Introduction
With the more detailed studies on the stem cells in
cervical carcinoma, we have successfully isolated and identified
the stem cells in cervical carcinoma from multiple types of
cervical carcinoma tissues and cell strains, and some scholars have
proposed that tumor stem cells in cervical carcinoma are involved
in the formation, infiltration, metastasis, chemotherapy tolerance
and recurrence of cervical carcinoma (1,2). Tumor
stem cells are characterized with the features of self-renewal,
infinite proliferation and the potential of multi-orientated
differentiation, which may be realized by the regulation of many
development-related signal transduction factors and transcriptional
activators (3–5).
The signal transducer and activator of transcription
3 (STAT3), as a kind of functional protein coupled with the
tyrosine phosphorylation signaling pathway (6–9), exerts an
important effect on regulating the expression of proteins relating
to various functions, such as cell proliferation, differentiation,
apoptosis and immune escape (10).
Active STAT3 is a key to the malignant changes of cells and the
abnormal high expression or activity enhancement of STAT3 has been
identified in a variety of tumor cell strains and tumor tissues
such as the cervical carcinoma tissues and HeLa cell strains
(7,11–13).
Suppression on the expression of STAT3 gene can inhibit cell
proliferation and induce cell apoptosis in HeLa strains of cervical
carcinoma.
NANOG, a kind of gene specifically expressed
in the embryonic stem cells (14,15). It is
a key gene capable of maintaining the self-renewal, proliferative
and sub-totipotent capability of stem cells, and together with
other key pluripotent factors, such as OCT4 and SOX2 it can control
the expression of a series of target genes (16–18) that
play key roles in sustaining the pluripotency of embryonic stem
cells. These factors can form a regulatory network to support or
limit the expression levels mutually (19,20).
Although it has been found that STAT3 signal
transduction factor is indispensable for the proliferation of tumor
stem cells, it can upregulate the expression of NANOG,
transcription factor of stem cells, by binding OCT3/4, to further
maintain the self-renewal capability of stem cells (21,22).
However, it remains unknown currently whether STAT3 can regulate
the characteristics of stem cells of cervical carcinoma through the
regulations of markers of stem cells, such as NANOG, OCT4 and SOX2.
Thus, after intervention of the STAT3 expression, we assayed the
changes in the expression of markers of stem cells, i.e., NANOG,
OCT4 and SOX2, using western blotting and RT-qPCR and observed the
formation of tumor sphere through the tumor sphere experiment and
detected the expression of STAT3, NANOG, OCT4 and SOX2 in clinical
samples using immunohistochemistry.
Materials and methods
Samples
SiHa cell strains of human cervical carcinoma were
preserved in the Scientific Research Center of Zhongnan Hospital of
Wuhan University; rabbit anti-human STAT3, OCT4, SOX2 and NANOG
polyclonal antibodies were purchased from Abcam (Cambridge, UK),
DMEM high-sugar medium was purchased from Hyclone (Logan, UT, USA),
10% fetal bovine serum was from Gibco (Grand Island, NY, USA),
Lipofectamine 2000 was from Invitrogen Life Technologies (Carlsbad,
CA, USA). The cervical carcinoma tissue samples were collected from
76 patients who were admitted to the Department of Obstetrics and
Gynecology, Xiangyang No. 1 People's Hospital, Hubei University of
Medicine for surgery between January 2013 and February 2014. Of the
samples 35 were cervical carcinoma and 31 were chronic cervicitis.
All patients gave their informed consent, and after surgery, the
samples were immediately preserved at −70°C after being processed
using liquid nitrogen. This study was approved by the Ethics
Committee of Xiangyang No. 1 People's Hospital, Hubei University of
Medicine.
Plasmid and primer
GV316-STAT3 plasmid, STAT3-specific siRNA and its
control gene segment were all purchased from New Cycle Biotech Co.,
Ltd. (Wuhan, China).
Cell culture and transfection
SiHa cells were cultured at 37°C in a thermostat
incubator containing 5% CO2. Then, SiHa cells were
inoculated on the 6-well plate, respectively, and when the cell
adhesion degree reached >90%, the liposome transfection
technique was performed according to the defined instructions of
Lipofectamine 2000. At 6 h of transfection, the medium was
replaced. After 24 h, G418 was added onto the medium and the
culture was continued. Following the cell proliferation, we
extracted the total protein and RNA to assay the expression of
STAT3 using western blotting and RT-qPCR.
The mRNA expression of OCT4, SOX2 and
NANOG using qRT-PCR
Cells in each group were collected after 48 h of
transfection followed by extraction of total RNA of cells in each
group using TRIzol reagent. Reverse transcription and gel
electrophoresis was performed to obtain the cDNA according to the
kit instructions. The mRNA expression of OCT4, SOX2
and NANOG were assayed using RT-qPCR after transfection. The
primer sequences are shown in Table
I.
 | Table I.Primers for RT-PCR and RT-qPCR. |
Table I.
