Introduction
Cancer is the greatest threat towards human lives
(1). Currently, the focus of
anticancer drug development has migrated from traditional
chemotherapies to molecularly targeted therapies with high
selections and few side effects (2).
In the 1970s, Widder et al (3)
first proposed the concept of a magnetic targeting drug delivery
system and performed experiments investigating drug-bearing
magnetic particles. Due to investigation into potential novel
targeted drug delivery systems, magnetic nanoparticles have been
developed rapidly in cancer-targeting therapies and have become the
research focus and hotspot of anticancer drugs in China and other
countries (4). In recent years,
magnetic nanoparticles have become increasingly widely used in
biomedical studies, including magnetic resonance imaging (MRI)
contrast enhancement, targeting drug delivery, tumor magnetic
thermotherapy and concentration tracing towards specific targeting
points (5). Among numerous control
delivery systems, magnetic nanoparticles exhibited the highest
targeting effectiveness (6).
The principle of magnetic transfection technique,
which combined magnetic targeting technology and RNA interference
(RNAi) technology, was to combine magnetic nanoparticles with
targeted genes by chemical covalent bonds or physical adhesion. The
formed magnetic nanoparticles would be able to accumulate directly
towards the target organs under external magnetic field, thus
serving its roles (7,8). Using this technique, our previous in
vitro experiments confirmed that angiopoietin 2-small
interfering RNA (Ang2-siRNA) chitosan magnetic nanoparticles could
inhibit the expression of Ang2 gene in human malignant
melanoma (MM) cells, and the inhibition efficiency was 59.56%
(9). In the present study, Ang2-siRNA
plasmid/chitosan magnetic nanoparticles were injected into the nude
mouse MM model to observe the targeting characteristic of these
particles under external magnetic field, in order to determine
certain foundations for further in vivo targeting
intervention studies investigating the tumor growth in
MM-transplanted nude mice.
Materials and methods
Preparation of chitosan magnetic
nanoparticles
A total of 0.15 g magnetic Fe3O4 nanoparticles was
dispersed into 20 ml of 1.5% chitosan (relative molecular weight:
1.38×106; deacetylation degree: 90%; Zhejiang Hisun Chemical Co.,
Ltd., Taizhou, China) under ultrasound and agitation. Subsequently,
this was added to 80 ml mixed phase solvent of liquid paraffin and
petroleum ether (volume ratio: 7/5) supplemented with 2 ml Span-80
(emulsifier). The solution was sufficiently emulsified and agitated
at 40°C for 30 min, then 10 ml glutaraldehyde solution (diluted 1
ml 25% glutaraldehyde to 10 ml) was slowly added drop-wise.
Following, the solution was incubated at 40°C in a water bath for
30 min, and then adjusted to pH 9.0 with 1 mol/NaOH solution. The
resulting solution was heated to 60°C. After standing for 1 h, the
precipitate was produced. Following thorough washing with anhydrous
ether, acetone, anhydrous ethanol and distilled water successively,
the chitosan magnetic nanoparticles were obtained.
Combination of Ang2-siRNA plasmid and
chitosan magnetic nanoparticles
A total of 1 mg chitosan magnetic nanoparticles were
added to 1 ml PBS buffer (pH 7.4) and ultrasonically agitated (200
W, 3 min). Subsequently, 2 ml polylysine (diluted with PBS buffer
to a concentration of 0.1 mg/ml) was added, mixed well and
incubated at room temperature for 10 min. The Ang2-siRNA plasmid
was then combined with the polylysine-modified chitosan magnetic
nanoparticles with ratios of 1:1, 1:10, 1:100 and 1:1,000 (quality
ratio), respectively, followed by incubation at room temperature
for 1 h. Routinely vaccinated and cultured MM A-375 cells
(purchased from Type Culture Collection of the Chinese Academy of
Sciences, Shanghai, China) were seeded into the 6-well plate
(1.0×105 cells/well). The Ang2-siRNA plasmid/chitosan magnetic
nanoparticles were added to the wells, followed by incubation at
37°C, 5% CO2 for 48 h. The expression of red fluorescent
protein was observed under DVM6 optical microscope (Leica Science
Lab, Leica Camera AG Berlin, Germany).
