Introduction
Hepatocellular carcinoma (HCC) is the most common
type of primary liver tumor and the fifth most common type of
cancer worldwide, representing the third leading cause of
cancer-associated mortality (1–3). Although
hepatic resection is currently regarded as the standard therapeutic
modality for HCC, its application is limited due to severe liver
dysfunction in the majority of hepatic cancer cases. Liver
transplantation is an alternative treatment for small, unresectable
HCC, although its application is restricted by the shortage of
liver graft donors (4,5). Consequently, non-surgical methods,
including image-guided radiofrequency ablation (RFA), have been
introduced for HCC treatment. As the most widely used non-surgical
treatment approach for HCC, RFA has numerous advantages, including
its definitive therapeutic effect, minimal damage to functioning
liver, simplicity, repeatability and shorter associated hospital
stays (4,6). However, increasing laboratory and
clinical studies have reported that residual tumor tissue following
RFA exhibits more malignant growth and accelerated local recurrence
compared with non-RFA treated tumors (5–8).
Therefore, further investigations into the molecular mechanisms
underlying the undesirable effects of RFA on HCC are warranted.
The epidermal growth factor receptor (EGFR)
signaling pathway is important for cancer cell oncogenesis,
proliferation, maintenance and survival. In addition to being
directly activated by its cognate ligand, epidermal growth factor
(EGF), increasing evidence has revealed that EGFR also serves as a
central element in the signal transduction networks activated by
other stimuli, including cytokines (9), ammonia (10), H2O2 (11) and G-protein-coupled receptors
(12). Currently, this nonclassical
EGFR stimulation process is generally termed ‘EGFR transactivation’
(12). Transactivated EGFR
subsequently integrates signals from the various extracellular
stimuli into a limited number of downstream signal transduction
molecules, including mitogen-activated protein kinase
(MAPK)/extracellular signal-regulated kinase (ERK),
phosphatidylinositol 3-kinase/Akt, phospholipase C/protein kinase
C, and Ca2+ (10,13). Our previous study demonstrated that
insufficient RFA promotes hepatoma cell malignancy via the
Ca2+/calmodulin-dependent protein kinase II
(CaMKII)/ERK-induced overexpression of vascular endothelial growth
factor (VEGF) (6). Thus, the current
study aimed to investigate whether EGFR signal transactivation is
involved in HCC malignancy induced by incomplete RFA, and determine
its association with the CaMKII/ERK-VEGF signaling cascade.
Materials and methods
Cell culture
Human HCCSMMC7721 cells were obtained from the
American Type Culture Collection (Manassas, VA, USA). The cancer
cells were maintained in RPMI-1640 medium (Corning Incorporated,
Corning, NY, USA) supplemented with 10% fetal bovine serum
(Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) in a
humidified atmosphere containing 5% CO2 at 37°C. The
medium was changed 2–3 times/week.
Heat treatment
Incomplete RFA was simulated in vitro
according to the authors' previously reported procedure (6). Briefly, SMMC7721 cells were seeded into
6-well plates at a density of 5×104 cells/well.
Following culture for 24 h in a CO2 incubator with a
humidified atmosphere containing 5% CO2 at 37°C, the
plates were sealed and submerged in a water bath at 47°C for 5 min.
Thereafter, the cells were transferred into the CO2
incubator for recovery. When the residual populations achieved 80%
confluence, they were passaged into the 6-well plates and exposed
to 47°C heat treatment for 10 min. This process was repeated with
the stimulation duration increasing to 15, 20 and finally 25 min.
Cells surviving the 47°C treatment regimen were designated as the
47°C treatment group. Cells in the control group underwent the same
procedures; however, the temperature of the water bath was set at
37°C instead of 47°C. Subsequently, 100 nM AG1478, a specific
inhibitor of EGFR, or 10 µM KN93, a specific inhibitor of CaMKII
(both purchased from Sigma-Aldrich, St. Louis, MO, USA) was added
to the 47°C treatment group, to investigate the roles of EGFR and
CaMKII in incomplete RFA-induced SMMC7721 malignancy.
