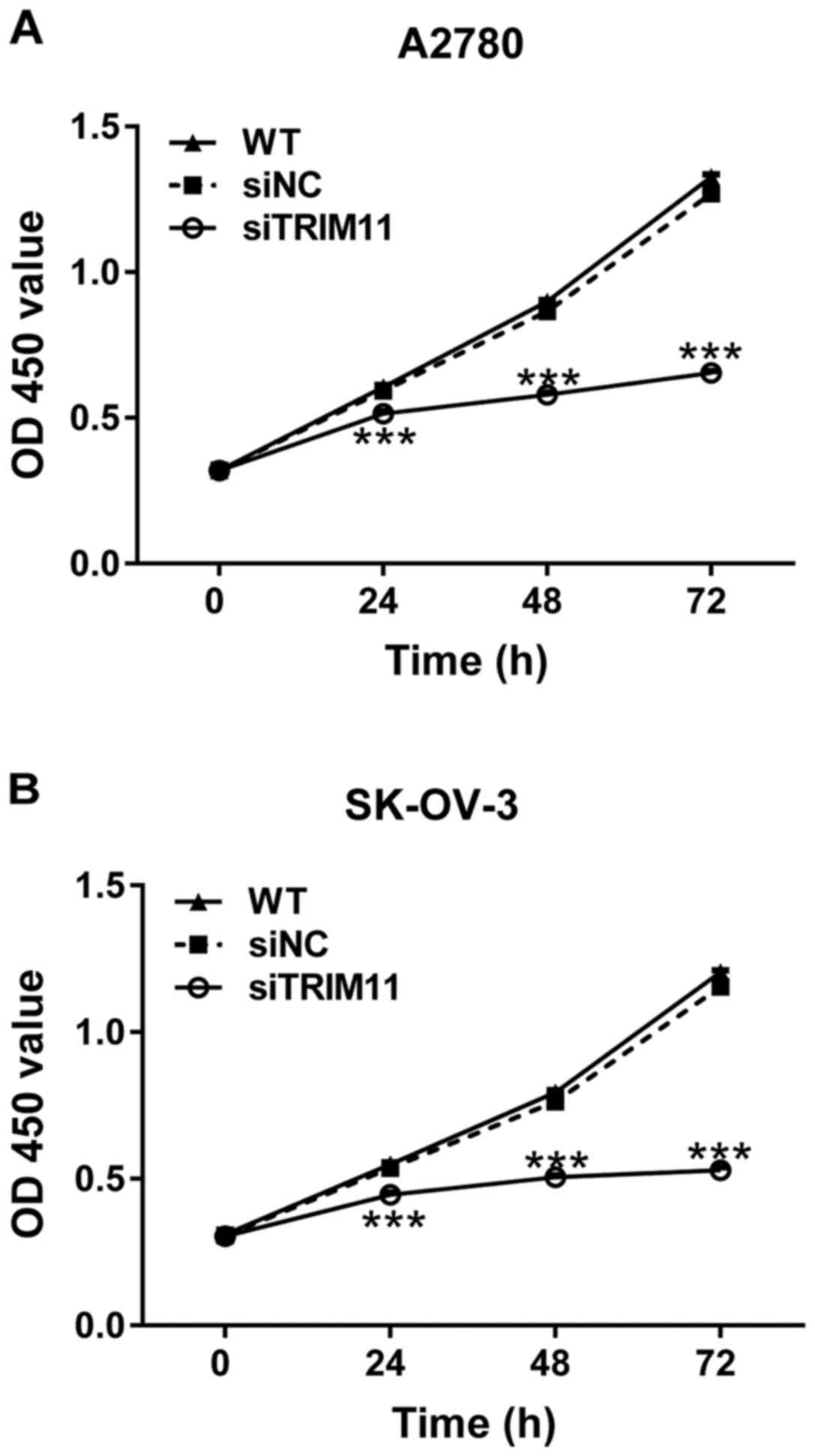
To explore whether TRIM11 plays a role in the growth
of ovarian cancer cells, CCK-8 assay was performed after siRNA
transfection. The proliferation of A2780 and SK-OV-3 cells was
remarkably repressed at 24, 48 and 72 h after siTRIM11 transfection
(Fig. 3). These results indicated the
inhibitory role of TRIM11-siRNA in the proliferation of ovarian
cancer cells.
