Introduction
In 2012, there were 782,500 new cases of
hepatocellular carcinoma (HCC) and >745,500 liver
cancer-associated mortalities (1).
Liver cancer is the second most common cause of cancer-associated
mortality worldwide and has a poor prognosis (mortality/incidence
ratio, 0.95) (1). There are a variety
of treatment guidelines for liver cancer depending on tumor stage
(2–4),
and at present, liver resection and localized treatment
[percutaneous ethanol injection surgery or radiofrequency ablation
(RFA)] are recommended as curative and localized treatments for
early-stage liver cancer (2–4). However, a number of patients harbor
chronic hepatitis B and C viral infections or cirrhosis in addition
to liver cancer (5). In these
patients, who have decreased liver function, less invasive and more
effective early treatment of liver cancer may be beneficial.
Surgical resection is considered to be the only potentially
curative therapy for HCC (6), but it
is a highly invasive procedure. In contrast, the less-invasive
percutaneous RFA is a standardized and widely used treatment
method, which has equal efficacy to liver resection in terms of
localized control (7). However, RFA
monotherapy may increase the risk of relapse in cases where the HCC
is comparatively large, when it exists near the surface of the
liver or near vessels that are hard to treat due to the risk of
coagulation necrosis (8). A variety
of methods, including imaging support such as Real-Time Virtual
Sonography (9), have been devised in
the past to counteract these difficulties.
Transarterial chemoembolization (TACE) is often used
prior to RFA in the treatment of early-phase HCC (10). However, the rate of local and ectopic
recurrence and the long-term effect on prognosis have not been
adequately investigated for this combination. The present study
examined the long-term effectiveness of RFA/TACE compared with RFA
alone.
Materials and methods
Patients
The present, retrospective study initially enrolled
192 patients, the median age was 72.0 years old (range 45–91 years
old) and 47.7% were female, with a total of 283 HCC tumors treated
with RFA between April 2007 and August 2014 at Kagoshima University
Medical and Dental Hospital (Kagoshima, Japan), Kagoshima Teishin
Hospital (Kagoshima, Japan) and Kagoshima City Hospital (Kagoshima,
Japan). Among these patients, 83 patients who met the following
inclusion criteria were selected for final analysis: Solitary HCC
nodules ≤30 mm in diameter; strong contrast compared with
surrounding liver parenchyma in early-phase dynamic
contrast-enhanced computed tomography (CT) and low-density areas in
the late phase; no imaging evidence of tumor invasion into the
major portal or hepatic vein branches; no extrahepatic metastasis;
a platelet count of >30,000 ×104/µl; and
post-treatment observation for ≥3 months.
The informed consent was waived due to the
retrospective nature of the study. The study protocol conformed to
the ethical guidelines of the World Medical Association Declaration
of Helsinki and was approved by the Ethics Committees of Kagoshima
University Medical and Dental Hospital, Kagoshima Teishin Hospital
and Kagoshima City Hospital (Kagoshima, Japan).
Diagnosis of HCC
In all patients, HCC was diagnosed based on typical
results by two or more imaging modalities [ultrasonography (US), CT
and magnetic resonance imaging (MRI)] and characteristic serum
levels of α-fetoprotein (AFP) and des-γ-carboxy prothrombin (DCP;
also termed PIVKA-II). Abdominal US was performed with a real-time
scanner using a 3.5-MHz transducer (HI VISION 900S; Hitachi, Ltd.,
Tokyo, Japan). The US diagnosis of HCC was based on the presence of
lesions with different echogenicity (hypoechoic, hyperechoic,
isoechoic or a mixed pattern) compared with the surrounding liver
parenchyma. Dynamic CT was performed with a multi-detector row
scanner (Aquilion PRIME; Toshiba Medical Systems Corporation,
Tokyo, Japan). Non-enhanced CT scans were obtained first, followed
by quadra-phase contiguous CT scans with 5 mm-thick sections. A
bolus injection of 100 ml 65% iopamidol (Iomeron 350; Eisai Co.,
Ltd., Tokyo, Japan) was then administered at a rate of 3 ml/sec.
Arterial-phase CT scans were obtained at 30 sec, portal-phase CT
scans were obtained at 60 sec and late-phase CT scans were obtained
at 90–120 sec. A radiologist diagnosed the CT results. The CT
diagnosis of HCC was based on the presence of an enhancing lesion
on arterial-phase CT scans and hypoattenuation on late-phase CT
scans. AFP and DCP were performed within the week prior to RFA.
Normal limits were defined as <10 ng/ml for AFP and <40
mAU/ml for DCP. The term ‘early HCC’ has two meanings, namely
clinical early HCC and histopathological early HCC. However, in the
present study it was used to mean histopathological early HCC. The
Barcelona Clinic Liver Cancer (BCLC) staging system was followed
(11,12), which is commonly used in the United
States and Europe, and early HCC was diagnosed as presenting with
hypervascularity in the arterial phase by contrast-enhanced CT and
classification as single-site Stage A (early stage). Patients with
multiple existing HCCs and single-site lesions >5 cm were
excluded, according to guidelines on The Japan Society of
Hepatology (4).
Treatment protocol
The treatment selection of RFA/TACE (with TACE
performed prior to RFA) or RFA monotherapy was performed by
specialists in HCC treatment (such as RFA and TACE) at Kagoshima
University Medical and Dental Hospital, Kagoshima Teishin Hospital
and Kagoshima City Hospital, according to the age, performance
status (PS), liver function, tumor size and tumor location of
patients.
TACE and RFA combination therapy
In the RFA/TACE group, TACE was first performed
using the Seldinger technique (13)
according to the following protocol. Subsequent to introducing a
3.5- or 4-Fr long sheath (Medikit Super Sheath; Medikit Co., Ltd.,
Tokyo, Japan) into the femoral artery, a 3.5- or 4-Fr pre-shaped
catheter (Selecon-PA Catheter; Terumo Clinical Supply Co., Ltd.,
Gifu, Japan) was inserted into a superior mesenteric artery and
30–40 ml 50% iopamidol (Iomeron 350; Eisai Co., Ltd.) was injected.