Establishment of MM-transplanted nude
mouse model
Routinely vaccinated and cultured MM A-375 cells
were seeded in 10 cm dishes (1.0×106 cells/dish). When the cells in
logarithmic growth phase grew to >90% confluency, 0.25% trypsin
was added for a 3 min digestion period, then Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) was added to terminate digestion. The cells were
transferred into a 1 ml centrifuge tube for 3 min centrifugation at
256 × g and 4°C. Subsequently, the supernatant was discarded and
cell culture medium was added to pipet the cells into tumor cell
suspensions, this was centrifuged (256 × g at 4°C for 3 min) and
the supernatant was discarded. Cells were washed twice with PBS by
centrifugation, then serum-free high glucose DMEM was added to
prepare the cell suspensions. The cells were counted using a BX61
fluorescent microscope (Olympus Corp., Tokyo, Japan) and the cell
concentration was adjusted to 5×107/ml. Subsequently, 100 µl cell
suspension was subcutaneously inoculated using a micro-injector
into the right armpit of nude mice. A total of 15 nude BALB/c male
mice (specific pathogen free, 6 weeks old, 20–25 g) were provided
by Shanghai Wu Animals Center, Shanghai, China (license number,
SCXK (Min) 2012-0001). They were raised in housing conditions
(22–25°C; 55±5% humidity) in a 12 h dark/light cycle with free
access to food and water. The present study was approved by the
Animal Ethics Committee of Fujian Medical University (Fuzhou,
China).
Magnetic targeting positioning
experiment of Ang2-siRNA plasmid vector/chitosan magnetic
nanoparticles in nude mice
Following successful establishment of the nude mouse
model and when the subcutaneous tumors grew to ~6×6 mm in size, the
mice were randomly divided into 3 groups, with 5 mice in each
group. The targeting group was injected with 0.4 ml chitosan
magnetic nanoparticle solution through the tail vein, then 4,000 GS
magnetic field was added close to the right armpit subcutaneously
following anesthesia using 4% chloral hydrate (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany), 60 min later the magnetic field was
removed and the mice were sacrificed. The non-targeting group was
injected with 0.4 ml of plasmid-coupled particles (35.35 mg/kg)
through the tail vein, this was not performed under a magnetic
field. Following 60 min, the mice were sacrificed. The control
group was injected with 0.4 ml saline through the tail vein, this
was performed under a magnetic field; following 60 min, the mice
were sacrificed by cervical dislocation. The tumor tissues were
stripped to prepare paraffin tissue sections, followed by
hematoxylin and eosin (H&E) staining and Prussian blue staining
in order to verify the particle distributions inside the tissues
using a DVM6 optical microscope (Leica Microsystems GmbH, Wetzlar,
Germany).
Results
Determination of suitable quality
ratio of Ang2-siRNA plasmids and chitosan magnetic
nanoparticles
Ang2-siRNA plasmid (Fig.