Primers for RT-PCR and RT-qPCR.
| Genes | Sequence (5′-3′) | Product size
(bp) |
|---|
| OCT4 |
CGTGAAGCTGGAGAAGGAGAAGCTG | 247 |
|
|
CAAGGGCCGCAGCTTACACATGTTC |
|
| SOX2 |
CGCCCCCAGCAGACTTCACA | 170 |
|
|
CTCCTCTTTTGCACCCCTCCCATTT |
|
| NANOG |
AGTCCCAAAGGCAAACAACCCACTTC | 164 |
|
|
ATCTGCTGGAGGCTGAGGTATTTCTGTCTC |
|
| GAPDH |
GAAGGTGAAGGTCGGAGTC | 226 |
|
|
GAAGATGGTGATGGGATTTC |
|
Detection of the protein expression of
OCT4, SOX2 and NANOG using western blotting
After the collection of transfected cells, the cells
were treated using 100 µl lysis buffer on ice for 30 min, followed
by centrifugation at 4°C at 11,500 × g for 30 min to obtain the
supernatant of the lysate. Then the supernatant served for
detecting the protein concentration using BCA method. After the 10%
SDS-PAGE, the samples were transferred to PVDF membrane and blocked
using 5% skimmed milk powder prepared by TBST at room temperature
for 1 h. The membrane was incubated at 4°C overnight using rabbit
monoclonal OCT4 antibody (dilution, 1:1,000; cat. no. ab18976);
rabbit monoclonal SOX2 antibody (dilution, 1:1,000; cat. no.
ab97959) and rabbit monoclonal NANOG antibody (dilution, 1:1,000;
cat. no. ab80892) (all from Abcam, Cambridge, MA, USA). Then the
membrane was washed three times using TBST, 5 min each time. The
secondary goat anti-rabbit (HRP) IgG antibody (dilution, 1:2,000;
cat. no. ab6721; Abcam, Cambridge, MA, USA) was added for 1 h of
incubation followed by rinsing the membrane using TBST 3 times.
Finally, color development was carried out for the membrane.
Culture of tumor sphere
Cells in the logarithm phase were digested and
rinsed with PBS twice, followed by suspending the culture on the
serum-free medium (DMEM:F12 = 1:1), in which 0.25 ml serum-free
fresh medium was added on a daily basis. The formation of tumor
sphere was observed, photographed and recorded. After 10 days, the
tumor sphere was collected and mechanically isolated for passaging
(1:2). Fresh medium (0.25 ml) was added into the medium each day.
After 7 days, sub-culturing was performed to determine cell
proliferation.
Immunohistochemistry
The pathological section of cervix was stained using
SP, an immunohistochemical staining method was carried out as
described below. A total of 30 ml/l H2O2 was
used to deactivate the endogenous peroxidase and antigen retrieval
was carried out in the boiled citrate acid buffer (pH 6.0) in an
autoclave. The section was blocked using regular goat serum for 30
min followed by incubation at 4°C overnight using polyclonal goat
anti-human STAT3, OCT4, SOX2 and NANOG antibody. The section
was incubated using biotin-labelled secondary antibody for 30 min
and HRP-labelled streptavidin for another 30 min (Bioss Inc.,
Woburn, MA, USA). Then the section was treated with DAB for color
development and hematoxylin for re-dyeing, followed by regular
dehydration, clearing, drying and sealing. Then, the results were
observed and analyzed under a light microscope (BX-42; Olympus,
Tokyo, Japan). For the negative control group, the primary antibody
was replaced by regular goat serum and the rest of the procedures
were the same as above.
Statistical analysis
SPSS 19.0 (Chicago, IL, USA) was used for data
analysis, and each experiment was repeated thrice. All experimental
data are presented as mean ± standard deviation. t-test was
performed for the comparison between the means of two samples.
Statistical significance was set at p<0.05.