MTT assay
The trypsin-dispersed parent SMMC7721 or
47°C-treated SMMC7721 cells were seeded into a 96-well plate
(3×103 cells/well in a volume of 100 µl RPMI-1640 medium
supplemented with 10% fetal bovine serum) and cultured for 48 h at
37°C. Subsequently, MTT solution was added to each well to give a
final concentration of 0.5 mg/ml, and incubated for 4 h at 37°C.
Finally, the culture medium was removed and 150 µl DMSO/well was
added. The absorbance was measured at a wavelength of 570 nm using
an automated ELISA plate reader (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
Colony formation assay
The SMMC7721 cells were seeded into 6-well dishes at
a concentration of 1×103/well and cultured in RPMI-1640
medium containing 10% fetal bovine serum for 2 weeks at 37°C. The
obtained colonies were washed with PBS and fixed in 4%
paraformaldehyde for 20 min at room temperature, and then washed
three times with PBS. Finally, colonies were stained with 0.1%
crystal violet for 10 min at room temperature. Stained cells were
washed with PBS and the colony number was counted.
Western blotting
Whole-cell lysates were collected using freshly
prepared lysis buffer [containing 150 mM NaCl, 50 mM Tris-HCl, pH
8.0, 0.1% sodium dodecyl sulfate, 1% Triton X-100, and 1%
proteinase inhibitors (1:100, Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany)]. Following incubation on ice for 30 min, the cell lysates
were centrifuged at 14,000 × g for 20 min at 4°C (Sorvall™ ST 16R;
Thermo Fisher Scientific, Inc.). The protein content was
subsequently determined using a Lowry protein assay with bovine
serum albumin (Sigma-Aldrich; Merck KGaA) as the standard. For
western blot analysis, solubilized proteins (30–50 µg/lane) were
separated using SDS-PAGE (10% gel) and eletrophoretically
transferred onto polyvinylidene difluoride membranes. Following
transfer, the membranes were blocked with 5% non-fat milk for 1 h
at room temperature and then incubated overnight at 4°C with
primary rabbit anti-human polyclonal anti-ERK (1:1,000; cat. no.
sc-292838; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-phosphorylated (phospho)-ERK (1:1,000; cat. no. sc-101760;
Santa Cruz Biotechnology, Inc.), anti-CaMKII (1:500; cat. no.
sc-13082; Santa Cruz Biotechnology, Inc.), anti-phospho-CaMKII
(1:1,000; cat. no. V1111; Promega Corporation, Madison, WI, USA),
anti-VEGF (1:200; cat. no. BA0407; Wuhan Boster Biological
Technology, Ltd., Wuhan, China), anti-EGFR (1:500; cat. no. sc-03;
Santa Cruz Biotechnology, Inc.), or anti-phospho-EGFR (1:500; cat.
no. sc-101668; Santa Cruz Biotechnology, Inc.) antibodies.