1) and chitosan magnetic nanoparticles were combined with the
quality ratios 1:1, 1:10, 1:100 and 1:1,000, respectively, then
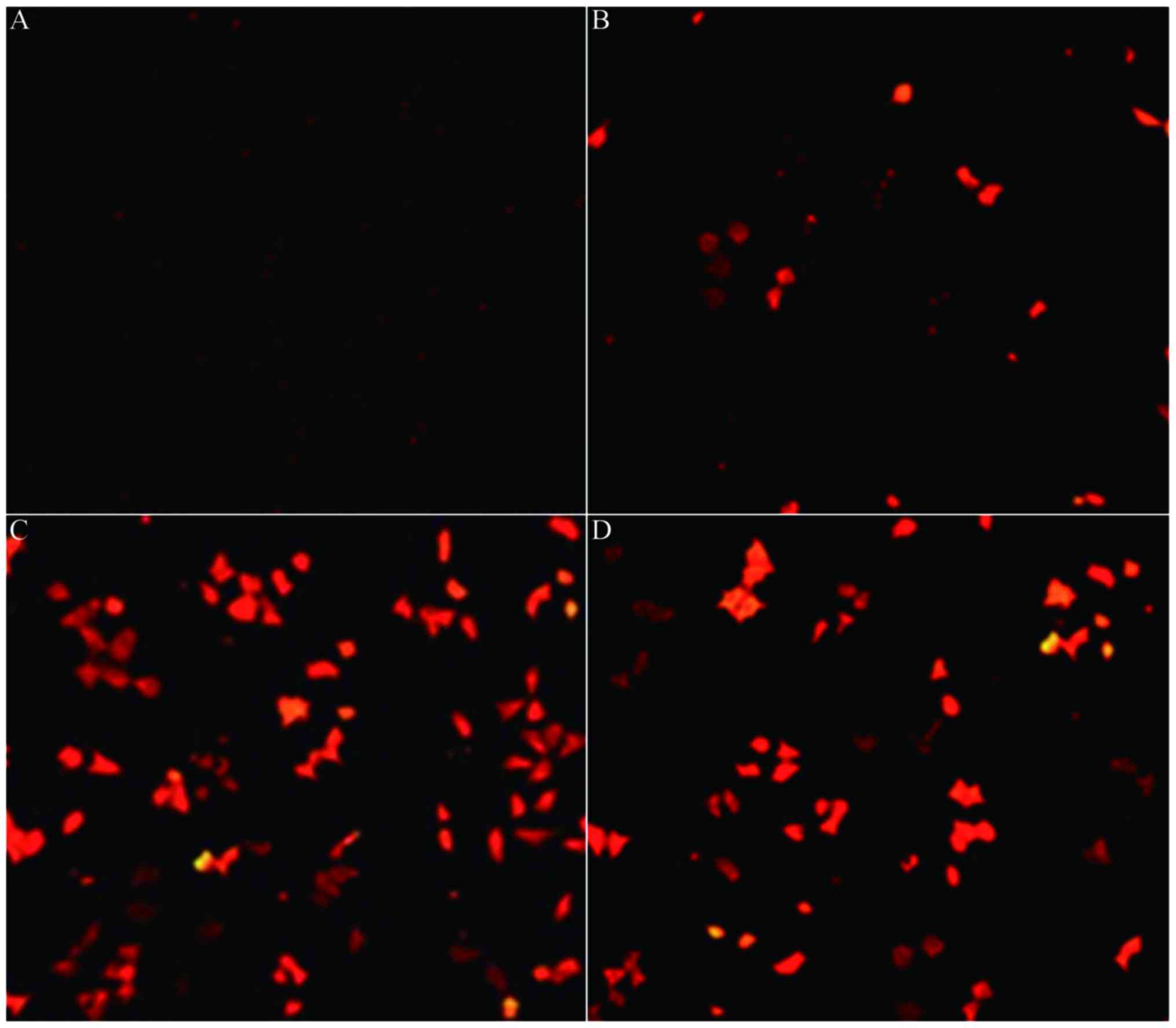
transfected into human MM cells. Fluorescence microscopy of A-375
cells (Fig. 2A-D) demonstrated that
when the quality ratio was 1:100, the red fluorescence emitted was
the strongest (Fig. 2C). The cells in
each group were digested into single cell suspensions for cell
counting (Table I) and the quality
ratio 1:100 was determined to be the appropriate ratio for
subsequent experiments as a result.
 | Table I.Transfection efficiency of
angiopoietin 2-small interfering RNA plasmid/chitosan magnetic
nanoparticles towards human malignant melanoma cells. |
Table I.