Results
Construction and identification of
STAT3 plasmid vector
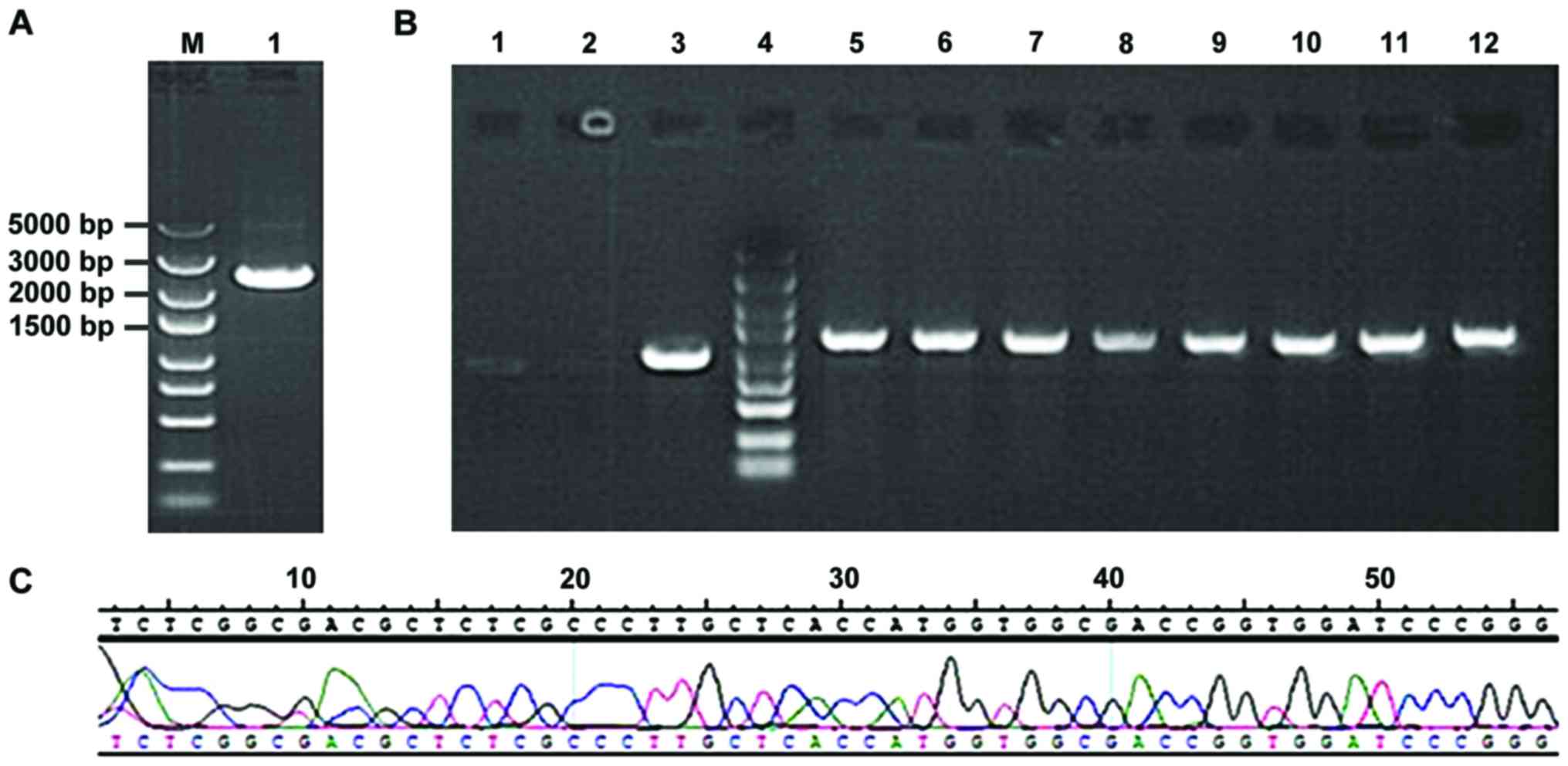
Following gel electrophoresis, STAT3 obtained
from amplification using PCR (Fig.
1A) was bound with GV316 vector using T4 DNA ligase. After the
transformation and LA plate-spread procedures, we selected the
monoclonal genes for PCR and found the results were coincident with
the anticipated 1265 bp, indicating that the binding was
successfully completed (Fig. 1B). The
sequencing results suggested that the clonal plasmid was inserted
correctly and the quality was in accordance with the standard
design (Fig. 1C).
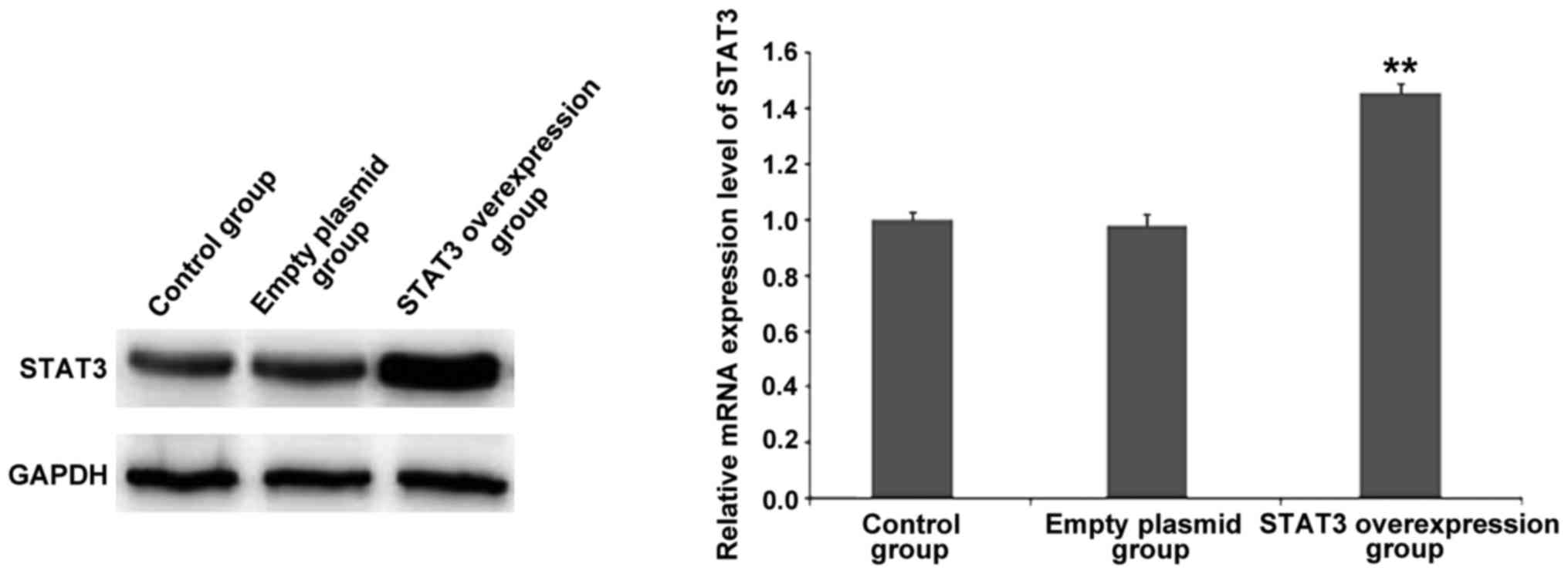
Verification of STAT3 overexpression
plasmid and siRNA- STAT3 efficiency
The results of RT-qPCR and western blot analysis
(Fig. 2) revealed that after the
successful transfection of GV316-STAT3 plasmid, the mRNA and
protein expression of STAT3 was significantly increased