Computed tomography during arterial-portography (CTAP) was
performed to determine whether there were one or more HCC lesions,
and to assess the patency of the portal vein. Computed tomography
during arteriography (CTA) was then performed to detect HCC; 15–20
ml of 50% iopamidol was injected via the same catheter placed in a
common hepatic artery. In addition, a 2-Fr microcatheter was
selectively placed in the tumor-bearing artery of the HCC (nutrient
artery), and an emulsified formulation of iodized oil (Lipiodol;
Laboratoire Guerbet, AulnaySous-Bois, France) was injected along
with the following three anticancer agents: 20 mg epirubicin
hydrochloride (Farmorubicin; Pfizer Japan, Inc., Tokyo, Japan) and
4 mg mitomycin C (Kyowa Hakko Kirin Co., Ltd., Tokyo, Japan);
miriplatin hydrate (Miripla; Dainippon Sumitomo Pharma Co., Ltd.,
Tokyo, Japan); and cisplatin (Nihon-Kayaku Co., Ltd., Tokyo,
Japan). Following injection of the emulsified formulation, gelatin
sponge particles (Gelpart; Nippon Kayaku, Tokyo, Japan) were
injected as an embolus into the same location. Hepatic
arteriography was performed following the embolus injection to
confirm the loss of blood flow to the tumor through the nutrient
artery prior to performing the surgery. The timing of RFA following
TACE varied according to the onset of side effects, overall patient
condition and degree of post-operative liver dysfunction, but
usually occurred within the week following TACE. RFA was performed
by locally anesthetizing the injection site using 1% xylocaine
(AstraZeneca, Tokyo, Japan) and the liver surface was assessed by
ultrasound. A 17-G internally-cooled electrode with a 2 or 3 cm
exposed tip (Radionics, Inc., Burlington, MA, USA) was then guided
to the HCC via ultrasound for the ablation. An abdominal CT was
performed 3–4 days post-RFA and the RFA treatment effect, in
particular the tumor and cauterization margins, was evaluated.
Treatment response was evaluated by dynamic CT within 1 week. When
HCC remained evident, additional ablation was performed. RFA was
performed so that margins of ≥5 mm were obtained in all patients,
and additional RFA was performed where possible when the ablation
area was insufficient. Patients with margins of 0–5 mm were
classified into those where it was possible to widen the ablation
area with additional RFA and those for whom additional RFA would be
difficult due to the radiator effect of surrounding blood vessels
or the original location of the lesion. For patients where
additional RFA was not expected to return benefits, follow-up
observations were performed. Patients with almost complete ablation
with certain lesions with margins <5 mm were included in the 0–5
mm margin group. All patients underwent two sessions or fewer.
RFA alone
RFA monotherapy was conducted within 1 week of
hospital admission using the exact procedure aforementioned. As
with the combined therapy, an abdominal CT was performed 3–4 days
post-RFA and the tumor and cauterization margins were evaluated
along with the RFA treatment effect. In the RFA alone group,
well-differentiated HCC was excluded, as well as HCC of other sites
confirmed by prior abdominal ultrasonography,
gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-MRI,
CTAP or CTA.
Clinical characteristics and
laboratory markers of patients
The clinical characteristics and laboratory markers
of patients assessed included age, sex, tumor size, observation
period, number of RFA sessions, TACE, RFA/TACE, previous treatment
and virus markers, including hepatitis B virus (HBV), hepatitis C
virus (HCV) and NBNC (HBV− and HCV−). Hepatic
function was assessed using the Child-Pugh classification (14) based on clinical (ascites and
encephalopathy) and laboratory (serum albumin, total bilirubin and
prothrombin time) parameters, body mass index, aspartate
transaminase (AST), alanine aminotransferase (ALT), γ-glutamyl
transpeptidase (γ-GTP), serum albumin, total bilirubin, prothrombin
time, platelets, AFP and DCP.
Comparison
Overall survival (OS) rates were compared using the
time from the beginning of treatment to the last follow-up CT
examination or mortality. RFA of a single HCC may still result in
multiple recurrences and progression to the intermediate stage
during follow-up. Post-treatment TACE may be performed for
intermediate-stage HCC, but this was not confirmed in the present
study. Local recurrence was defined as the presence of one or more
recurrent lesions within the RFA-ablated area. A patient who
presented with HCC adjacent to the site of ablation after several
years was excluded, and was not classified as having local
recurrence. Local recurrence rates in each nodule were compared
using the time from the beginning of treatment to the last
follow-up CT examination. The same period was used to compare the
intrahepatic distant recurrence rate, including multicentric
occurrences and intrahepatic metastases in each nodule. Comparison
of tumor-free survival rates among patients was conducted using the
time from the beginning of treatment to local tumor progression,
progression of other tumors at the last follow-up CT examination or
mortality. Naïve and recurrent patients were examined together in
the present study, since the two types of patients exhibited
similar trends (data not shown). The present study included
patients with early HCC, defined at the time of treatment based on
the earliest stage according to each of the following
classification systems: Tumor-node-metastasis stage (15); Child-Pugh grade (14); Japan integrated staging score
(4); and the Cancer of the Liver
Italian Program score (16). There
were also a number of confounding factors, including tumor size,
number and presence of extrahepatic metastasis. Thus, prognostic
factors were not analyzed using these items.
Statistical analysis
Statistical analyses were performed using the
χ2-test or the Mann-Whitney U test, as appropriate. The
Kaplan-Meier method was used to estimate cumulative survival and
progression of local and other tumors, and these distribution
curves were compared using the log-rank test. Univariate and
multivariate analyses of patient outcome risk ratios were performed
using Cox's proportional hazards model. All statistical analyses
were conducted using IBM SPSS Statistics version 21.0 (IBM SPSS,
Armonk, NY, USA). Results were expressed as the median, with
minimum and maximum values. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of patients
A total of 83 patients met the aforementioned
inclusion criteria. Table I
summarizes the baseline clinical characteristics of the 83 patients
with early, solitary and hypervascular HCC.
 | Table I.Clinical characteristics of patients
with early, solitary and hypervascular hepatocellular
carcinoma. |
Table I.
Clinical characteristics of patients
with early, solitary and hypervascular hepatocellular
carcinoma.