Transfection efficiency of
angiopoietin 2-small interfering RNA plasmid/chitosan magnetic
nanoparticles towards human malignant melanoma cells.
| Quality ratio | Total no. of
cellsa | Total no. of
cellsb | Transfection
efficiencyc, % |
|---|
| 1:1 | 0 | 118 | 0.00 |
| 1:10 | 10 | 107 | 9.35 |
| 1:100 | 63 | 103 | 61.17 |
| 1:1,000 | 35 | 84 | 41.67 |
Construction of MM-transplanted nude
mouse model
Fig. 3 revealed that
subsequent to subcutaneous inoculation for 5–7 days, subcutaneous
tiny nodules (~1 mm) were observed and obvious subcutaneous nodules
were observed following 14 days. When the tumor grew to ~6 mm, the
tumor-bearing mice were grouped and the success rate of tumor
formation by subcutaneous injection was 100%.
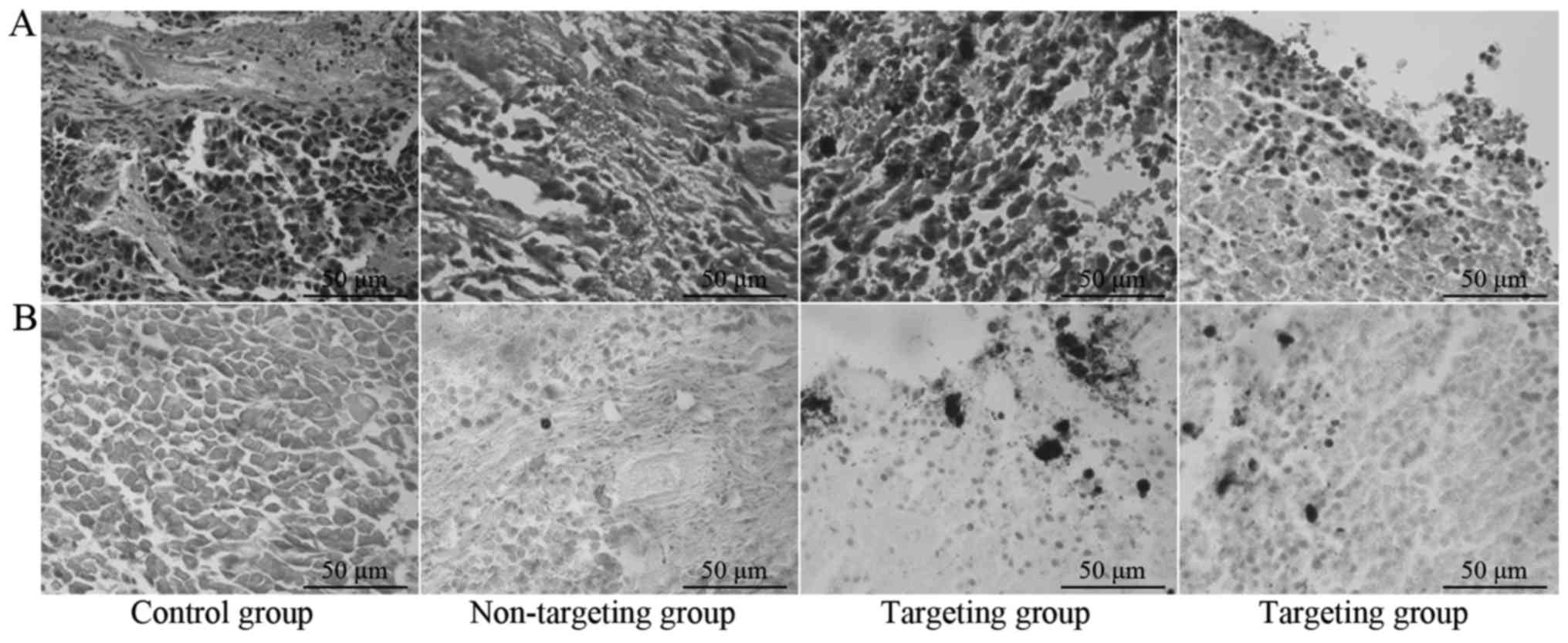
H&E staining and Prussian blue
staining
Fig. 4A and B
demonstrated that there were no particles inside the tumor tissues
of the control group and Prussian blue staining was negative. The
non-targeting group exhibited rare particles inside tumor tissues
occasionally and Prussian blue staining was weakly positive. The
targeting group exhibited aggregation of numerous particles on the
capsule of tumor tissues and inside blood vessels and Prussian blue
staining was strongly positive.