compared to that in the control group and empty plasmid group,
indicating that STAT3 was successfully overexpressed.
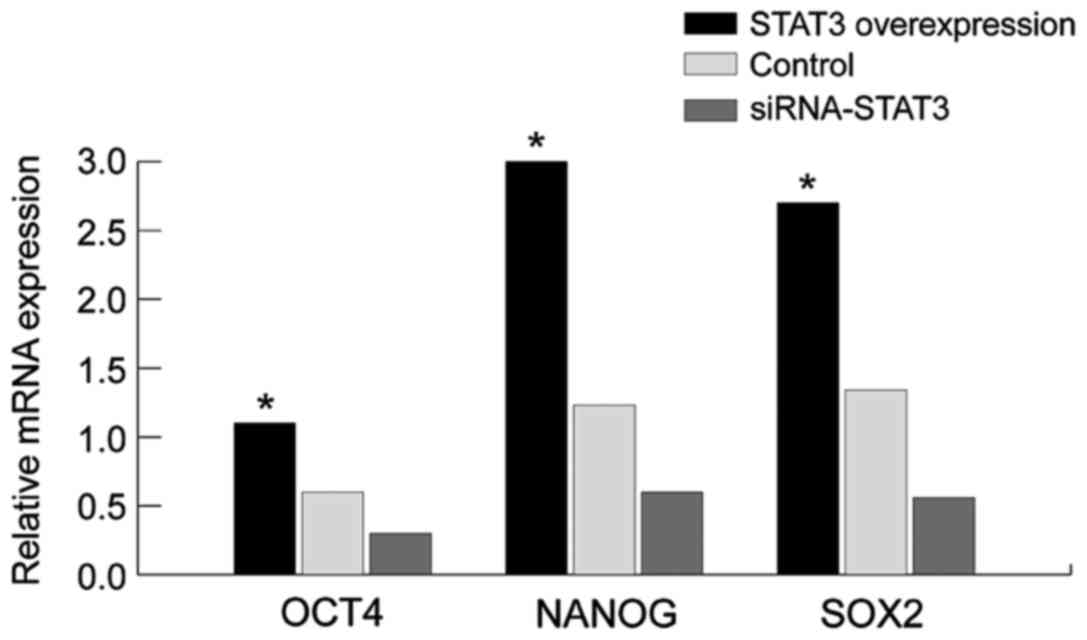
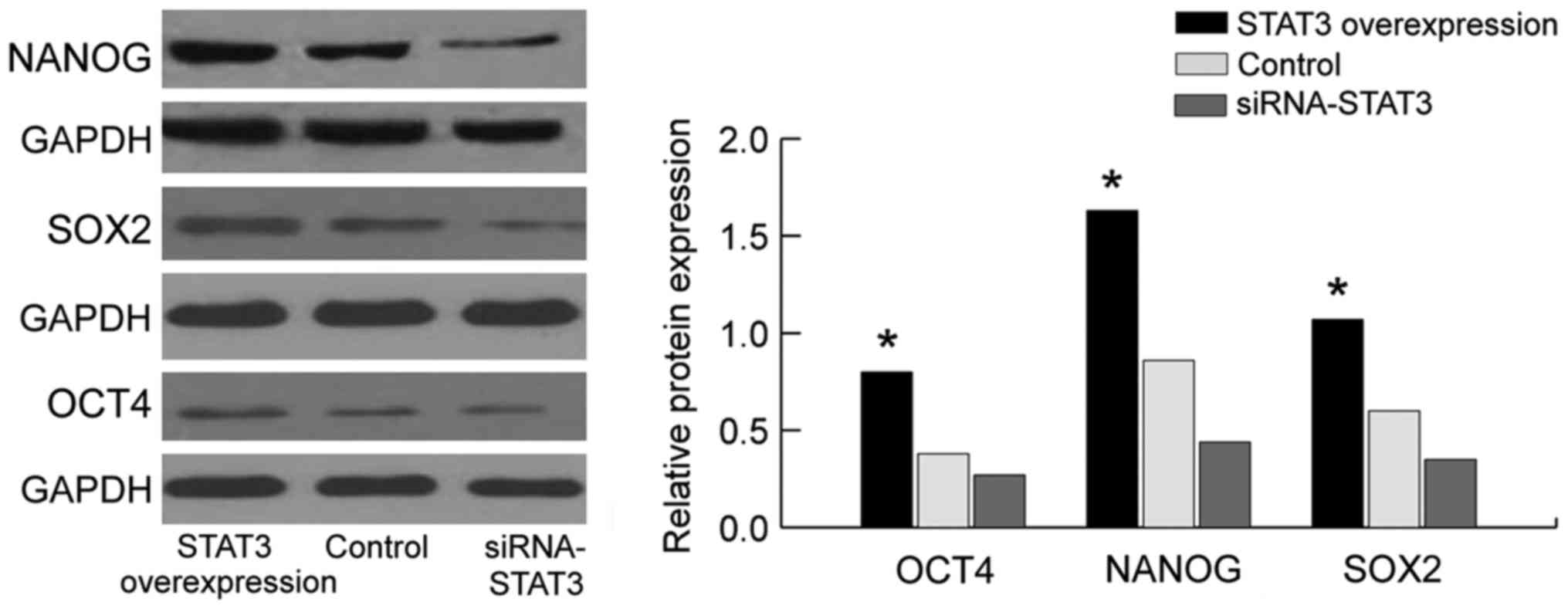
Detection of the effect of STAT3 on
the mRNA and protein expression of stem cell markers NANOG, OCT4
and SOX2, using western blot analysis and RT-qPCR
We employed the western blot analysis and RT-qPCR to
assay the expression of NANOG, OCT4 and SOX2 (Figs. 3 and 4).
Compared with those in the empty plasmid group and STAT3
overexpression group, the mRNA and protein expression of stem cell
markers (NANOG, OCT4 and SOX2) in the siRNA-STAT3 group was
significantly decreased with statistically significant difference
(P<0.05). By contrast, the expression of NANOG, OCT4 and SOX2 in
the STAT3 overexpression group was significantly increased compared
to those in the empty plasmid group and siRNA-STAT3 group with
statistically significant differences (P<0.05). The results