| Characteristic | Number of
patients/mean ± SD |
|---|
| Age, years | 71.6±9.2 |
| Sex,
male/female | 41/42 |
| Tumor size, mm | 17.2±5.5 |
| Observation period,
months | 17.2±5.5 |
| Number of RFA
sessions |
1.2±0.4 |
| RFA/TACE, −/+ | 27/56 |
| Previous treatment,
−/+ | 41/42 |
| Virus marker,
HBV/HCV/NBNC | 7/60/16 |
| Child-Pugh
classification, A/B/C | 73/10/0 |
| BMI,
kg/m2 | 24.1±3.4 |
| Biochemical
analysis |
|
| AST, IU/l |
49.9±21.2 |
| ALT, IU/l | 39.8±22 |
| γ-GTP, IU/l | 24.1±3.4 |
| Serum albumin,
g/dl |
3.6±0.5 |
| Total bilirubin,
mg/dl |
1.1±0.5 |
| Prothrombin time,
% |
81.5±14.5 |
| Platelets,
×104/µl | 10.2±4.8 |
| AFP, ng/ml | 277.7±1,289.9 |
| DCP, mAU/ml | 419.6±1,474.4 |
Rate of OS, local recurrence and
tumor-free survival (TFS) in all 83 patients
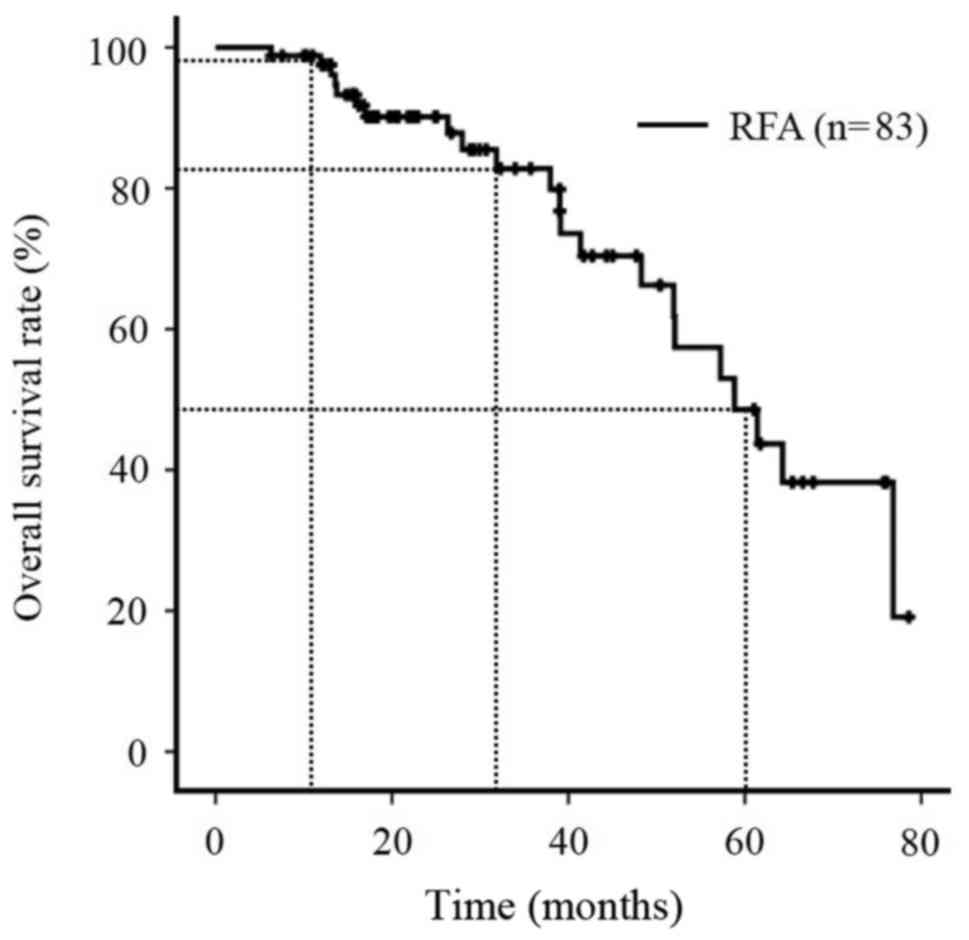
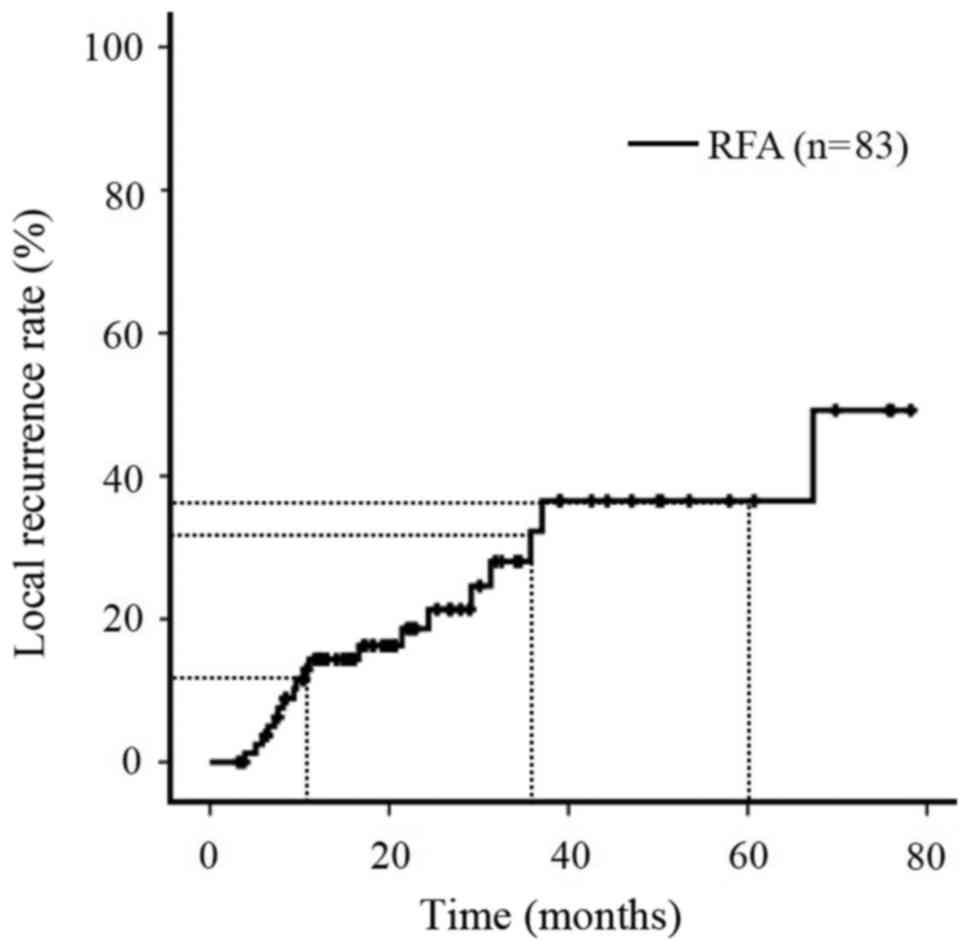
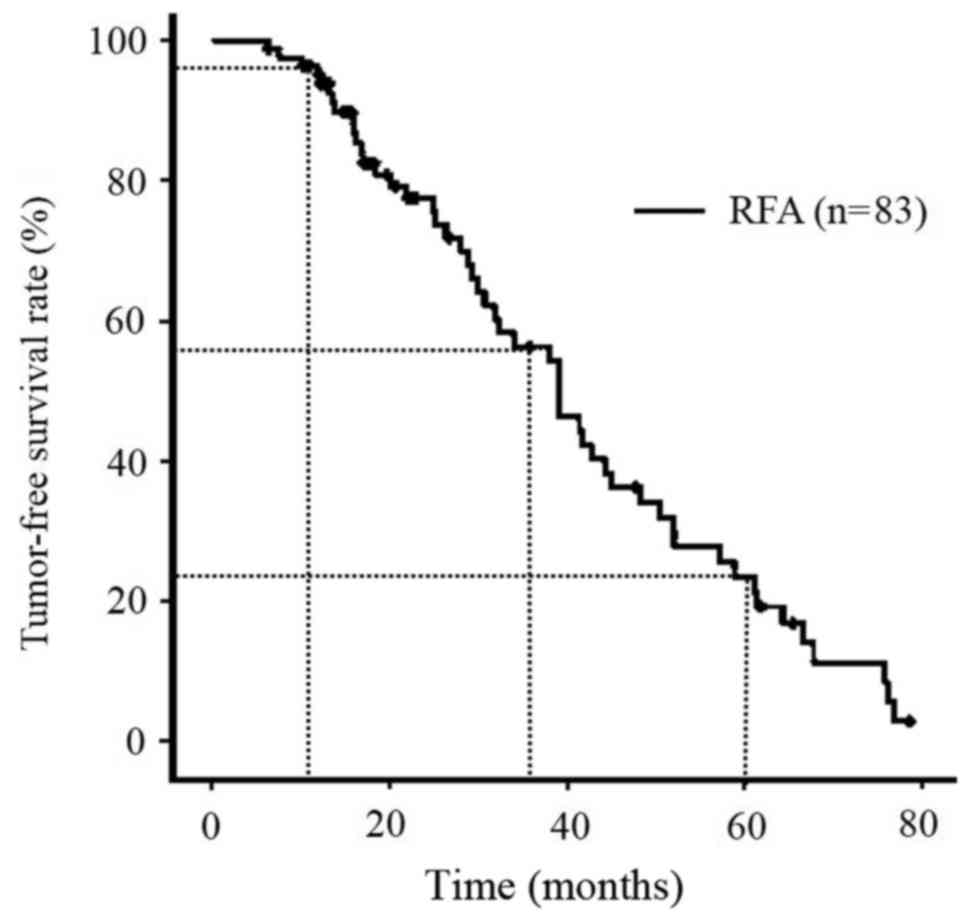
The OS rates of all patients during the follow-up
period were 97.5% at 1 year, 82.8% at 3 years and 48.6% at 5 years
(Fig. 1), and the local recurrence
rates of all patients (n=83) were 14.3% at 1 year, 32.3% at 3 years
and 36.5% at 5 years (Fig. 2). The
TFS rates of all patients (n=83) were 95.1% at 1 year, 56.3% at 3
years and 23.4% at 5 years (Fig.
3).
RFA/TACE compared with RFA alone
A total of 56 patients with 56 HCC nodules were
treated with RFA/TACE, while 27 patients with 27 HCC nodules were
treated with RFA alone. Table II
summarizes the following baseline clinical characteristics of the
two groups with early, solitary and hypervascular HCC, stratified
by treatment categories: Age; sex; virus markers, including HBV,
HCV and NBNC; clinical laboratory parameters, including prothrombin
time, bilirubin, serum albumin, AST, ALT, γ-GTP, platelets, AFP and