Discussion
Cancer is one of the three diseases that pose a
serious threat to human health (10).
MM is a superficial tumor with high malignancy and difficult
treatment (11). The current
conventional clinical treatments have shortcomings including, low
specificity and numerous side effects (12). Therefore, increasing the specificity
of anticancer drugs is necessary to reduce drug side effects and
improve efficacies (13). The study
of antitumoral drugs has progressed greatly and targeted drug
delivery systems have become a focus in China and other countries
(14). A magnetic targeting drug
delivery system (MTDDS) is a stable system composed of magnetic
substances and drugs with a suitable carrier; the drugs move,
re-position, concentrate and accumulate around lesion tissues when
exposed to external magnetic field with a specific intensity
(15). The high specific
characteristic of targeting drug therapy may significantly reduce
the side effects of treatment (16).
The targeting of MTDDS included active and passive
targeting (17). The active targeting
exhibited higher specificity compared with the passive targeting,
which mainly involved coupling with the ligand or antibody of
targeted cells (18). Otherwise, the
particles with magnetic properties migrated directly to the
targeted tissues and achieved targeting under an external magnetic
field (19). Hsieh et al
(20) established a mouse model with
colon cancer, and injected specific antibodies-containing
Fe3O4 particles into mice. This revealed that
the particles accumulated in the lesions and little was observed in
other tissues (20). Zhou et
al (21) used
liposome-encapsulated adriamycin to prepare adriamycin magnetic
microspheres, which could specifically accumulate inside tumor
tissues under the action of an external magnetic field.
A previous study demonstrated that angiogenesis
within tumors was in a chaotic state and generated a large number
of immature blood vessels, performing as vascular network
distribution disorder, vascular smooth muscle insufficiency,
incomplete basement membrane structures and large gaps among
endothelial cells (100–600 nm) (22).
This type of immature blood vessel is the important cause of
continuous aggravation and metastasis of tumors (23). These features increased vascular
permeability inside tumors in order to facilitate the penetration
of large macromolecules and nanoparticles through the gaps in
vascular endothelial cells (24).
However, tumor tissues were found to lack a lymphatic system, thus
the venous return was slow, resulting in the accumulation of
molecules and nanoparticles; this phenomenon demonstrates the high
permeability and retention effect of tumors (24). By utilizing this effect, nanoparticles
were able to pass through highly permeable tumor blood vessels,
resulting in accumulation inside tumor tissues (1).
The sizes of nanoparticles are closely associated
with their in vivo distribution: When the size of
nanoparticles are <400 nm, they are able to penetrate tumor
vascular endothelial cells; when the size is >100 nm, they are
absorbed by the hepatolienal endothelial reticular system; and when
the size is <10 nm, they are mainly excreted by the kidneys
(25). Therefore, the nanoparticle
sizes should be within 10–100 nm for use as a tumor targeting drug
delivery system. The particles resist the permeability reduction of
nanoparticles induced by the increased tumor interstitial pressure
when particle size is small, thereby increasing the targeting of
nanoparticles (26). The average
particle size of Ang2-siRNA plasmid vector/chitosan magnetic
nanoparticles prepared by the present study was 67 nm (9). Therefore, they were used as a suitable
targeting drug delivery system. A previous study revealed that
magnetic nanoparticles could generate heat in an alternating
magnetic field, thus inducing the apoptosis of tumor cells
(27), and achieving the purpose of
inhibiting tumor growth with a mechanism alternative to
conventional cancer therapy.