showed that the expression of stem cell markers is concurrently
accompanied with the overexpression of STAT3, indicating that there
is a correlation between them, and STAT3 may affect the expression
of stem cell markers (NANOG, OCT4 and SOX2). Thus, STAT3 can affect
the biological characteristics of stem cells in cervical carcinoma
through the stem cell markers (NANOG, OCT4 and SOX2).
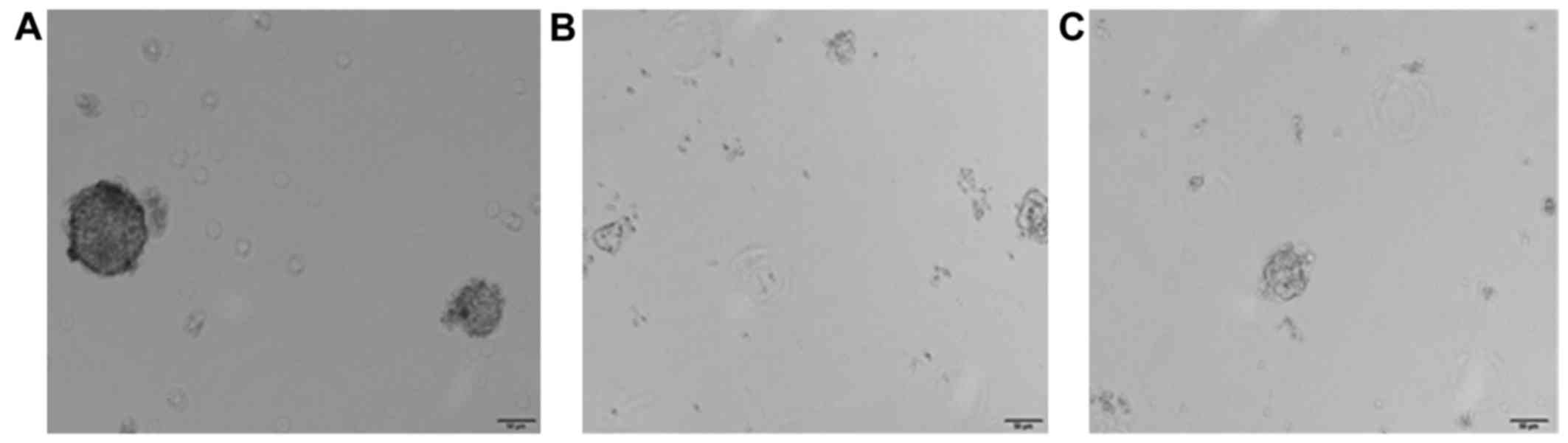
Comparison of the formation rate of
tumor spheres through the culture of tumor cells in serum-free
medium
The formation rates of first generation tumor
spheres in empty plasmid group, STAT3 overexpression group and
siRNA-STAT3 group were 5.2±0.38, 12.3±0.72 and 2.3±0.012%,
respectively. After 3 weeks, the formation rate of tumor spheres in
the STAT3 overexpression group was continuously increased, and
relatively loose tumor spheres were found to be formed in the
siRNA-STAT3 group (P<0.05; Fig.