Knockdown of TRIM11 induces ovarian
cancer cell apoptosis
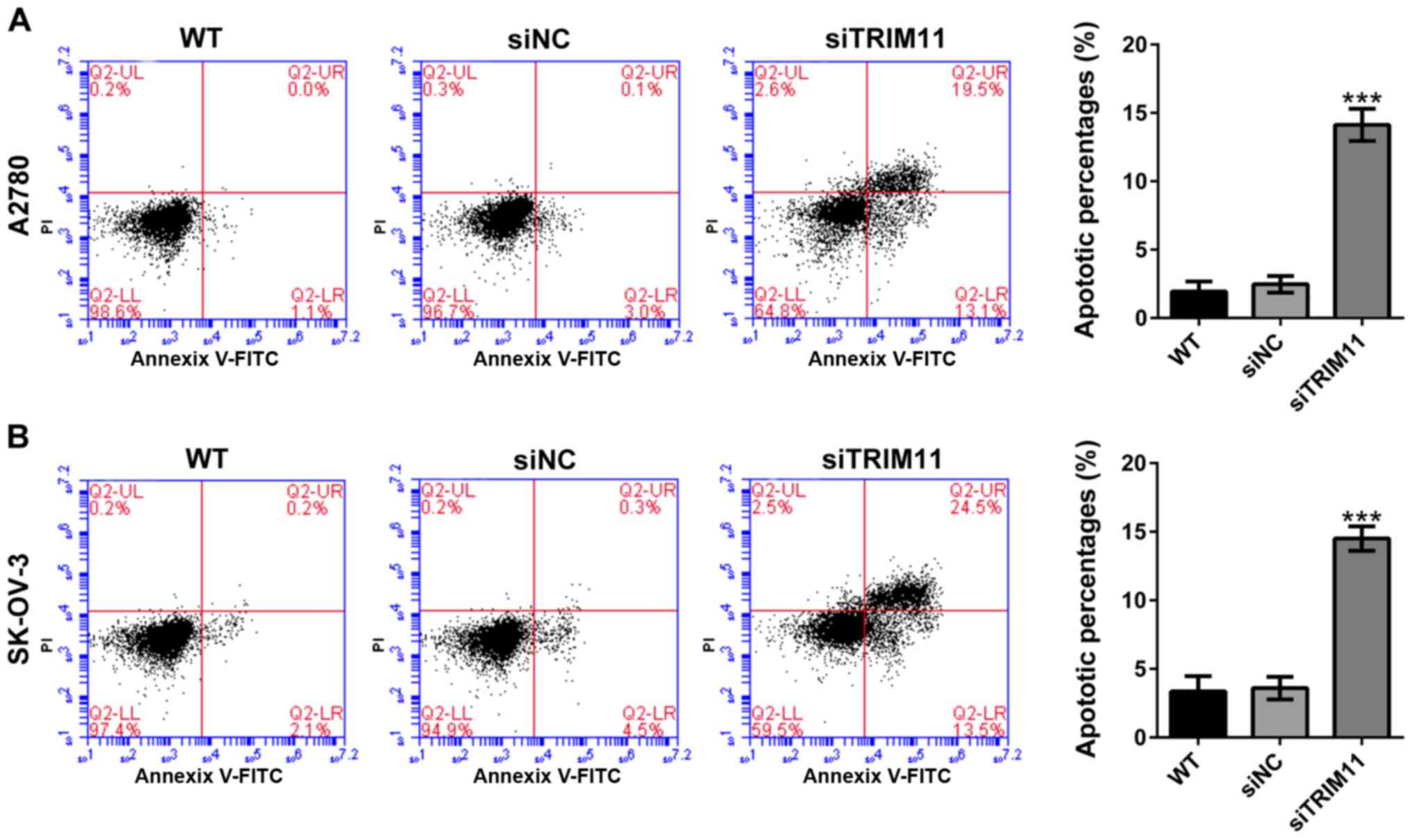
Determining whether TRIM11 affects apoptosis of
ovarian cancer cells, percentages of apoptotic cells were assessed
in TRIM11 knockdown cells by Annexin V-FITC/PI staining assay. From
flow cytometry analysis, it can be seen that compared with
corresponding control cells (siNC), knockdown of TRIM11 in A2780
cells (Fig. 4A) markedly reduced cell
apoptosis. Similar results were received in SK-OV-3 cells (Fig. 4B).