In the present study, the histopathological analysis
demonstrated that the non-targeting group exhibited occasional
particle distribution inside the vessels of tumor tissues and
Prussian blue staining was weakly positive. The control group
exhibited negative Prussian blue staining inside tumor tissues.
Whilst, the targeting group exhibited the aggregation of a large
number of particles under the capsule and blood vessels in tumor
tissues, and Prussian blue staining was strongly positive,
suggesting that the non-targeting group may have blood rich vessels
inside tumor tissues. The Ang2-siRNA plasmid vector/chitosan
magnetic nanoparticles enter the systemic blood circulation through
the tail vein and partial particles may enter tumor tissues due to
blood flow, whereas the distribution in the targeting group was due
to the external magnetic field. Ang2-siRNA plasmid vector/chitosan
magnetic nanoparticles may specifically migrate towards tumor
tissues due to the magnetic field.
In conclusion, the present study demonstrated that
Ang2-siRNA plasmid vector/chitosan magnetic nanoparticles exhibited
a good targeting characteristic and may be considered as a vector
for gene therapies. The present study also provided foundations for
further in vivo targeting intervention studies investigating
the angiogenesis and tumor growth of MM in nude mice.
Acknowledgements
The present study was supported by the Foundation of
National Key Clinical Specialty Discipline Construction Program
(China; grant no. 2013-GJLCZD), the National Health Planning
Scientific Research Foundation-Joint Research Projects of Fujian
Provincial Health and Education (Fuzhou, China; grant no.
WKJ-FJ-03) and the Projects of Fujian Provincial Natural Science
Foundation (Fuzhou, China; grant no. 2016J01527).
References
|
1
|
Dong X and Mumper RJ: Nanomedicinal
strategies to treat multidrug-resistant tumors: Current progress.
Nanomedicine (Lond). 5:597–615. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Bono JS and Ashworth A: Translating
cancer research into targeted therapeutics. Nature. 467:543–549.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Widder KJ, Senyel AE and Scarpelli GD:
Magnetic microshperes: A model system for site specific drug
delivery in vivo. Proc Soc Exp Biol Med. 158:pp. 141–146. 1978;
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang X, Chen Y, Yuan R, Chen G, Blanco E,
Gao J and Shuai X: Folate-encoded and Fe3O4-loaded polymeric
micelles for dual targeting of cancer cells. Polymer. 49:3477–3485.
2008. View Article : Google Scholar
|
|
5
|
Kim KY: Nanotechnology platforms and
physiological challenges for cancer therapeutics. Nanomedicine.
3:103–110. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao J and Xu B: Applications of
nanomaterials inside cells. Nano Today. 4:37–51. 2009. View Article : Google Scholar
|
|
7
|
Berry CC, Wells S, Charles S and Curtis
AS: Dextran and albumin derivatised iron oxide nanoparticles:
Influence on fibroblasts in vitro. Biomaterials. 24:4551–4557.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Perets A, Baruch Y, Weisbuch F, Shoshany
G, Neufeld G and Cohen S: Enhancing the vascularization of
three-dimensional porous alginate scaffolds by incorporating
controlled release basic fibroblast growth factor microspheres. J
Biomed Mater Res A. 65:489–497. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu ZL, You CL, Wang B, Lin JH, Hu XF,
Shan XY, Wang MS, Zheng HB and Zhang YD: Construction of Ang2-siRNA
chitosan magnetic nanoparticles and the effect on Ang2 gene
expression in human malignant melanoma cells. Oncol Lett.