Subsequently, the membranes were incubated with polyclonal
horseradish peroxide-conjugated secondary goat anti-rabbit
antibodies (1:3,000; cat. no. sc-2004; Santa Cruz Biotechnology,
Inc.) diluted in TBS with Tween-20 (20 mM Tris-HCl, 150 mM NaCl, pH
7.4 and 0.1% Tween-20) for 2 h at room temperature. Finally, the
membrane was rinsed with TBS and visualized using Pierce™ Enhanced
Chemiluminescence Western Blotting Substrate (Thermo Fisher
Scientific, Inc.). The optical density for each band was assessed
using QuantityOne software (version 4.6.9; Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Statistical analysis
Statistical analyses were performed using SPSS
software (version 17.0; SPSS, Inc., Chicago, IL, USA). All data are
expressed as the mean ± standard error of the mean from ≥3
independent experiments. Statistical analysis for multiple
comparisons was performed by one-way analysis of variance followed
by Fisher's least significant difference test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Incomplete RFA promotes SMMC7721
proliferation, which is significantly inhibited by AG1478
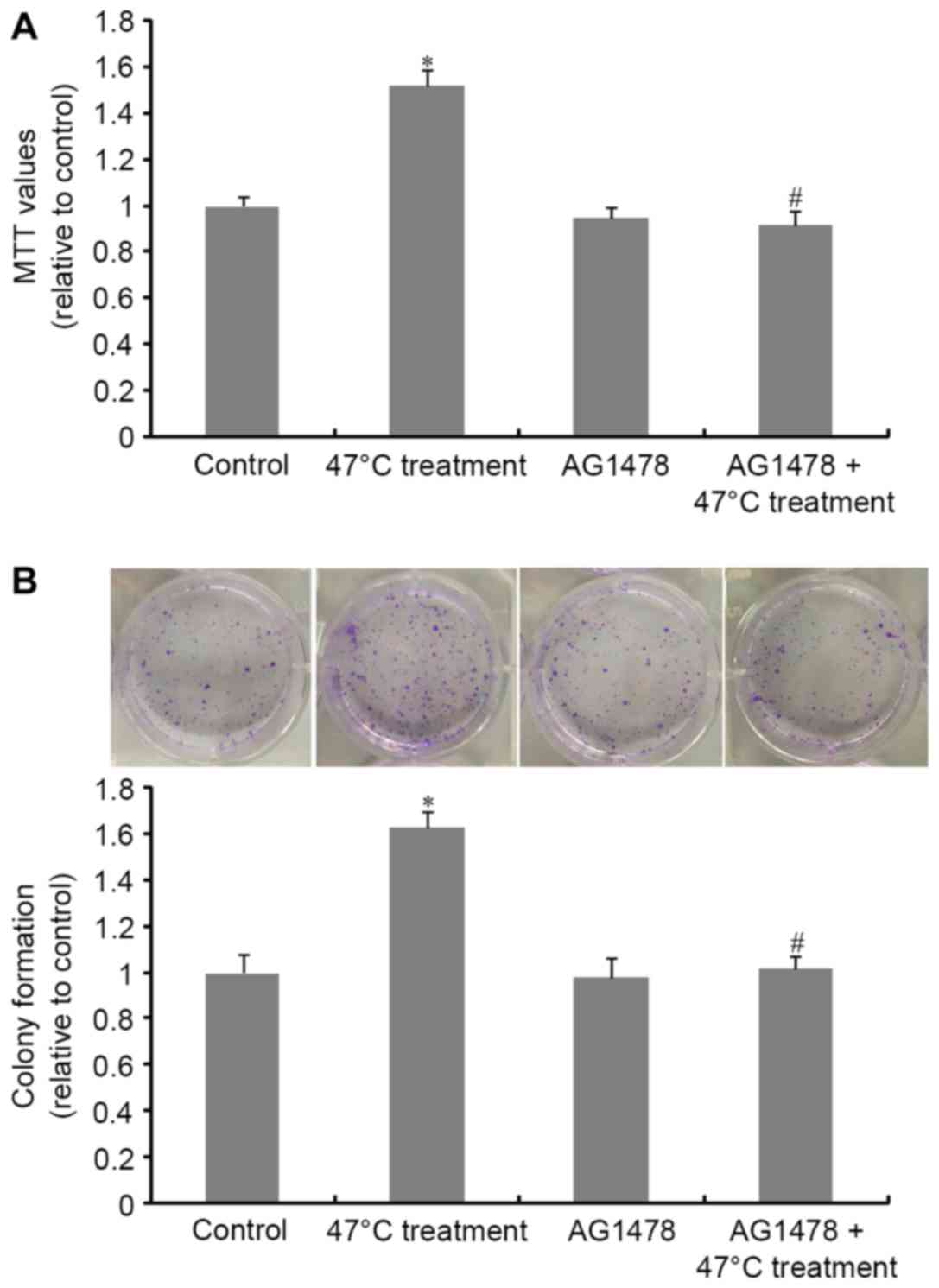
The MTT assay results presented in Fig. 1A demonstrated that the 47°C treatment
regimen significantly promoted SMMC7721 cell proliferation compared
with untreated cells. This effect was abrogated by treatment with
AG1478, when compared with cells that had received incomplete RFA
treatment alone. A similar result was obtained in the colony
formation assay (Fig. 1B). These
observations demonstrate the crucial role of EGFR transactivation
in heat stimulation-induced tumor cell proliferation.