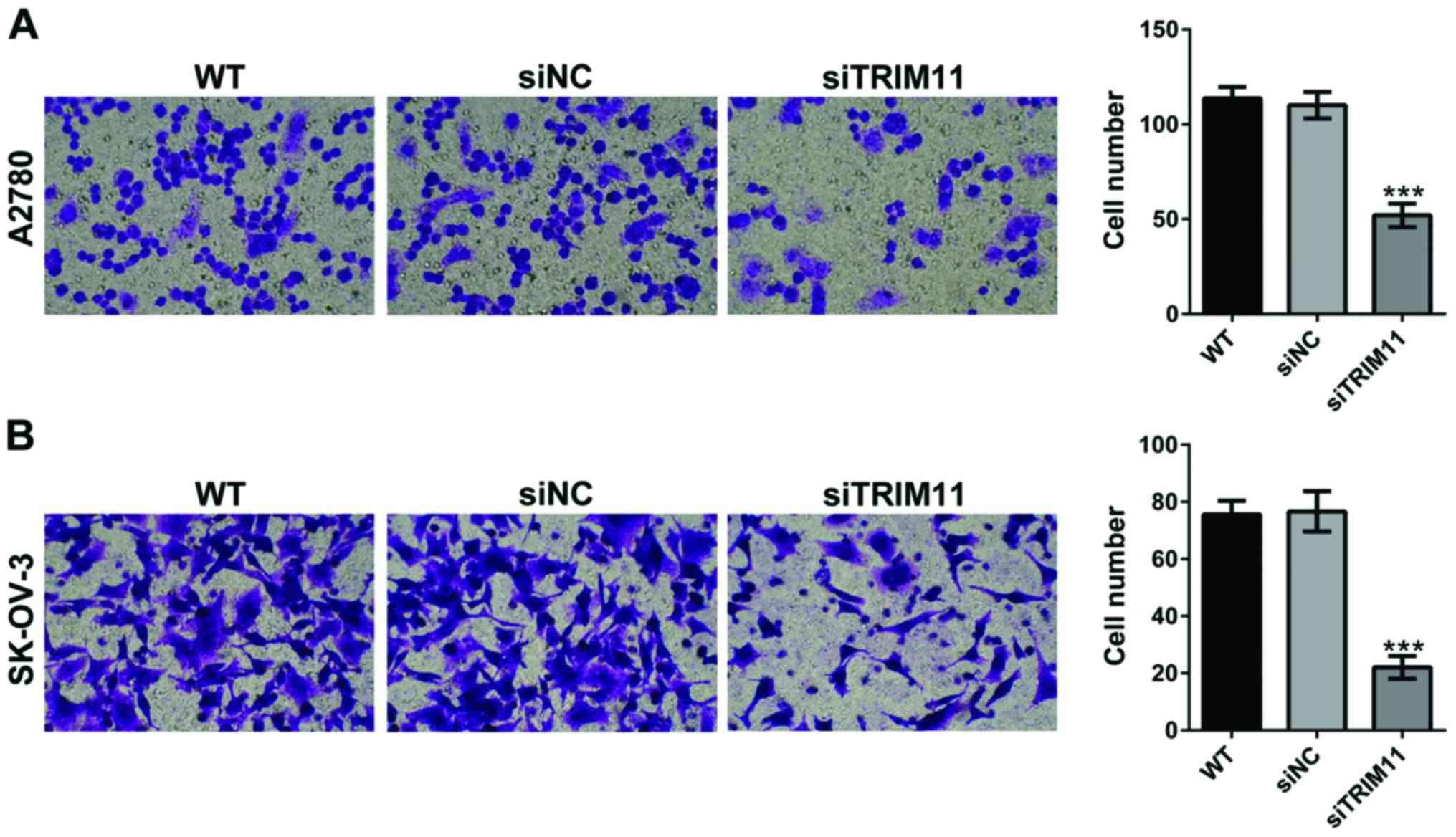
Depletion of TRIM11 inhibits ovarian
cancer cell invasion
Using a Matrigel-coated Transwell, we evaluated the
changes in ovarian cancer cell invasion after TRIM11 siRNA
transfection. As shown in Fig. 5,
depletion of TRIM11 in A2780 and SK-OV-3 cells led to markedly
reduced cell apoptosis of invasion. These data revealed that
TRIM11-siRNA play an inhibitory role on ovarian cancer cell
invasion.
