Introduction
Resistance to thyroid hormone (RTH) is a rare
autosomal dominant or recessive hereditary disorder resulting from
decreased responsiveness of the pituitary and/or peripheral target
tissues to thyroid hormone (TH) (1).
Thyroid function of the RTH is characterized by unsuppressed
(normal or slightly increased) thyroid-stimulating hormone (TSH)
levels, despite increased serum free thyroxine (FT4) and
free tri-iodothyronine (FT3) levels. The first case of
RTH was identified in 1967 by Refetoff et al (2) who described the clinical features of the
disorder, including deaf-mutism and goiter. The association between
RTH and mutation of the hormone-binding domain in the TH receptor β
gene (THRB) was revealed in 1989 by Sakurai et al (3). In total, ~85% of RTH cases result from a
number of mutations of the THRB gene (4), located on chromosome 3, with the
remaining ~15% of cases arising due to defects on alternative
genes, including the TH receptor α gene (THRA) located on
chromosome 17, and genes involved in the transport and metabolism
of TH (4). RTH exhibits variable
clinical presentations; however, the most common clinical feature
is goiter with a euthyroid state. On occasion, patients with RTH
may suffer from either hyperthyroidism or hypothyroidism. RTH is
diagnosed on the basis of clinical findings and laboratory results,
and a definite diagnosis relies on the identification of associated
gene mutations.
The association between RTH and autoimmune thyroid
diseases (AITDs) remains a matter of debate. A previous study
(5) demonstrated that RTH is free of
autoantibodies against thyroglobulin (anti-TgAb) and thyroid
peroxidase (anti-TpoAb), whereas Barkoff et al (6) hypothesized that patients with RTH
possess an increased risk of developing AITDs including Hashimoto
thyroiditis (HT). On the other hand, HT has been associated with
papillary thyroid carcinoma (PTC) and may constitute a risk factor
for this type of cancer (7). There
are a number of studies of adult patients with PTC and HT; however,
there are a limited number of studies demonstrating the coexistence
of these two diseases in pediatric patients (8,9). In
addition, there are a number of case studies that describe RTH with
PTC (10–19), which identify the possible association
between these diseases. In the present case report, a pediatric
patient with newly diagnosed RTH and coexisting PTC and HT is
discussed. Additionally, a literature review of PTC in RTH subjects
(Table I) is provided.
 | Table I.Literature review of papillary
carcinoma with resistance to thyroid hormone. |
Table I.
Literature review of papillary
carcinoma with resistance to thyroid hormone.
| Author, year | Sex/age | Order of
diagnosis | TSH levels | Gene analysis | Histology of
DTC | Diameter, mm | HT | Therapy | Follow up for DTC,
year/result | (Refs.) |
|---|
| Taniyama et
al (2001) | F/46 | 1. TMNG; 2. DTC; 3.
RTH | Unsuppressed | Amino acid
substitution at codon 429 (R429Q) of THRB | Follicular variant
of papillary carcinoma | 5 | − | 1. ATD (MMI); 2.
subtotal thyroidectomy | NR | (10) |
| Siristatidis et
al (2004) | F/26 | 1. PTC; 2. TSHoma;
3. RTH | Increased | NR | Papillary
carcinoma | NR | NR | Total thyroidectomy
and L-T4 | 0.75/remission | (11) |
| Kim et al
(2010) | F/38 | 1. TRH; 2. PTC | Unsuppressed | Amino acid
substitution at codon 310 (M310T) in exon 9 of THRB | Papillary
carcinoma | 4 (multifocal) | − | 1. Total
thyroidectomy; 2. L-T4 | NR | (12) |
| Paragliola et
al (2011) | M/48 | 1. RTH; 2. MNG; 3.
PTC | Increased | no mutation
identified in THRB | Papillary
carcinoma | 24 | − | 1. Total
thyroidectomy; 2. L-T4 | 9.5/remission | (13) |
| Paragliola et
al (2011) | M/63 | 1. MNG; 2. RTH; 3.