DCP; previous treatment; intrahepatic recurrence; tumor size and
ablated size. A significant difference was observed only in tumor
size (P=0.004; Table II). No further
statistically significant differences were observed between the two
groups.
 | Table II.Clinical characteristics of 83
patients with early, solitary and hypervascular hepatocellular
carcinoma. |
Table II.
Clinical characteristics of 83
patients with early, solitary and hypervascular hepatocellular
carcinoma.
| Factor | RFA/TACE group,
n | RFA alone group,
n | P-value |
|---|
| Total no. of
patients | 56 | 27 |
|
| Age, years | 73.0 (45–91) | 73.0 (57–89) | 0.218 |
| Sex,
male/female | 30/26 | 11/16 | 0.350 |
| Virus marker,
HBV/HCV/NBNC | 6/39/11 | 1/21/5 | 0.538 |
| Prothrombin time,
% | 82.8 (35–110) | 83.4 (51–101) | 0.857 |
| Total bilirubin,
mg/dl | 0.9 (0.3–2.6) | 1.1 (0.4–2.5) | 0.205 |
| Serum albumin,
g/dl | 3.6 (2.8–4.8) | 3.6 (2.4–4.9) | 0.934 |
| AST, IU/l | 44.0 (19–110) | 45.0 (17–84) | 0.613 |
| ALT, IU/l | 35.0 (13–116) | 31.0 (9–84) | 0.644 |
| γ-GTP, IU l | 81.7
(34.7–110) | 83.4 (51–101) | 0.166 |
| Platelet count,
×104/µl | 9.5 (3.3–27.6) | 9.3 (3.1–19.8) | 0.637 |
| AFP, ng/ml | 13.7
(1.5–7,931) | 13.4
(3.6–8,609) | 0.996 |
| DCP, mAU/ml | 33.5
(12–9,455) | 33.0
(8.0–2,314) | 0.656 |
| Previous treatment,
−/+ | 29/27 | 12/15 | 0.641 |
| Intrahepatic
recurrence, −/+ | 29/27 | 12/15 | 0.641 |
| Tumor size, mm | 17.8 (10–30) | 13.3 (6–30) | 0.004 |
| Ablated size,
mm | 31.0 (19–52) | 28.5
(20–48) | 0.527 |
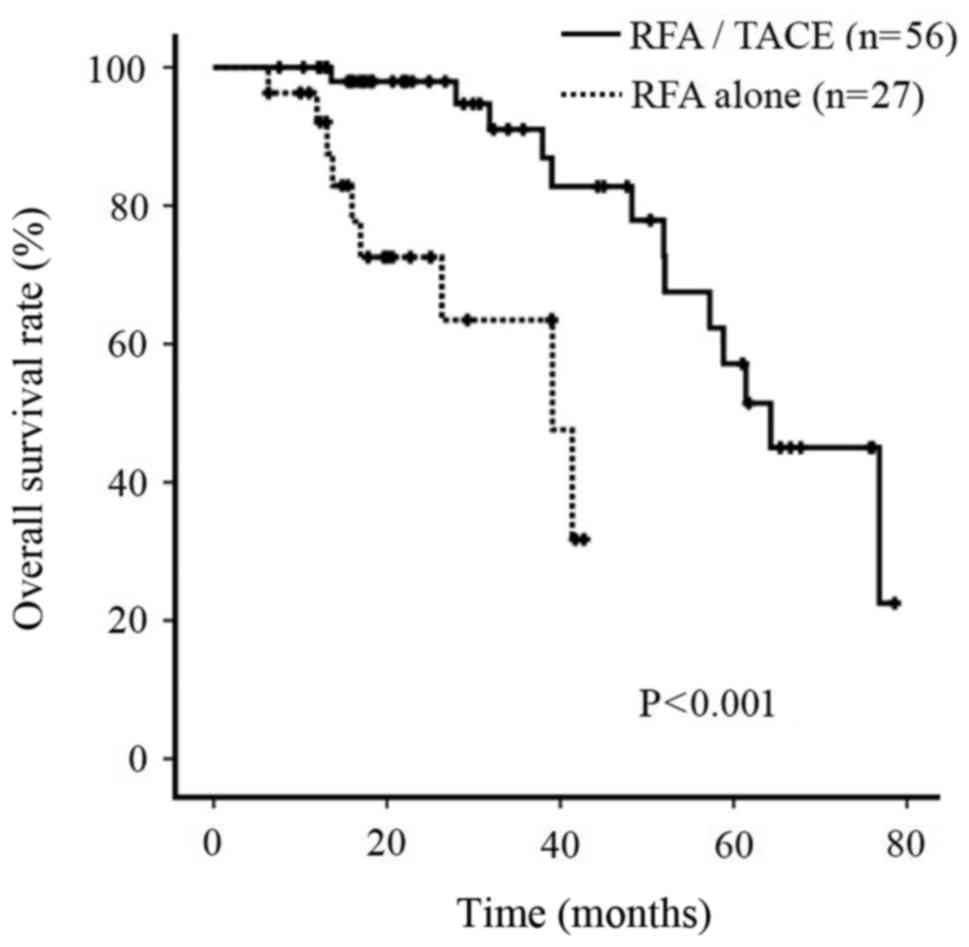
OS rate
During the follow-up period, the cumulative survival
rate of patients treated with RFA/TACE was significantly improved
compared with that of patients treated with RFA alone (P<0.001;
Fig. 4).