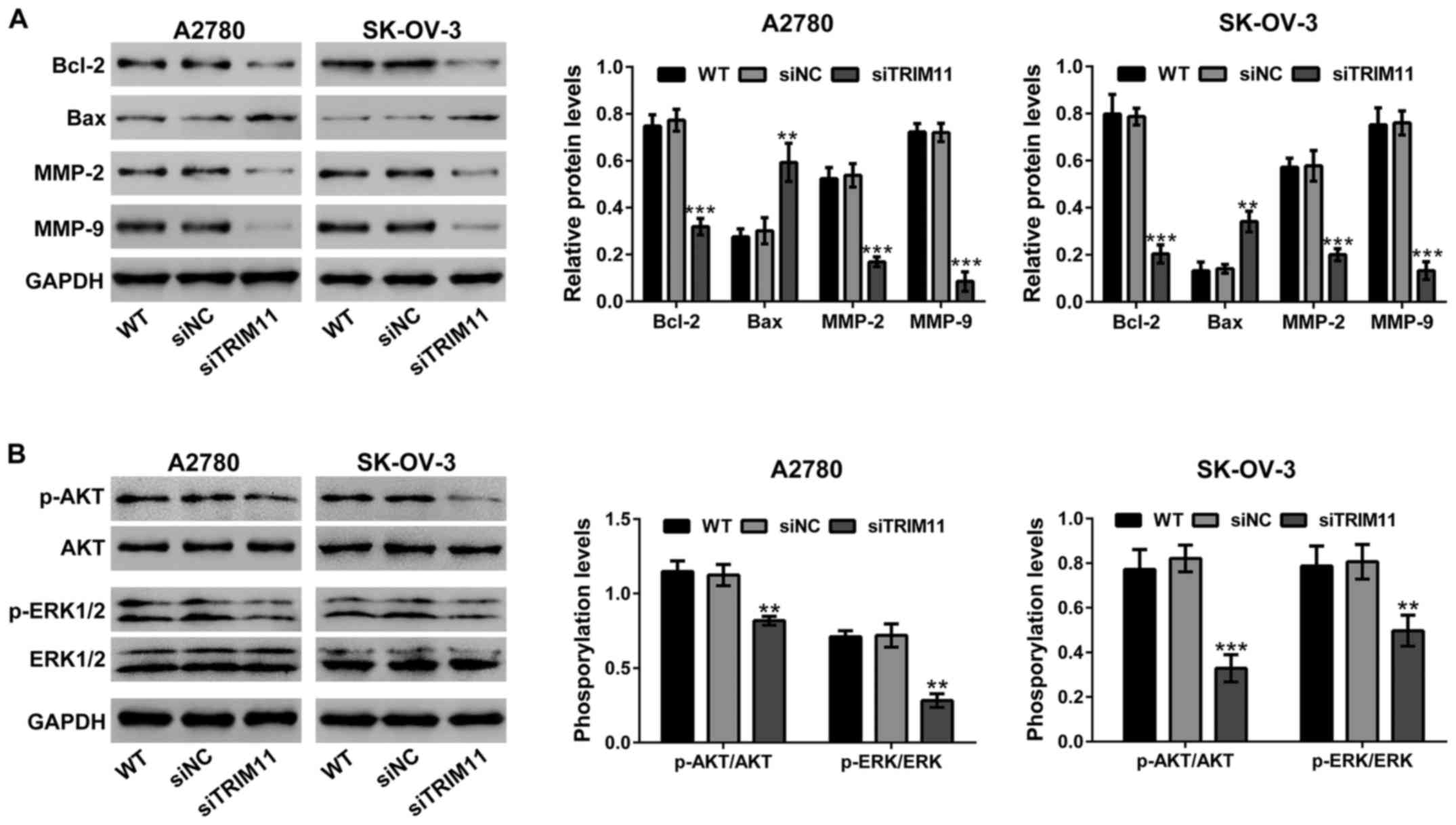
Effects of TRIM11 knockdown on the
expression of apoptosis and invasion related proteins
The protein levels of cell apoptosis and invasion
related proteins by western blot analysis (Fig. 6A) were evaluated. The levels of
protein of Bcl-2, MMP-2 and MMP-9 were markedly suppressed in A2780
and SK-OV-3 cells with TRIM11 knockdown, but Bax was remarkably
increased in TRIM11 knockdown cells.