5). In addition, the results of this study suggested that STAT3
overexpression can activate the tumor cells to form spheres, which,
signifies at cellular level that STAT3 can affect the biological
characteristics of stem cells in cervical carcinoma.
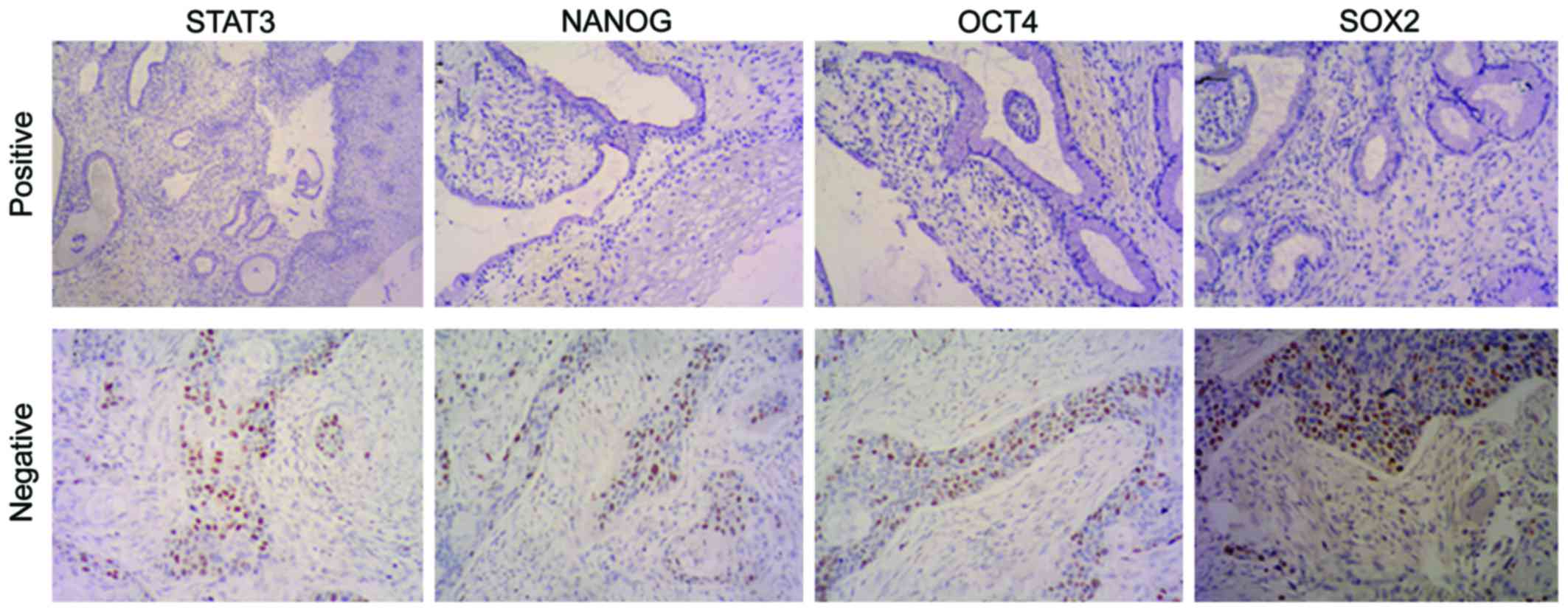
Expression of STAT3, NANOG, OCT4 and
SOX2 in cervical carcinoma tissues and chronic cervicitis
STAT3, NANOG, OCT4 and SOX2 are mainly expressed in
the nucleus of cervical carcinoma cells, and scarcely in the
cytoplasm. The nuclei that are presented as tan or sepia-colored
granules represent the positive staining which, however, is hardly
to be found in the regular cervical squamous epithelial tissues, or
only mildly stained area is evident (Fig.