AG1478 significantly inhibits
RFA-induced overexpression of VEGF and activation of ERK
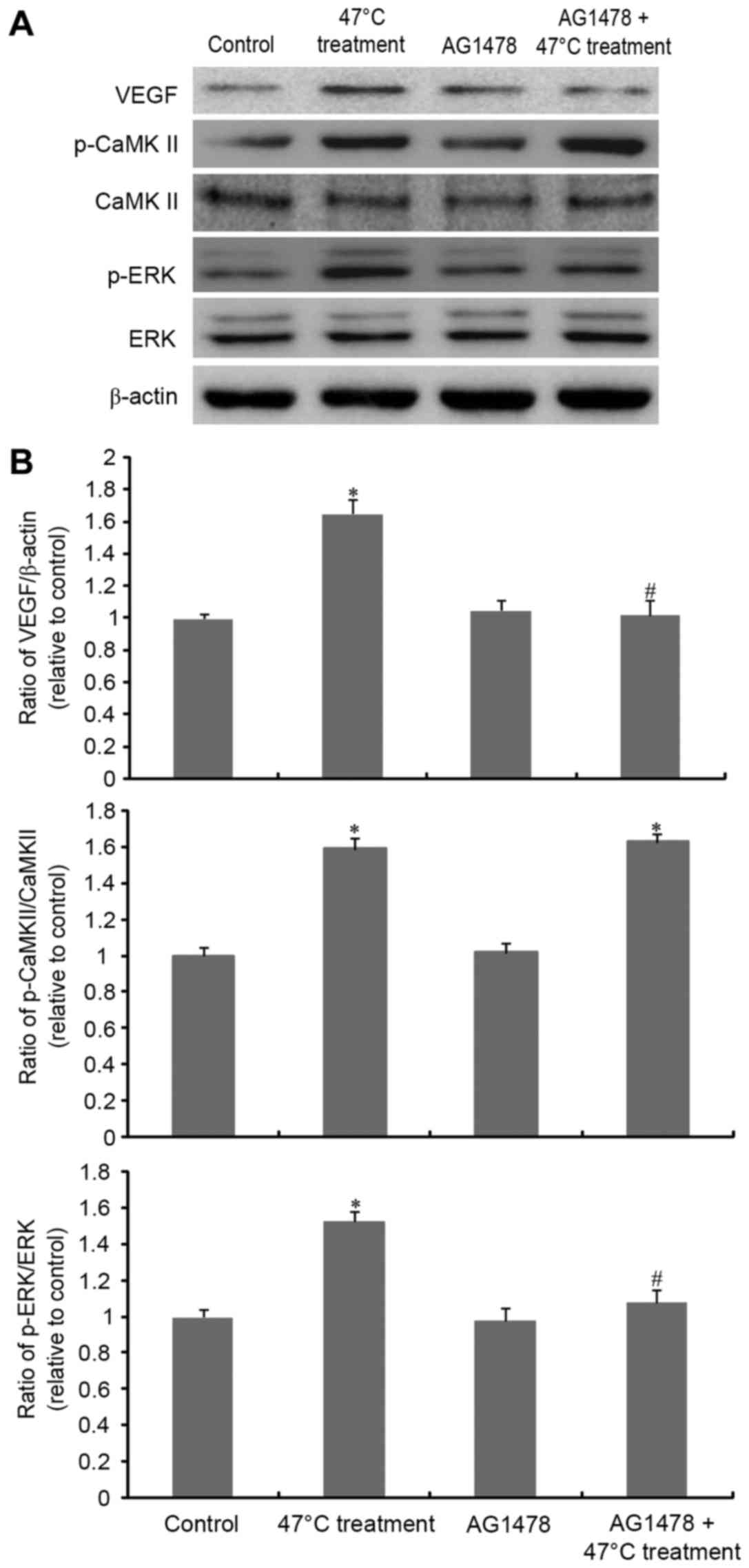
Our previous study demonstrated that
CaMKII/ERK-dependent VEGF overexpression was involved in the 47°C
treatment-induced malignant proliferation of SMMC7721 cells
(6). Thus, the present study further
investigated the association between EGFR transactivation and the
CaMKII/ERK/VEGF signaling cascade. As Fig. 2 demonstrates, VEGF expression was
significantly increased upon 47°C treatment stimulation. AG1478
exposure significantly inhibited the overexpression of VEGF induced
by incomplete RFA. Furthermore, AG1478 abrogated heat
stress-induced ERK phosphorylation. However, CaMKII phosphorylation
(activation) was not affected by EGFR inhibition.