Knockdown of TRIM11 suppresses the
activity of AKT and ERK in ovarian cancer cells
The effects of TRIM11 on AKT and ERK signaling have
been studied in glioma cells (8) and
lung cancer cells (9). Fig. 6B, shows that transfection of TRIM11
siRNA in ovarian cancer cells obviously suppressed the
phosphorylation of AKT and ERK, but does not show any effects on
the levels of AKT and ERK expression.
Discussion
Dysregulated expression of several members of TRIM
proteins has been found in ovarian cancer tissues (11–14). The
diagnostic and prognostic values of TRIM11 have been reported in
gliomas (8), lung cancer (9) and colon cancer (10). Here, we reported that TRIM11 mRNA was
efficient in ovarian cancer tissues (Fig.
1). Next, we studied the functions of TRIM11 in ovarian cancer
by knockdown its expression with specific siRNA. Reduced expression
of TRIM11 significantly suppressed proliferation of cells (Fig. 3) and invasion (Fig. 5), and induced apoptosis (Fig. 4). These data were consistent with
previous investigation in other types of human cancer cells
(8–10). The present study together with the
previous findings suggested the important role of TRIM11 in cancer
biology.
Members of Bcl-2 family are critical mediators for
cell apoptosis promoting cell survival (e.g., Bcl-2 and Bcl-xL) and
initiation of apoptosis (e.g., Bax and Bak) (15). Members of MMP family degrade
extracellular matrix and cell surface proteins, thus playing an
important role in cancer cell invasion (16). High levels of MMP-2 and MMP-9 predict
poor survival in patients at advanced-stage of ovarian cancer
(17). The activation of AKT and ERK
pathways is involved in the regulation of Bcl-2 family (18,19) and
MMP family proteins (20,21). Selective targeting of AKT or ERK
pathways may block cancer progression (22–24). The
effects of TRIM11 on AKT and ERK signaling have been studied in
gliomas (8) and lung cancer cells
(9). In the present study, we showed
that knockdown of TRIM11 reduced phosphorylated levels of AKT and
ERK, and the protein levels of Bcl-2, MMP-2 and MMP-9, whereas
increased the protein levels of Bax in ovarian cancer cells
(Fig. 6), suggesting that in ovarian
cancer cells, targeting TRIM11 could be a successful anticancer
strategy.
In conclusion, the expression of TRIM11 in ovarian
cancer tissues was remarkably increased compared to normal adjacent
tissues. The proliferation and invasion of ovarian cancer cells can
be suppressed in ovarian cancer cells by depleting of TRIM11. We
also illustrated that TRIM11 plays a key role and perform the
functions by adjusting the levels of AKT and ERK.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Heintz AP, Odicino F, Maisonneuve P, Quinn
MA, Benedet JL, Creasman WT, Ngan HY, Pecorelli S and Beller U:
Carcinoma of the ovary. FIGO 26th annual report on the results of
treatment in gynecological cancer. Int J Gynaecol Obstet. 95 Suppl
1:161–192. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hatakeyama S: TRIM proteins and cancer.
Nat Rev Cancer. 11:792–804. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Niikura T, Hashimoto Y, Tajima H, Ishizaka
M, Yamagishi Y, Kawasumi M, Nawa M, Terashita K, Aiso S and
Nishimoto I: A tripartite motif protein TRIM11 binds and
destabilizes Humanin, a neuroprotective peptide against Alzheimers
disease-relevant insults. Eur J Neurosci. 17:1150–1158. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hong SJ, Chae H, Lardaro T, Hong S and Kim
KS: Trim11 increases expression of dopamine beta-hydroxylase gene
by interacting with Phox2b. Biochem Biophys Res Commun.