6). The positive expression rates of STAT3, NANOG, OCT4 and
SOX2 in the cervical carcinoma tissues and chronic cervicitis were
respectively 6.45% (2/31) and 45.7% (16/35), 12.9% (4/31) and 57.1%
(20/35), 9.67% (3/31) and 62.9% (22/35), 9.67% (3/31) and 54.3%
(19/35), 9.67% (3/31) and 62.9% (22/35), 9.67% (3/31) and 54.3%
(19/35). We found that the differences of the positive expression
rate of STAT3, NANOG, OCT4 and SOX2 in cervical carcinoma and
chronic cervicitis were statistically significant (P<0.05).
Discussion
In this study, to investigate the mutually dependent
correlations among the proteins STAT3, NANOG, OCT4 and SOX2 and to
confirm whether STAT3 was capable of regulating the biological
characteristics of stem cells in cervical carcinoma by affecting
the proteins NANOG, OCT4 and SOX2, we constructed the STAT3
overexpression plasmid (Figs. 1 and
2), which served as the basis for
research into the regulatory effect of STAT3 on the biological
characteristics of stem cells in cervical carcinoma. The results of
western blotting and RT-qPCR revealed that the mRNA and protein
expression of NANOG, OCT4 and SOX2 in the STAT3 overexpression
group was significantly increased compared to those in the empty
plasmid group and STAT3 low-expression group. However, when the
STAT3 expression was inhibited using siRNA, the expression of
NANOG, OCT4, and SOX2 was decreased with statistically significant
differences (P<0.05). The result indicates that STAT3 can affect
the mRNA and protein expressions of NANOG, OCT4 and SOX2, and that
NANOG, OCT4 and SOX2 may be the key downstream proteins of
STAT3.
To further verify whether STAT3 in the cell strain
of cervical carcinoma regulated the biological characteristics of
stem cells by affecting the proteins of NANOG, OCT4 and SOX2, we
employed the tumor sphere suspension culture in serum-free medium
and the results revealed that the highest formation rate of tumor
sphere was found in the cervical carcinoma cells in the STAT3
overexpression group (P<0.05), and only loose tumor spheres or
no formation of tumor spheres were found in the siRNA-STAT3 group,
signifying that STAT3 overexpression can activate the formation of
tumor spheres, and suggesting at a cellular level that STAT3 can
affect the biological characteristics of stem cells in cervical
carcinoma.
Furthermore, we detected the expression of STAT3,
NANOG, OCT4 and SOX2 in clinical samples using
immunohistochemistry. The results showed that the positive
expression rates of STAT3, NANOG, OCT4 and SOX2 in the cervical
carcinoma tissues and chronic cervicitis were respectively 6.45%
(2/31) and 45.7% (16/35), 12.9% (4/31) and 57.1% (20/35), 9.67%
(3/31) and 62.9% (22/35), 9.67% (3/31) and 54.3% (19/35), 9.67%
(3/31) and 62.9% (22/35), 9.67% (3/31) and 54.3% (19/35). Thus,
STAT3, NANOG, OCT4 and SOX2, mostly expressed in chronic cervicitis
tissues, are presented as negative, while some expression of NANOG,
OCT4 and SOX2 in all the levels of cervical carcinoma tissues is
positive, which is coincident with the feature that tumor stem
cells are partially expressed in the tumor tissues. Thus, the
results of the clinical experiments in this study conform to those
of the molecular biology experiments.
Based on these cellular and molecular experiments,
we subsequently investigated and analyzed the roles of the
STAT3/NANOG pathway on the xenograft models of cervical
carcinoma.
References
|
1
|
Feng D, Peng C, Li C, Zhou Y, Li M, Ling
B, Wei H and Tian Z: Identification and characterization of cancer
stem-like cells from primary carcinoma of the cervix uteri. Oncol
Rep. 22:1129–1134. 2009.PubMed/NCBI
|
|
2
|
Bortolomai I, Canevari S, Facetti I, De
Cecco L, Castellano G, Zacchetti A, Alison MR and Miotti S: Tumor
initiating cells: development and critical characterization of a
model derived from the A431 carcinoma cell line forming spheres in
suspension. Cell Cycle. 9:1194–1206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takebe N, Harris PJ, Warren RQ and Ivy SP:
Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog
pathways. Nat Rev Clin Oncol. 8:97–106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Song L, Turkson J, Karras JG, Jove R and
Haura EB: Activation of Stat3 by receptor tyrosine kinases and
cytokines regulates survival in human non-small cell carcinoma
cells. Oncogene. 22:4150–4165. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang P, Li H, Yang B, Yang F, Zhang LL,
Kong QY, Chen XY, Wu ML and Liu J: Biological significance and
therapeutic implication of resveratrol-inhibited Wnt, Notch and
STAT3 signaling in cervical cancer cells. Genes Cancer. 5:154–164.