KN93 significantly inhibits
RFA-induced EGFR phosphorylation
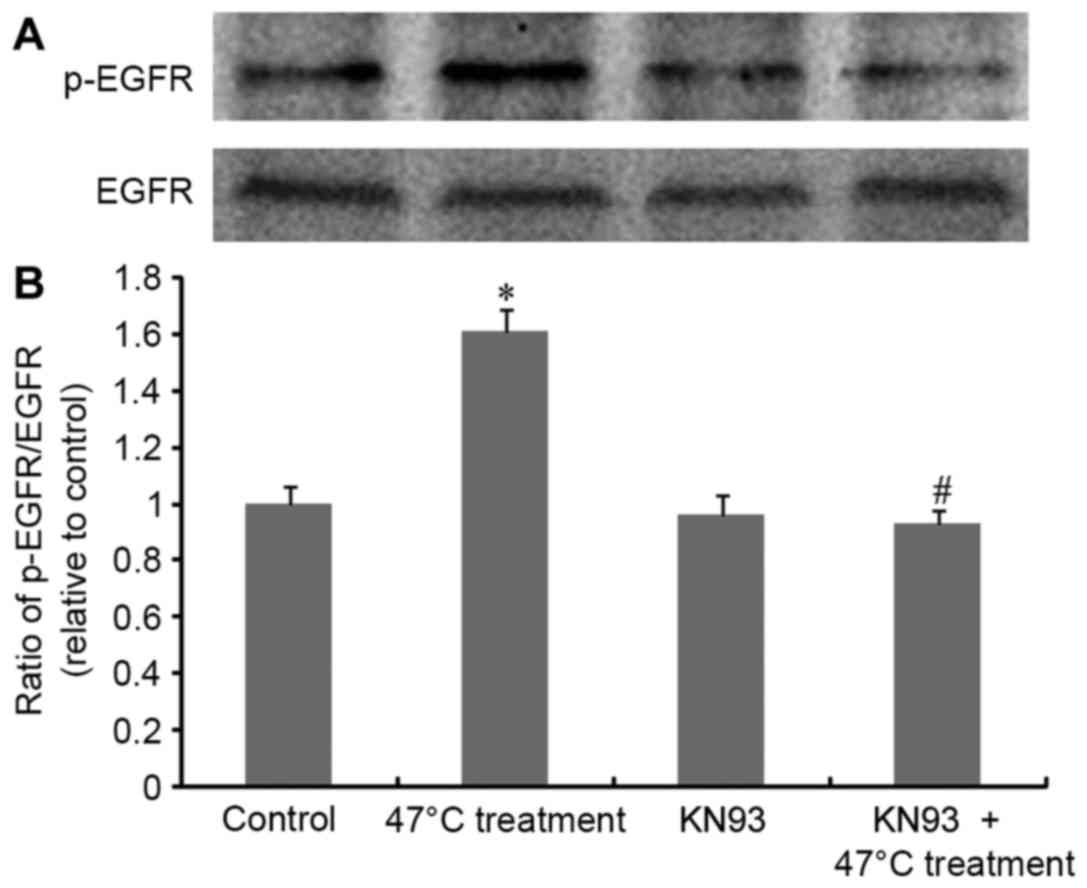
Upon exposure of SMMC7721 cells to the 47°C regimen,
EGFR phosphorylation levels were determined and the results are
presented in Fig. 3. The heat
stimulation regimen triggered significant activation
(phosphorylation) of EGFR, with no influence on total EGFR
expression, confirming that 47°C treatment induces EGFR
transactivation. Notably, this transactivation was significantly
inhibited by KN93. This result, combined with the data in Fig. 2, suggests a CaMKII-dependent EGFR
transactivation cascade upon 47°C treatment in SMMC7721 cells.