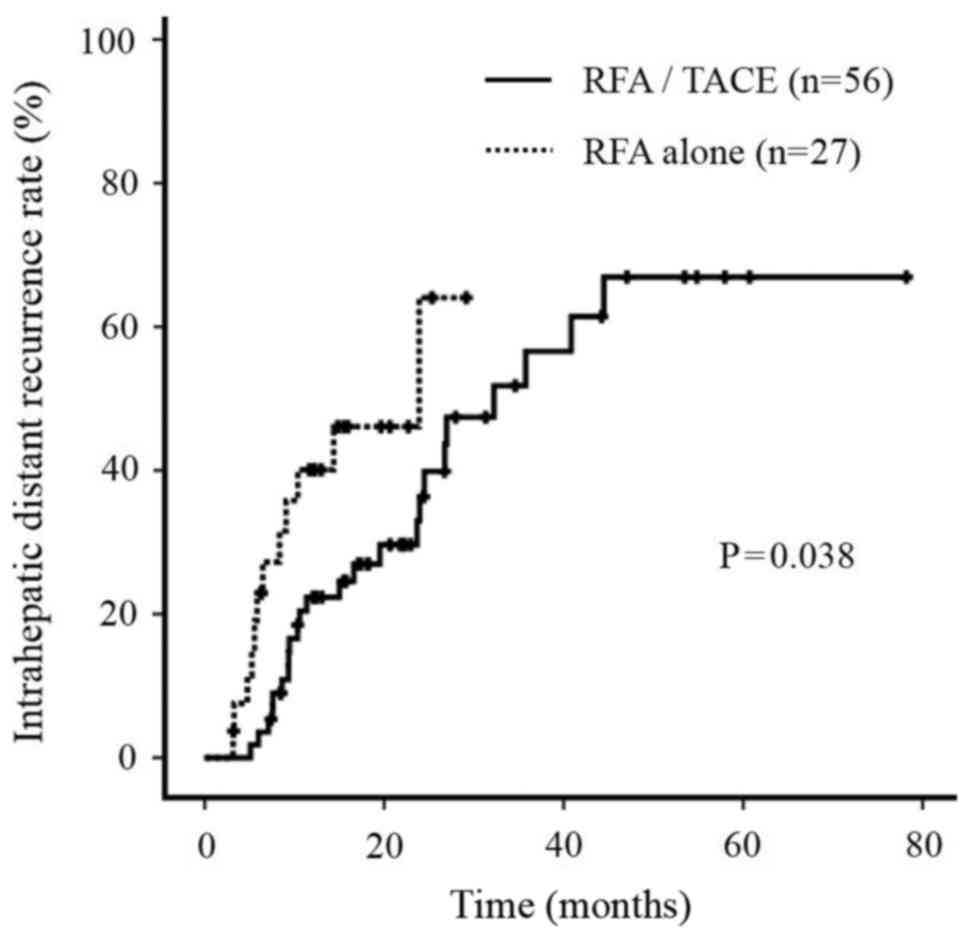
Intrahepatic distant recurrence
rate
During the follow-up period, the intrahepatic
distant recurrence rate in patients treated with RFA/TACE was
significantly improved compared with patients treated with RFA
alone (P=0.038; Fig. 5). No
significant differences were observed in the site of recurrence
between the two groups (data not shown).
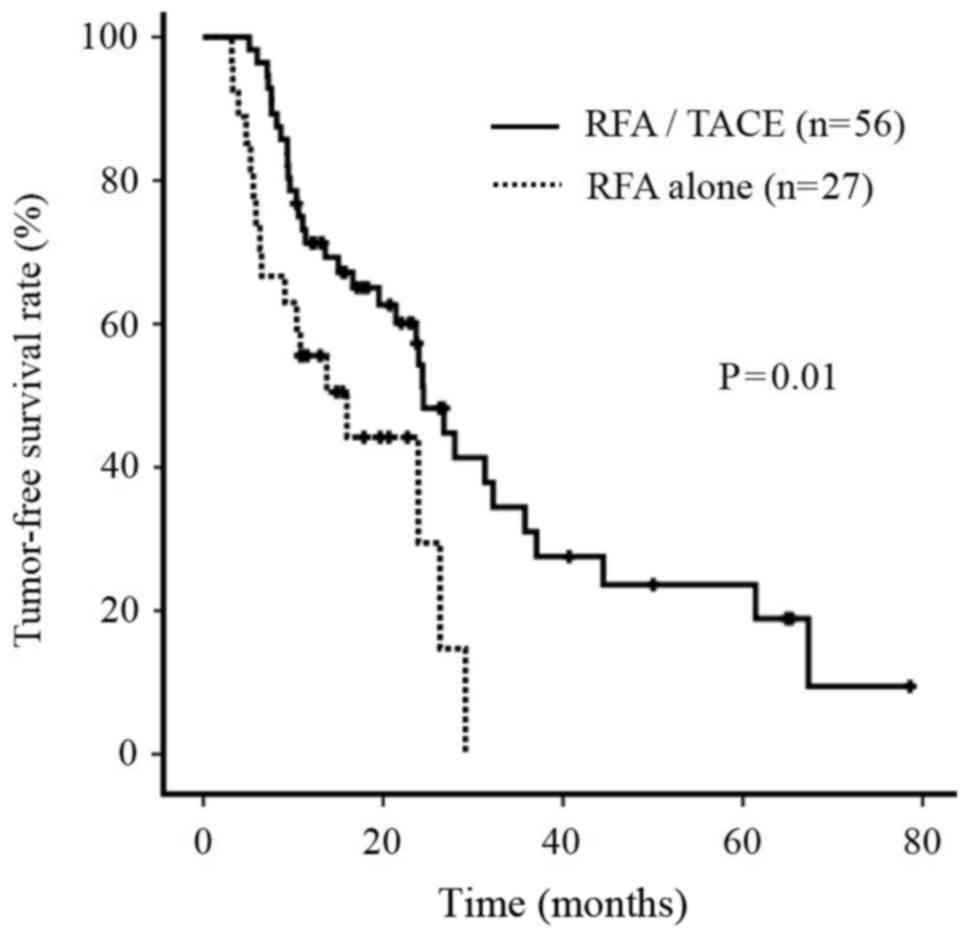
TFS rate
During the follow-up period, the TFS rate of
patients treated with RFA/TACE was significantly improved compared
with that of patients treated with RFA alone (P=0.01; Fig. 6).