PTC | Increased | Missense mutation
at codon 453 (P453T) in exon 10 of THRB | Papillary
carcinoma | 6 | − | 1. Total
thyroidectomy; 2. L-T4 | 5/remission | (13) |
| Sugita et al
(2012) | F/26 | 1. PTC; 2. RTH | Unsuppressed | Mutation at codon
447 (P447L) of THRB | Papillary
carcinoma | NR | − | 1. ATD (MMI); 2.
L-T4 and T3 | 8/remission | (14) |
| Ramos-Prol et
al (2013) | F/9 | 1. ADHD; 2. AITD
and RTH; 3. PTC | Increased | Missense mutation
at codon 243 (R243W) of THRB | Papillary
carcinoma | 24
(multifocal) | + | 1. ATD (first
cabimazole, then propylthiouracil) and βB; 2. TRIAC; 3. total
thyroidectomy; 4. L-T4 and TRIAC | 3/remission | (15) |
| Unluturk et
al (2013) | F/29 | 1.
Hyper-thyroidism; 2. PTC; 3. RTH | Unsuppressed | Missense mutation
at codon 334 (T334C) of THRB | Papillary
carcinoma | 8 | − | 1. ATD; 2. subtotal
thyroidectomy; 3. completion thyroidectomy and radioiodine; 4.
L-T4 and bromocriptine; 5. L-T4 and βB | 21/remission | (16) |
| Unluturk et
al (2013) | M/33 | 1. MNG; 2. PTC; 3.
RTH | Unsuppressed | Amino acid
substitution at codon 364 (I364F) of TSHR | Papillary
carcinoma | 12 | − | 1. Total
thyroidectomy and radioiodine; 2. L-T4 | 0.75/remission | (16) |
| Vinagre et
al (2014) | F/19 | 1. Hyper-
thyroidism; 2. PTC and follicular adenoma; 3. RTH | Unsuppressed | Mutation at codon
320 (R320C) in exon 9 of THRB and BRAFV600E mutation in
PTC by gene sequence | Papillary
carcinoma | 4 | − | 1. ATD (MMI); 2.
total thyroidectomy and radioiodine; 3. L-T4 and βB |
11.00/remission | (17) |
| Aoyama et al
(2015) | F/54 | 1. PTC; 2. RTH | Unsuppressed | Point mutation at
codon 453 (P453S) of THRB | Papillary
carcinoma | 10
(multifocal) | − | 1. Total
thyroidectomy; 2. L-T4 | 2.25/remission | (18) |
| Karakose et
al (2015) | F/56 | 1. MNG; 2. RTH; 3.
PTC | Unsuppressed | Missense mutation
at codon 234 (A234D) in exon 8 of THRB | Papillary
carcinoma | 2 | − | 1. Subtotal
thyroidectomy; 2. total thyroidectomy; 3. L-T4 and
T3 | 0.33/remission | (19) |
| Karakose et
al (2015) | M/33 | 1. RTH; 2. PTC | Increased | Missense mutation
at codon 234 (A234D) in exon 8 of THRB and BRAFV600E
mutation negative | Papillary
carcinoma | 4 (two focus) | − | 1. Total
thyroidectomy and radioiodine; 2. L-T4 | 0.17/remission | (19) |
| Present case
(2015) | F/12 | 1. Hyper-
thyroidism; 2. PTC; 3. RTH | Unsuppressed | Mutation at codon
454 (L454FS) in exon 10 of THRB and BRAFV600E mutation
in PTC | Papillary
carcinoma | 10
(multifocal) | + | 1. ATD (MMI); 2.
total thyroidectomy and radioiodine; 3. L-T4, βB and
bromocriptine | 0.25/remission |
|
Case report
A female Chinese patient, aged 11 years, was
examined following clinical presentation of mild thyroid
enlargement. Thyroid ultrasonography (US) revealed marked
heterogeneity of the parenchyma, 1 nodule (8×7 mm) with clear
margins and no blood flow signal in the right lobe. Fine needle
assay (FNA) of the thyroid nodule revealed no malignancy. The
patient was diagnosed with hyperthyroidism following a thyroid
function assessment in Heze Municipal Hospital (Shandong, China);
however, the patient exhibited a poor response to initial
methimazole (MMI) treatment (10 mg, once daily) so the dose was
increased to 10 mg twice daily. Baseline thyroid function
information and during MMI therapy are presented in Table II.
 | Table II.Alterations in thyroid function at
the initial visit and during MMI therapy. |
Table II.