Discussion
As a novel therapeutic strategy, RFA exhibits
numerous advantages and is now widely used for HCC treatment as an
alternative to traditional surgical approaches. However, one of the
major challenges associated with RFA is the rapid growth of
residual HCC, thus leading to high recurrence rates associated with
the cancer (6,8). As reported, post-RFA local recurrence
rates can reach up to 60% (14).
Previous studies have demonstrated that the rapid progression of
residual HCC may be associated with a more malignant hepatoma
cellular phenotype due to the activation of several signaling
pathways. For example, a recent study revealed that Akt and ERK
signaling activation served as an important mechanism underlying
insufficient-RFA-induced HCC malignancy (8). Another study reported that suboptimal
RFA accelerated residual tumor proliferation by inducing the
overexpression of hypoxia inducible factor-1α and VEGFA (14). Similarly, our previous report showed
that insufficient RFA could promote residual hepatoma cell
proliferation via triggering the CaMKII/ERK-mediated overexpression
of VEGF (6). In order to obtain a
more detailed understanding of the signaling mechanisms, the
involvement of EGFR transactivation in RFA-induced hepatoma cell
malignancy and its association with CaMKII/ERK-VEGF signaling
cascades were investigated in the present study.
In addition to being directly activated by its
cognate ligand EGF, EGFR is involved in the signal transduction
networks of other stimuli. A previous study has revealed that EGFR
transactivation is required for the mediation of mitogenic effects
of epoxyeicosatrienoic acid in various types of cancer cells
(15). Previously, it was reported
that heat stress induced a decrease in EGFR expression in
intestinal epithelial-6 cells, representing a protective mechanism
in response to inflammation and/or injury in intestinal mucosa
(16). However, the EGFR signaling
response and its role in RFA-induced HCC malignant proliferation
remain unclear. In the present study, it was demonstrated that 47°C
heat stimulation significantly induced the proliferation of HCC
cells, which was abrogated by the EGFR-specific inhibitor AG1478.
Additionally, 47°C heat treatment resulted in significantly
increased the levels of EGFR phosphorylation, as shown by western
blot analysis. These data demonstrate the essential role of EGFR
transactivation in 47°C treatment-enhanced malignant proliferation
of liver cancer cells. This is further supported by preliminary
data that revealed a significant inhibitory effect of genistein, a
broad-spectrum tyrosine kinase inhibitor, on 47°C treatment-induced
HCC over-proliferation (Dai et al, unpublished data).
In the present study, the association between
CaMKII/ERK-VEGF signaling cascades and EGFR transactivation was
investigated. The MAPK kinase (MEK)/ERK pathway is a common
signaling pathway downstream of direct or indirect (trans-)
activation of EGFR. In the majority of cases, EGFR-mediated MEK/ERK
activation results in the malignant proliferation of tumors
(17–19). Based on this, in addition to the
significant inhibitory effect of AG1478 on HCC proliferation, it
was hypothesized that 47°C treatment-induced ERK activation is
mediated by EGFR transactivation. This hypothesis was confirmed by
the significant inhibitory effect of AG1478 on heat
stimulation-triggered ERK activation. Although it was initially
hypothesized that CaMKII activation also occurred following EGFR
activation, the data of the present study excluded this
possibility. It is more likely that CaMKII serves as an upstream
operator of EGFR activation, as its phosphorylation was eliminated
by KN93, a specific inhibitor of CaMKII. This is in contrast with
observations of transforming growth factor β1-stimulated CaMKII
activation downstream of EGFR transactivation during the
differentiation of fibroblasts to myofibroblasts (20). However, a CaMKII-dependent EGFR
activation in the present study has been observed to underlie the
following: i) The effect of hydrogen peroxide on lung alveolar
epithelial A549 cells (21); ii) the
effect of α1A-adrenoceptorstimulation on Chinese hamster
ovary cells (22); and iii) the
effect of adenosine triphosphate on vascular smooth muscle cells
(23).