Univariate analysis and multivariate
statistics for OS rate
A univariate analysis using the log-rank test
revealed that the survival rate varied significantly with age, AST,
ALT, platelet count (Plt) and RFA/TACE (Table III). Multivariate analysis using a
Cox proportional hazard model for four of these markers (AST, ALT,
Plt and RFA/TACE), as well as age and sex, revealed that RFA/TACE
was the only independent risk factor associated with good patient
prognosis [hazard ratio, 0.108; 95% confidential interval (CI),
0.029–0.401; P=0.001; Table
III].
 | Table III.Evaluation of the prognostic factors
in the early, solitary and hypervascular hepatocellular carcinoma
cases. |
Table III.
Evaluation of the prognostic factors
in the early, solitary and hypervascular hepatocellular carcinoma
cases.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Factor
(categories) | n=83 | P-value | HR | (95% CI) | P-value |
|---|
| Age (<70/≥70
years) | 28/55 | 0.008 | 0.459 | 0.162–1.302 | 0.143 |
| Sex
(male/female) | 41/42 | 0.601 | 0.601 | 0.213–1.700 | 0.337 |
| Total bilirubin,
mg/dl (<1.0/≥1.0) | 37/46 | 0.066 |
|
|
|
| AST, IU/l
(<50/≥50) | 49/34 | 0.003 |
|
|
|
| ALT, IU/l
(<40/≥40) | 48/35 | 0.006 |
|
|
|
| γ-GTP, IU/l
(<45/≥45) | 54/29 | 0.162 |
|
|
|
| Serum albumin, g/dl
(<3.6/≥3.6) | 47/36 | 0.102 |
|
|
|
| Platelet count,
×104/µl (<10/≥10) | 47/36 | 0.05 |
|
|
|
| Prothrombin time, %
(<85/≥85) | 48/35 | 0.134 |
|
|
|
| AFP, ng/ml
(<15/≥15) | 43/40 | 0.291 |
|
|
|
| DCP, mAU/ml
(<35/≥35) | 44/39 | 0.400 |
|
|
|
| Tumor size, mm
(<20/≥20) | 54/29 | 0.064 |
|
|
|
| Ablated margin, mm
(<5/≥5) | 59/24 | 0.496 |
|
|
|
| RFA/TACE (−/+) | 27/56 | <0.001 | 0.108 | 0.029–0.401 | 0.001 |
Univariate analysis and multivariate
statistics for intrahepatic distant recurrence and TFS rate
A univariate analysis using the log-rank test
revealed that the intrahepatic distant recurrence and TFS rate were
only positively associated with RFA/TACE (data not shown). A
multivariate analysis using a Cox proportional hazard model,
including age and sex, for six markers (ALT, γ-GTP, Plt, DCP,
ablation margin and RFA/TACE) selected based on P<0.500 in
univariate analysis, revealed that RFA/TACE was the only
independent risk factor associated with intrahepatic distant
recurrence factors (odds ratio, 0.467; 95% CI, 0.225–0.973;
P=0.042; Table IV) and for seven
markers (total bilirubin, AST, ALT, γ-GTP, tumor size, ablated
margin and RFA/TACE) selected based on P<0.500 in univariate
analysis, revealed that RFA/TACE was the only independent risk
factor associated with tumor-free survival factors (odds ratio,
0.452; 95% CI, 0.224–0.842; P=0.012; Table IV).
 | Table IV.Multivariate statistics for the
intrahepatic distant recurrence and tumor-free survival factors in
the early, solitary and hypervascular hepatocellular carcinoma
cases. |
Table IV.
Multivariate statistics for the
intrahepatic distant recurrence and tumor-free survival factors in
the early, solitary and hypervascular hepatocellular carcinoma
cases.
| RFA/TACE (−/+) | OR | 95% CI | P-value |
|---|
| The intrahepatic
distant recurrence | 0.467 | 0.225–0.973 | 0.042 |
| The tumor-free
survival | 0.452 | 0.224–0.842 | 0.012 |
Discussion
In the treatment algorithm for HCC, based on the
Japan Society of Hepatology consensus, liver resection or localized
therapy is recommended based on remaining liver function (4). In contrast, in the treatment algorithm
based on the BCLC staging system, solitary HCCs <2 cm in
diameter were classified as very early stage (stage 0) (3). The 5-year OS rate of patients undergoing
liver resection and liver transplant was reported as 80–90%, while
it was 70% in those undergoing localized ablation (6,17,18). In addition, patients with single
tumors >2 cm or three nodules <3 cm in diameter were
classified as early HCC (BCLC stage A) with a 5 year OS rate of
50–70% for liver resection, liver transplant and localized ablation
(16,19). Although this was a retrospective
study, to reduce bias, only subjects suspected of having
moderately-differentiated liver cancer identified via imaging as
single hypervascular tumors were included. As a result, patients
with single HCC tumors <3 cm that exhibited early-phase staining
were enrolled, and it was demonstrated that the rates of overall
and tumor-free survival were not inferior to previous studies
(20). In addition, results were
obtained during long-term follow-up of over 5 years. In terms of
TFS, the 5-year survival rate was slightly low at 23.4%; however,
multiple patients with HCC also had concomitant HCV infections.
Therefore, even if local factors were controlled, there may still
have been patients who relapsed.
At Kagoshima University Medical and Dental Hospital,
Kagoshima Teishin Hospital and Kagoshima City Hospital, RFA is
often performed as a curative therapy following TACE in the
tumor-bearing area. The main reasons for recommending RFA/TACE are
as follows: When performing TACE in the tumor-bearing area, an
antitumor effect is expected in the primary lesion as well as the
surrounding area; lipiodol accumulates in the tumor, serving as a
marker when performing the RFA, and the post-treatment
identification of the ablation area is easier (21); expansion of the ablation area is
expected in areas in which lipiodol accumulates following TACE,
making it appropriate for slightly larger HCC (22); and the combination of TACE and RFA
results in improved local control even when lesions occur on the
surface of the liver or near blood vessels.
Multiple studies have been conducted on RFA/TACE,
and a systematic review (23)
performed a meta-analysis of eight clinical trials (24–30).