2014.PubMed/NCBI
|
|
6
|
Kruczyk M, Przanowski P, Dabrowski M,
Swiatek-Machado K, Mieczkowski J, Wallerman O, Ronowicz A,
Piotrowski A, Wadelius C, Kaminska B and Komorowski J: Integration
of genome-wide of Stat3 binding and epigenetic modification mapping
with transcriptome reveals novel Stat3 target genes in glioma
cells. Biochim Biophys Acta. 1839:1341–1350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yao J, Qian CJ, Ye B, Zhao ZQ, Wei J,
Liang Y and Zhang X: Signal transducer and activator of
transcription 3 signaling upregulates fascin via nuclear factor-κB
in gastric cancer: Implications in cell invasion and migration.
Oncol Lett. 7:902–908. 2014.PubMed/NCBI
|
|
8
|
Bao W, Wang HH, Tian FJ, He XY, Qiu MT,
Wang JY, Zhang HJ, Wang LH and Wan XP: A TrkB-STAT3-miR-204-5p
regulatory circuitry controls proliferation and invasion of
endometrial carcinoma cells. Mol Cancer. 12:1552013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng GZ, Zhang WZ, Sun M, Wang Q, Coppola
D, Mansour M, Xu LM, Costanzo C, Cheng JQ and Wang LH: Twist is
transcriptionally induced by activation of STAT3 and mediates STAT3
oncogenic function. J Biol Chem. 283:14665–14673. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qin A, Yu Q, Gao Y, Tan J, Huang H, Qiao Z
and Qian W: Inhibition of STAT3/cyclinD1 pathway promotes
chemotherapeutic sensitivity of colorectal caner. Biochem Biophys
Res Commun. 457:681–687. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takemoto S, Ushijima K, Kawano K,
Yamaguchi T, Terada A, Fujiyoshi N, Nishio S, Tsuda N, Ijichi M,
Kakuma T, et al: Expression of activated signal transducer and
activator of transcription-3 predicts poor prognosis in cervical
squamous-cell carcinoma. Br J Cancer. 101:967–972. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Devarajan E and Huang S: STAT3 as a
central regulator of tumor metastases. Curr Mol Med. 9:626–633.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao C, Zhao G, Yu W, Xie X, Wang W, Yang
R, Lv X and Liu D: Activation of STAT3 stimulates AHSP expression
in K562 cells. Sci China Life Sci. 57:488–494. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chambers I, Colby D, Robertson M, Nichols
J, Lee S, Tweedie S and Smith A: Functional expression cloning of
Nanog, a pluripotency sustaining factor in embryonic stem cells.
Cell. 113:643–655. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ye F, Zhou C, Cheng Q, Shen J and Chen H:
Stem-cell-abundant proteins Nanog, Nucleostemin and Musashi1 are
highly expressed in malignant cervical epithelial cells. BMC
Cancer. 8:1082008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsai LL, Yu CC, Chang YC, Yu CH and Chou
MY: Markedly increased Oct4 and Nanog expression correlates with
cisplatin resistance in oral squamous cell carcinoma. J Oral Pathol
Med. 40:621–628. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Z, Oron E, Nelson B, Razis S and
Ivanova N: Distinct lineage specification roles for NANOG, OCT4,
and SOX2 in human embryonic stem cells. Cell Stem Cell. 10:440–454.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chiou SH, Yu CC, Huang CY, Lin SC, Liu CJ,
Tsai TH, Chou SH, Chien CS, Ku HH and Lo JF: Positive correlations
of Oct-4 and Nanog in oral cancer stem-like cells and high-grade
oral squamous cell carcinoma. Clin Cancer Res. 14:4085–4095. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu XF, Yang WT, Xu R, Liu JT and Zheng
PS: Cervical cancer cells with positive Sox2 expression exhibit the
properties of cancer stem cells. PLoS One. 28:870–892. 2014.
|
|
20
|
Li SW, Wu XL, Dong CL, Xie XY, Wu JF and
Zhang X: The differential expression of OCT4 isoforms in cervical
carcinoma. PLoS One. 10:e01180332015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bourguignon LY, Peyrollier K, Xia W and
Gilad E: Hyaluronan-CD44 interaction activates stem cell marker
Nanog, Stat-3-mediated MDR1 gene expression, and ankyrin-regulated
multidrug efflux in breast and ovarian tumor cells. J Biol Chem.
283:17635–17651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Do DV, Ueda J, Messerschmidt DM,
Lorthongpanich C, Zhou Y, Feng B, Guo G, Lin PJ, Hossain MZ, Zhang
W, et al: A genetic and developmental pathway from STAT3 to the
OCT4-NANOG circuit is essential for maintenance of ICM lineages in
vivo. Genes Dev. 27:1378–1390. 2013. View Article : Google Scholar : PubMed/NCBI
|