In conclusion, the results of the current study
suggest an important molecular mechanism involving EGFR
transactivation in the incomplete RFA-induced proliferation of
residual HCC cells. Thus, EGFR has been identified as a potential
target for the prevention and treatment of RFA-induced HCC
progression, and recurrence.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Liaoning Province (grant no. 2015020360); the
President Foundation, Aohongboze Foundation of Liaoning Medical
University (grant no. XZJJ20140111); and the President Foundation,
Aohongboze Foundation for Undergraduate Sci-Technology Innovation
of Liaoning Medical University (grant no. 2015D20).
References
|
1
|
Che YH, Chongsuvivatwong V, Li L, Sriplung
H, Wang YY, You J, Ma SJ, Yan Y, Zhang RY, Shen T, et al: Financial
burden on the families of patients with hepatitis B virus-related
liver diseases and the role of public health insurance in Yunnan
province of China. Public Health. 130:13–20. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fouad AA, Al-Mulhim AS and Jresat I:
Therapeutic effect of coenzyme Q10 against experimentally-induced
hepatocellular carcinoma in rats. Environ Toxicol Pharmacol.
35:100–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Venook AP, Papandreou C, Furuse J and de
Guevara LL: The incidence and epidemiology of hepatocellular
carcinoma: A global and regional perspective. Oncologist. 15 Suppl
4:S5–S13. 2010. View Article : Google Scholar
|
|
4
|
Lau WY and Lai EC: The current role of
radiofrequency ablation in the management of hepatocellular
carcinoma: A systematic review. Ann Surg. 249:20–25. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang N, Wang L, Chai ZT, Zhu ZM, Zhu XD,
Ma DN, Zhang QB, Zhao YM, Wang M, Ao JY, et al: Incomplete
radiofrequency ablation enhances invasiveness and metastasis of
residual cancer of hepatocellular carcinoma cell HCCLM3 via
activating β-catenin signaling. PLoS One. 9:e1159492014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Z, Dai H, Jia G, Li Y, Liu X and Ren
W: Insufficient radiofrequency ablation promotes human hepatoma
SMMC7721 cell proliferation by stimulating vascular endothelial
growth factor overexpression. Oncol Lett. 9:1893–1896.
2015.PubMed/NCBI
|
|
7
|
Ouyang Y, Liu K, Hao M, Zheng R, Zhang C,
Wu Y, Zhang X, Li N, Zheng J and Chen D: Radiofrequency
ablation-increased CXCL10 is associated with earlier recurrence of
hepatocellular carcinoma by promoting stemness. Tumour Biol.
37:3697–3704. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dong S, Kong J, Kong F, Kong J, Gao J, Ke
S, Wang S, Ding X, Sun W and Zheng L: Insufficient radiofrequency
ablation promotes epithelial-mesenchymal transition of
hepatocellular carcinoma cells through Akt and ERK signaling
pathways. J Transl Med. 11:2732013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moreno-Càceres J, Caja L, Mainez J,
Mayoral R, Martín-Sanz P, Moreno-Vicente R, Del Pozo MÁ, Dooley S,
Egea G and Fabregat I: Caveolin-1 is required for TGF-β-induced
transactivation of the EGF receptor pathway in hepatocytes through
the activation of the metalloprotease TACE/ADAM17. Cell Death Dis.
5:e13262014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dai H, Song D, Xu J, Li B, Hertz L and
Peng L: Ammonia-induced Na,K-ATPase/ouabain-mediated EGF receptor
transactivation, MAPK/ERK and PI3K/AKT signaling and ROS formation
cause astrocyte swelling. Neurochem Int. 63:610–625. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhuang S and Schnellmann RG: H2O2-induced
transactivation of EGF receptor requires Src and mediates ERK1/2,
but not Akt, activation in renal cells. Am J Physiol Renal Physiol.