However, the majority of these studies examined HCC tumors >3 cm
in diameter, and only one examined small HCCs (28). The meta-analysis revealed that rate of
local tumor progression, OS, local progression-free survival and
event-free survival were not significantly different between the
combination therapy and RFA alone. There are no details regarding
the inclusion criteria aside from the fact that the analyzed trials
enrolled patients with HCC with three tumors that were <3 cm in
diameter, which differs from the present study. Kim et al
(10) also published retrospective
data, but they reported on a small number of early-stage HCCs of
2–3 cm diameter. Furthermore, although RFA/TACE demonstrated a
higher rate of local progression-free survival and event-free
survival compared with RFA alone, no significant difference in OS
was observed, which contradicts the results of the present study.
Finally, multivariate analysis was not performed in this previous
study, and therefore it is possible to consider the present study
as the first to demonstrate that RFA/TACE is an independent
determining factor of prognosis and relapse.
Nakashima et al (31) investigated 209 nodules <3 cm in
diameter that were surgically resected, and revealed that ‘single
nodular type with extranodular growth’ and ‘confluent multinodular
type’ demonstrated higher frequencies of portal vein invasion and
intrahepatic metastases compared with ‘single nodular type’. In
addition, they reported that among 149 metastatic lesions, the
distance from the primary tumor was ≤10 mm in 118 (79.2%) cases.
Furthermore, Nishikawa et al (21) proposed a method for grading HCC based
on ablative margins and its use in predicting local recurrence. For
Grade A (absolutely curative) tumors the ablative margin around the
tumor was >5 mm, for Grade B (relatively curative) the margin
was <5 mm, for Grade C (relatively non-curative) there was no
complete ablative margin although no residual tumor was apparent,
and for Grade D (absolutely non-curative) the tumor had not been
entirely ablated. The cumulative localized rate of recurrence was
significantly improved for Grades A and B compared with Grades C
and D, and it was extremely important that the ablation range
(cauterization margin) was adequately achieved by RFA. In the
present study, the average tumor size was larger in the RFA/TACE
group compared with the RFA-only group. In addition, multiple
patients had inadequate tumor and ablation margins following RFA
alone. Despite these drawbacks, favorable results for cumulative
survival rate, intrahepatic tumor progression rate and tumor-free
survival were obtained, indicating that even small HCCs <3 cm in
diameter possess microscopic disseminated disease, depending on
gross morphology. Patients with cirrhosis commonly have fine
arterioportal shunts and hepatofugal blood flow (32). Anticancer drugs administered during
TACE are thought to be washed out immediately, except for Miripla
(33), but these drugs are likely to
be carried in blood that flows outside subsegments or the entire
liver, via the same hemodynamic route used in the dissemination of
liver cancer cells. Anticancer drugs may suppress recurrence of
micro-level dissemination of cells during TACE that cannot be
detected at the macro level (34,35). These
observations supported the idea that treatment with TACE in the
tumor-bearing zone not only affects the primary lesion, but also
suppresses local and ectopic recurrences.
There are several limitations to the present study.
First, it included a small number of patients. Second, there may be
slight biases in treatment approaches and patient selection due to
this being a multicenter study. Third, the study design was not a
prospective randomized controlled trial. Fourth, propensity
matching was not performed for either group despite the
retrospective nature of the study. Tumor size was significantly
larger in patients treated with RFA/TACE, which was an analytical
disadvantage; however, no significant differences in baseline
characteristics of patients were observed. Propensity matching may
have eliminated the biases between the groups, but would have
reduced the usable patient population, making adequate analysis
difficult. Furthermore, for more rigorous analysis, a randomized
controlled trial is desirable.
In conclusion, the present study revealed that
treatment with RFA/TACE improved prognosis, the rate of
intrahepatic recurrence and tumor-free survival compared with RFA
alone. The present study therefore demonstrated that RFA/TACE is
effective in patients with small HCC. However, a large-scale
randomized controlled trial is required to compare the results with
those obtained from therapy using RFA alone.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bruix J and Sherman M; American
Association for the Study of Liver Diseases, : Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
European Association For The Study Of The
Liver; European Organisation For Research And Treatment Of Cancer:
EASL-EORTC clinical practice guidelines: Management of
hepatocellular carcinoma. J Hepatol. 56:908–943. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kudo M, Matsui O, Izumi N, Iijima H,
Kadoya M, Imai Y, Okusaka T, Miyayama S, Tsuchiya K, Ueshima K, et
al: JSH consensus-based clinical practice guidelines for the
management of hepatocellular carcinoma: 2014 Update by the Liver
Cancer Study Group of Japan. Liver Cancer. 3:458–468. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lafaro KJ, Demirjian AN and Pawlik TM:
Epidemiology of hepatocellular carcinoma. Surg Oncol Clin N Am.
24:1–17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takayama T, Makuuchi M, Hirohashi S,
Sakamoto M, Yamamoto J, Shimada K, Kosuge T, Okada S, Takayasu K
and Yamasaki S: Early hepatocellular carcinoma as an entity with a
high rate of surgical cure. Hepatology. 28:1241–1246. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hong SN, Lee SY, Choi MS, Lee JH, Koh KC,
Paik SW, Yoo BC, Rhee JC, Choi D, Lim HK, et al: Comparing the
outcomes of radiofrequency ablation and surgery in patients with a
single small hepatocellular carcinoma and well-preserved hepatic
function. J Clin Gastroenterol. 39:247–252. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ueno M, Hayami S, Shigekawa Y, Kawai M,
Hirono S, Okada K, Tamai H, Shingaki N, Mori Y, Ichinose M and
Yamaue H: Prognostic impact of surgery and radiofrequency ablation
on single nodular HCC 5 cm: Cohort study based on serum HCC
markers. J Hepatol. 63:1352–1359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakai M, Sato M, Sahara S, Takasaka I,
Kawai N, Minamiguchi H, Tanihata H, Kimura M and Takeuchi N:
Radiofrequency ablation assisted by real-time virtual sonography
and CT for hepatocellular carcinoma undetectable by conventional
sonography. Cardiovasc Intervent Radiol. 32:62–69. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim JW, Kim JH, Won HJ, Shin YM, Yoon HK,
Sung KB and Kim PN: Hepatocellular carcinomas 2–3 cm in diameter:
Transarterial chemoembolization plus radiofrequency ablation vs.
Radiofrequency ablation alone. Eur J Radiol. 81:e189–e193. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Llovet JM, Brú C and Bruix J: Prognosis of
hepatocellular carcinoma: The BCLC staging classification. Semin
Liver Dis. 19:329–338. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Llovet JM, Di Bisceglie AM, Bruix J,
Kramer BS, Lencioni R, Zhu AX, Sherman M, Schwartz M, Lotze M,
Talwalkar J, et al: Design and endpoints of clinical trials in
hepatocellular carcinoma. J Natl Cancer Inst. 100:698–711. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seldinger SI: Catheter replacement of the
needle in percutaneous arteriography; a new technique. Acta Radiol.