Alterations in thyroid function at
the initial visit and during MMI therapy.
| Variable | FT3,
ng/ml | FT4,
ng/ml | TSH, µIU/ml |
|---|
| Normal range | 1.82–3.86 | 0.78–1.86 | 0.38–5.57 |
| Initial | 6.56 | 1.85 | 10.02 |
| 2 months after MMI
therapy (5 mg, bid) | 8.65 | 3.84 | 17.00 |
| 6 months after MMI
therapy (10 mg, tid) | 9.54 | 4.09 | >100 |
| 3 months after
withdrawal of MMI | 6.60 | 2.84 | 18.91 |
| 1 month after MMI
therapy (10 mg, bid) | 9.08 | 4.79 | 5.22 |
When first seen at The General Hospital of Jinan
Military Command (Shandong, China) on 31 August 2015, the patient's
height and weight were 156 cm and 45 kg, respectively, with a pulse
rate of 106 beats/min, blood pressure of 100/75 mmHg and a basal
metabolic rate of 20%. On observation, the patient's thyroid gland
was asymmetrically enlarged with palpable nodules on the two lobes
(the largest was located in the left lobe, diameter ~1 cm). The
patient was without exophthalmos or myxedematous skin lesions and
hepatic function, renal function, sex hormone levels, parathyroid