368:650–655. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tuoc TC and Stoykova A: Trim11 modulates
the function of neurogenic transcription factor Pax6 through
ubiquitin-proteosome system. Genes Dev. 22:1972–1986. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishikawa H, Tachikawa H, Miura Y and
Takahashi N: TRIM11 binds to and destabilizes a key component of
the activator-mediated cofactor complex (ARC105) through the
ubiquitin-proteasome system. FEBS Lett. 580:4784–4792. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Di K, Linskey ME and Bota DA: TRIM11 is
overexpressed in high-grade gliomas and promotes proliferation,
invasion, migration and glial tumor growth. Oncogene. 32:5038–5047.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang X, Shi W, Shi H, Lu S, Wang K, Sun C,
He J, Jin W, Lv X, Zou H and Shu Y: TRIM11 overexpression promotes
proliferation, migration and invasion of lung cancer cells. J Exp
Clin Cancer Res. 35:1002016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yin Y, Zhong J, Li S-W, Li JZ, Zhou M,
Chen Y, Sang Y and Liu L: TRIM11, a direct target of miR-24-3p,
promotes cell proliferation and inhibits apoptosis in colon cancer.
Oncotarget. 7:86755–86765. 2016.PubMed/NCBI
|
|
11
|
Sakuma M, Akahira J, Suzuki T, Inoue S,
Ito K, Moriya T, Sasano H, Okamura K and Yaegashi N: Expression of
estrogen-responsive finger protein (Efp) is associated with
advanced disease in human epithelial ovarian cancer. Gynecol Oncol.
99:664–670. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma Y, Wei Z, Bast RC Jr, Wang Z, Li Y, Gao
M, Liu Y and Wang X, Guo C, Zhang L and Wang X: Downregulation of
TRIM27 expression inhibits the proliferation of ovarian cancer
cells in vitro and in vivo. Lab Invest. 96:37–48. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Santin AD, Zhan F, Bellone S, Palmieri M,
Cane S, Bignotti E, Anfossi S, Gokden M, Dunn D, Roman JJ, et al:
Gene expression profiles in primary ovarian serous papillary tumors
and normal ovarian epithelium: identification of candidate
molecular markers for ovarian cancer diagnosis and therapy. Int J
Cancer. 112:14–25. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tan H, Liu Z, Qi J and Chu G: Tripartite
motif 16 inhibits the migration and invasion in ovarian cancer
cells. Oncol Res. 25:551–558. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reed JC: Regulation of apoptosis by bcl-2
family proteins and its role in cancer and chemoresistance. Curr
Opin Oncol. 7:541–546. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leber MF and Efferth T: Molecular
principles of cancer invasion and metastasis (Review). Int J Oncol.
34:881–895. 2009.PubMed/NCBI
|
|
17
|
Davidson B, Goldberg I, Gotlieb WH,
Kopolovic J, Ben-Baruch G, Nesland JM, Berner A, Bryne M and Reich
R: High levels of MMP-2, MMP-9, MT1-MMP and TIMP-2 mRNA correlate
with poor survival in ovarian carcinoma. Clin Exp Metastasis.
17:799–808. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Downward J: PI 3-kinase, Akt and cell
survival. Semin Cell Dev Biol. 15:177–182. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boucher MJ, Morisset J, Vachon PH, Reed
JC, Lainé J and Rivard N: MEK/ERK signaling pathway regulates the
expression of Bcl-2, Bcl-X(L), and Mcl-1 and promotes survival of
human pancreatic cancer cells. J Cell Biochem. 79:355–369. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jung C-H, Kim EM, Park JK, Hwang SG, Moon
SK, Kim WJ and Um HD: Bmal1 suppresses cancer cell invasion by
blocking the phosphoinositide 3-kinase-Akt-MMP-2 signaling pathway.
Oncol Rep. 29:2109–2113. 2013.PubMed/NCBI
|
|
21
|
Adya R, Tan BK, Punn A, Chen J and Randeva
HS: Visfatin induces human endothelial VEGF and MMP-2/9 production
via MAPK and PI3K/Akt signalling pathways: novel insights into
visfatin-induced angiogenesis. Cardiovasc Res. 78:356–365. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morgensztern D and McLeod HL:
PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer
Drugs. 16:797–803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kohno M and Pouyssegur J: Targeting the
ERK signaling pathway in cancer therapy. Ann Med. 38:200–211. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hill MM and Hemmings BA: Inhibition of
protein kinase B/Akt. implications for cancer therapy. Pharmacol
Ther. 93:243–251. 2002. View Article : Google Scholar : PubMed/NCBI
|