286:F858–F865. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Forrester SJ, Kawai T, O'Brien S, Thomas
W, Harris RC and Eguchi S: Epidermal growth factor receptor
transactivation: Mechanisms, pathophysiology, and potential
therapies in the cardiovascular system. Annu Rev Pharmacol Toxicol.
56:627–653. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Michailov Y, Ickowicz D and Breitbart H:
Zn2+-stimulation of sperm capacitation and of the acrosome reaction
is mediated by EGFR activation. Dev Biol. 396:246–255. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu M, Xie XH, Xie XY, Xu ZF, Liu GJ, Zheng
YL, Huang GL, Wang W, Zheng SG and Lü MD: Sorafenib suppresses the
rapid progress of hepatocellular carcinoma after insufficient
radiofrequency ablation therapy: An experiment in vivo. Acta
Radiol. 54:199–204. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng LM, Jiang JG, Sun ZY, Chen C, Dackor
RT, Zeldin DC and Wang DW: The epoxyeicosatrienoic acid-stimulated
phosphorylation of EGF-R involves the activation of
metalloproteinases and the release of HB-EGF in cancer cells. Acta
Pharmacol Sin. 31:211–218. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Niederlechner S, Baird C, Petrie B,
Wischmeyer E and Wischmeyer PE: Epidermal growth factor receptor
expression and signaling are essential in glutamine's
cytoprotective mechanism in heat-stressed intestinal epithelial-6
cells. Am J Physiol Gastrointest Liver Physiol. 304:G543–G552.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li L, Qi L, Liang Z, Song W, Liu Y, Wang
Y, Sun B, Zhang B and Cao W: Transforming growth factor-β1 induces
EMT by the transactivation of epidermal growth factor signaling
through HA/CD44 in lung and breast cancer cells. Int J Mol Med.
36:113–122. 2015.PubMed/NCBI
|
|
18
|
Gao M, Zhan YQ, Yu M, Ge CH, Li CY, Zhang
JH, Wang XH, Ge ZQ and Yang XM: Hepassocin activates the EGFR/ERK
cascade and induces proliferation of L02 cells through the
Src-dependent pathway. Cell Signal. 26:2161–2166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dai B, Zhan Y, Qi J and Zhang Y:
Eupolyphaga sinensis Walker inhibits human chronic myeloid leukemia
cell K562 growth by inducing G2-M phase cell cycle arrest and
targeting EGFR signaling pathway and in S180 tumor-bearing mice.
Environ Toxicol Pharmacol. 37:1177–1185. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Midgley AC, Rogers M, Hallett MB, Clayton
A, Bowen T, Phillips AO and Steadman R: Transforming growth
factor-β1 (TGF-β1)-stimulated fibroblast to myofibroblast
differentiation is mediated by hyaluronan (HA)-facilitated
epidermal growth factor receptor (EGFR) and CD44 co-localization in
lipid rafts. J Biol Chem. 288:14824–14838. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nishi H, Maeda N, Izumi S, Higa-Nakamine
S, Toku S, Kakinohana M, Sugahara K and Yamamoto H: Differential
regulation of epidermal growth factor receptor by hydrogen peroxide
and flagellin in cultured lung alveolar epithelial cells. Eur J
Pharmacol. 748:133–142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ulu N, Henning RH, Guner S, Zoto T,
Duman-Dalkilic B, Duin M and Gurdal H: Intracellular
transactivation of epidermal growth factor receptor by
α1A-adrenoceptor is mediated by phosphatidylinositol 3-kinase
independently of activation of extracellular signal regulated
kinases 1/2 and serine-threonine kinases in Chinese hamster ovary
cells. J Pharmacol Exp Ther. 347:47–56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ginnan R, Pfleiderer PJ, Pumiglia K and
Singer HA: PKC-delta and CaMKII-delta 2 mediate ATP-dependent
activation of ERK1/2 in vascular smooth muscle. Am J Physiol Cell
Physiol. 286:C1281–C1289. 2004. View Article : Google Scholar : PubMed/NCBI
|