39:368–376. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Albers I, Hartmann H, Bircher J and
Creutzfeldt W: Superiority of the Child-Pugh classification to
quantitative liver function tests for assessing prognosis of liver
cirrhosis. Scand J Gastroenterol. 24:269–276. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Novák J and Fabian P: Comments on the TNM
classification of malignant tumours-7th edition. Klin Onkol.
24:149–150. 2011.(In Czech). PubMed/NCBI
|
|
16
|
Llovet JM and Bruix J: Novel advancements
in the management of hepatocellular carcinoma in 2008. J Hepatol.
48 Suppl 1:S20–S37. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roayaie S, Blume IN, Thung SN, Guido M,
Fiel MI, Hiotis S, Labow DM, Llovet JM and Schwartz ME: A system of
classifying microvascular invasion to predict outcome after
resection in patients with hepatocellular carcinoma.
Gastroenterology. 137:850–855. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livraghi T, Meloni F, Di Stasi M, Rolle E,
Solbiati L, Tinelli C and Rossi S: Sustained complete response and
complications rates after radiofrequency ablation of very early
hepatocellular carcinoma in cirrhosis: Is resection still the
treatment of choice? Hepatology. 47:82–89. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arii S, Yamaoka Y, Futagawa S, Inoue K,
Kobayashi K, Kojiro M, Makuuchi M, Nakamura Y, Okita K and Yamada
R: Results of surgical and nonsurgical treatment for small-sized
hepatocellular carcinomas: A retrospective and nationwide survey in
Japan. The Liver Cancer Study Group of Japan. Hepatology.
32:1224–1229. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Waki K, Aikata H, Katamura Y, Kawaoka T,
Takaki S, Hiramatsu A, Takahashi S, Toyota N, Ito K and Chayama K:
Percutaneous radiofrequency ablation as first-line treatment for
small hepatocellular carcinoma: results and prognostic factors on
long-term follow up. J Gastroenterol Hepatol. 25:597–604. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nishikawa H, Inuzuka T, Takeda H, Nakajima
J, Sakamoto A, Henmi S, Matsuda F, Eso Y, Ishikawa T, Saito S, et
al: Percutaneous radiofrequency ablation therapy for hepatocellular
carcinoma: A proposed new grading system for the ablative margin
and prediction of local tumor progression and its validation. J
Gastroenterol. 46:1418–1426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamakado K, Nakatsuka A, Akeboshi M,
Shiraki K, Nakano T and Takeda K: Combination therapy with
radiofrequency ablation and transcatheter chemoembolization for the
treatment of hepatocellular carcinoma: Short-term recurrences and
survival. Oncol Rep. 11:105–109. 2004.PubMed/NCBI
|
|
23
|
Chen QW, Ying HF, Gao S, Shen YH, Meng ZQ,
Chen H, Chen Z and Teng WJ: Radiofrequency ablation plus
chemoembolization versus radiofrequency ablation alone for
hepatocellular carcinoma: A systematic review and meta-analysis.
Clin Res Hepatol Gastroenterol. 40:309–314. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peng ZW, Zhang YJ, Chen MS, Xu L, Liang
HH, Lin XJ, Guo RP, Zhang YQ and Lau WY: Radiofrequency ablation
with or without transcatheter arterial chemoembolization in the
treatment of hepatocellular carcinoma: A prospective randomized
trial. J Clin Oncol. 31:426–432. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Peng ZW, Zhang YJ, Liang HH, Lin XJ, Guo
RP and Chen MS: Recurrent hepatocellular carcinoma treated with
sequential transcatheter arterial chemoembolization and RF ablation
versus RF ablation alone: A prospective randomized trial.
Radiology. 262:689–700. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morimoto M, Numata K, Kondou M, Nozaki A,
Morita S and Tanaka K: Midterm outcomes in patients with
intermediate-sized hepatocellular carcinoma: A randomized
controlled trial for determining the efficacy of radiofrequency
ablation combined with transcatheter arterial chemoembolization.
Cancer. 116:5452–5460. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang W, Chen MH, Wang MQ, Cui M, Gao W, Wu
W, Wu JY, Dai Y and Yan K: Combination therapy of radiofrequency
ablation and transarterial chemoembolization in recurrent
hepatocellular carcinoma after hepatectomy compared with single
treatment. Hepatol Res. 39:231–240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shibata T, Isoda H, Hirokawa Y, Arizono S,
Shimada K and Togashi K: Small hepatocellular carcinoma: Is
radiofrequency ablation combined with transcatheter arterial
chemoembolization more effective than radiofrequency ablation alone
for treatment? Radiology. 252:905–913. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shen SQ, Xiang JJ, Xiong CL, Wu SM and Zhu
SS: Intraoperative radiofrequency thermal ablation combined with
portal vein infusion chemotherapy and transarterial
chemoembolization for unresectable HCC. Hepatogastroenterology.
52:1403–1407. 2005.PubMed/NCBI
|
|
30
|
Zhang Z, Wu M, Chen H, Chen D and He J:
Percutaneous radiofrequency ablation combined with transcatheter
arterial chemoembolization for hepatocellular carcinoma. Zhonghua
Wai Ke Za Zhi. 40:826–829. 2002.(In Chinese). PubMed/NCBI
|
|
31
|
Nakashima Y, Nakashima O, Tanaka M, Okuda
K, Nakashima M and Kojiro M: Portal vein invasion and intrahepatic
micrometastasis in small hepatocellular carcinoma by gross type.
Hepatol Res. 26:142–147. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim TK, Choi BI, Han JK, Chung JW, Park JH
and Han MC: Nontumorous arterioportal shunt mimicking hypervascular
tumor in cirrhotic liver: Two-phase spiral CT findings. Radiology.
208:597–603. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Imai Y, Chikayama T, Nakazawa M, Watanabe
K, Ando S, Mizuno Y, Yoshino K, Sugawara K, Hamaoka K, Fujimori K,
et al: Usefulness of miriplatin as an anticancer agent for
transcatheter arterial chemoembolization in patients with
unresectable hepatocellular carcinoma. J Gastroenterol. 47:179–186.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang YX, De Baere T, Idée JM and Ballet S:
Transcatheter embolization therapy in liver cancer: An update of
clinical evidences. Chin J Cancer Res. 27:96–121. 2015.PubMed/NCBI
|
|
35
|
Fang ZT, Zhang W, Wang GZ, Zhou B, Yang
GW, Qu XD, Liu R, Qian S, Zhu L, Liu LX, et al: Circulating tumor
cells in the central and peripheral venous compartment-assessing
hematogenous dissemination after transarterial chemoembolization of
hepatocellular carcinoma. Onco Targets Ther. 7:1311–1318. 2014.
View Article : Google Scholar : PubMed/NCBI
|