11:3992–3998. 2016.PubMed/NCBI
|
|
10
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Venza M, Visalli M, Beninati C, De Gaetano
GV, Teti D and Venza I: Cellular mechanisms of oxidative stress and
action in melanoma. Oxid Med Cell Longev. 2015:4817822015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Häfeli UO: Magnetically modulated
therapeutic systems. Int J Pharm. 277:19–24. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee H, Yu MK, Park S, Moon S, Min JJ,
Jeong YY, Kang HW and Jon S: Thermally cross-linked
superparamagnetic iron oxide nanoparticles: Synthesis and
application as a dual imaging probe for cancer in vivo. J Am Chem
Soc. 129:12739–12745. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Unger E, Porter T, Lindner J and Grayburn
P: Cardiovascular drug delivery with ultrasound and microbubbles.
Adv Drug Deliv Rev. 72:110–126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sehrig F Zeinali, Majidi S, Nikzamir N,
Nikzamir N, Nikzamir M and Akbarzadeh A: Magnetic nanoparticles as
potential candidates for biomedical and biological applications.
Artif Cells Nanomed Biotechnol. 44:918–927. 2016.PubMed/NCBI
|
|
16
|
Ishii T, Okahata Y and Sato T: Mechanism
of cell transfection with plasmid/chitosan complexes. Biochim
Biophys Acta. 1514:51–64. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu B, Tai HC, Xue W, Lee LJ and Lee RJ:
Receptor-targeted nanocarriers for therapeutic delivery to cancer.
Mol Membr Biol. 27:286–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Torchilin VP: Passive and active drug
targeting: Drug delivery to tumors as an example. Handb Exp
Pharmacol. 1–53. 2010.PubMed/NCBI
|
|
19
|
Wang HH, Wang YX, Leung KC, Au DW, Xuan S,
Chak CP, Lee SK, Sheng H, Zhang G, Qin L, et al: Durable
mesenchymal stem cell labelling by using polyhedral
superparamagnetic iron oxide nanoparticles. Chemistry.
15:12417–12425. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hsieh WJ, Liang CJ, Chieh JJ, Wang SH, Lai
IR, Chen JH, Chang FH, Tseng WK, Yang SY, Wu CC and Chen YL: In
vivo tumor targeting and imaging with anti-vascular endothelial
growth factor antibody-conjugated dextran-coated iron oxide
nanoparticles. Int J Nanomedicine. 7:2833–2842. 2012.PubMed/NCBI
|
|
21
|
Zhou X, Zhang M, Yung B, Li H, Zhou C, Lee
LJ and Lee RJ: Lactosylated liposomes for targeted delivery of
doxorubicin to hepatocellular carcinoma. Int J Nanomedicine.
7:5465–5474. 2012.PubMed/NCBI
|
|
22
|
Maruyama K: Intracelluar targeting
delivery of liposomal drugs to solid tumors based on EPR effect.
Adv Drug Deliv Rev. 63:161–169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Payne SJ and Jones L: Influence of the
tumor microenvironment on angiogenesis. Future Oncol. 7:395–408.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Danquah MK, Zhang XA and Mahtao RI:
Extravasation of polymeric nanomedicines across tumor vasculature.
Adv Drug Deliv Rev. 63:623–639. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Neuberger T, Schöpf B, Hofmann H, Hofmann
M and Rechenberg BV: Superparamagnetic nanoparticles for biomedical
applications: Possibilities and limitations of a new drug delivery
system. J Magn Magn Mater. 293:483–496. 2005. View Article : Google Scholar
|
|
26
|
Lammers T, Kiessling F, Hennink WE and
Storm G: Drug targeting to tumors: Principles, pitfalls and (pre-)
clinical progress. J Control Release. 161:175–187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yi GQ, Gu B and Chen LK: The safety and
efficacy of magnetic nano-iron hyperthermia therapy on rat brain
glioma. Tumour Biol. 35:2445–2449. 2014. View Article : Google Scholar : PubMed/NCBI
|