hormone and prolactin concentrations were all within the normal
range. Psychological assessment by Raven's Standard Progressive
Matrices revealed an intelligence quotient score of 68, an
intelligence percentile ranked in the lower 5%, which indicated
mild mental retardation.
The laboratory results of thyroid function and
autoimmune assays were as follows: FT3, 14.74 (normal
range, 3.8–6.0 pmol/l); FT4, 44.86 (range, 7.86–14.1
pmol/l); TSH, 3.30 (range, 0.34–5.6 µIU/ml); anti-TgAb, 16.10
(range, 0–4.0); anti-TpoAb, 477.40 (range, 0–9.0 IU/ml). Thyroid US
evaluation revealed diffused enlargement with a heterogeneous
echotexture and multiple nodules in the two lobes. The dominant
nodules were 5×4 and 14×6 mm in size in the left lobe and 15×5,
15×6, 5×5 and 6×5 mm in the right lobe, with micro-calcifications
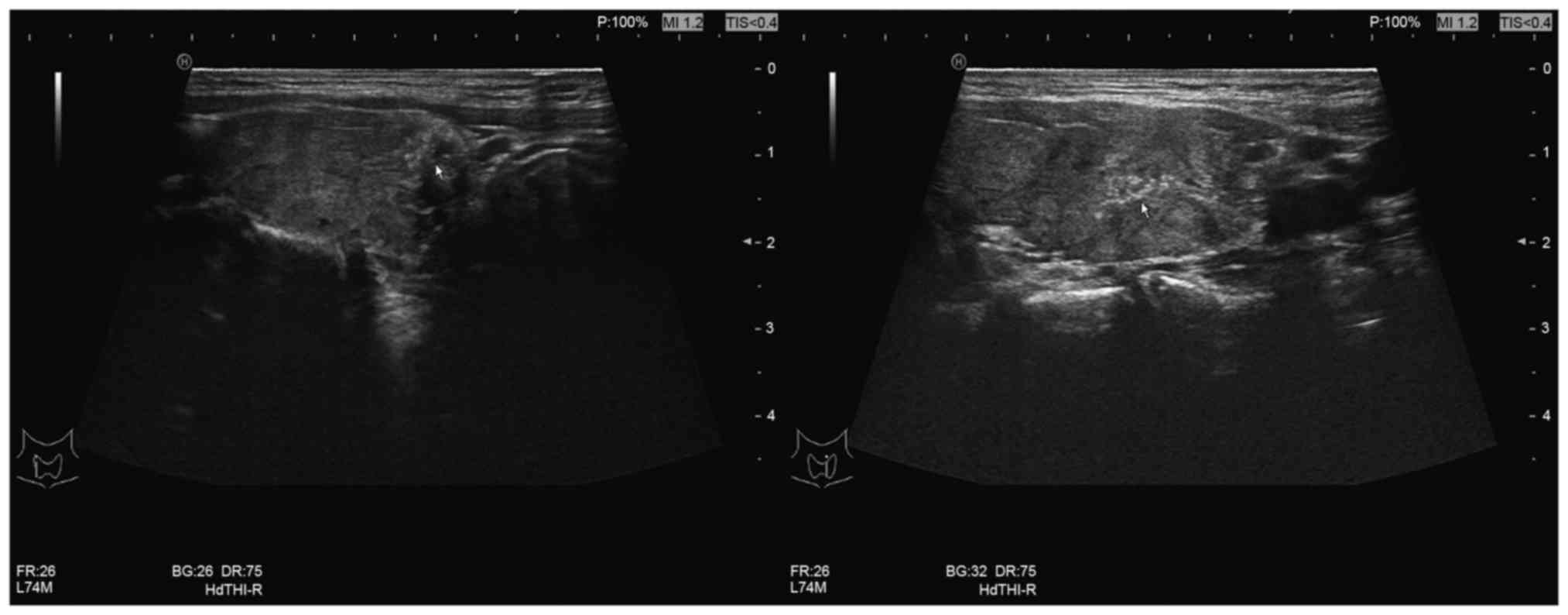
and unclear margins (Fig. 1). FNA
identified atypical results of undetermined significance and
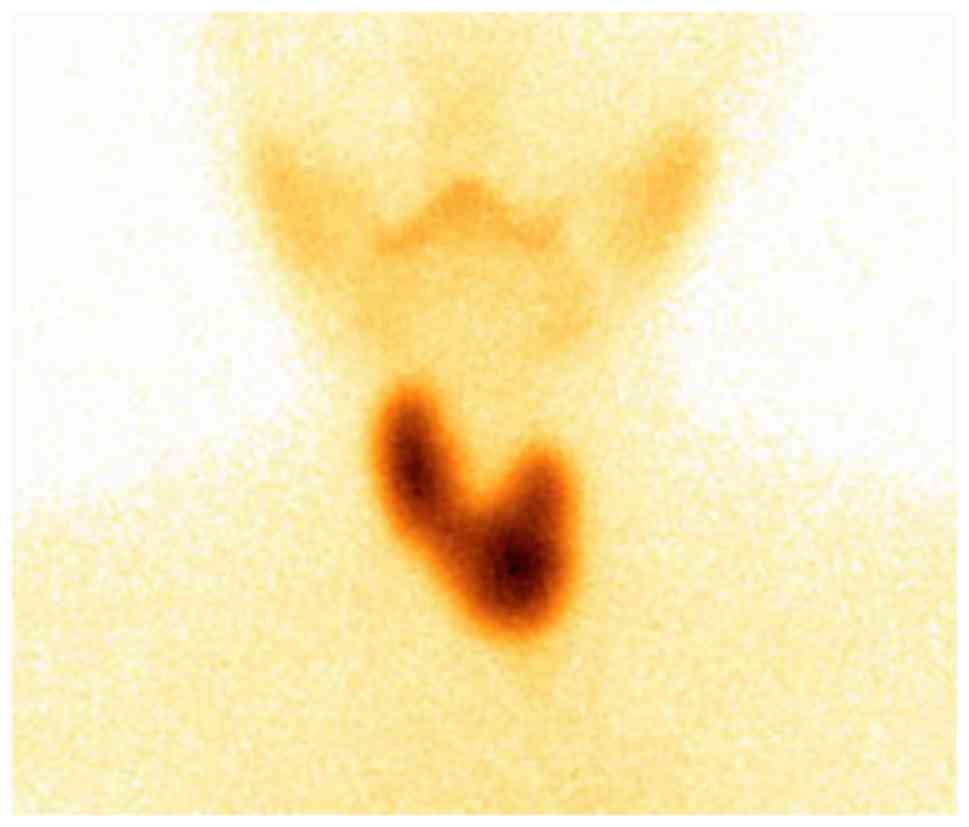
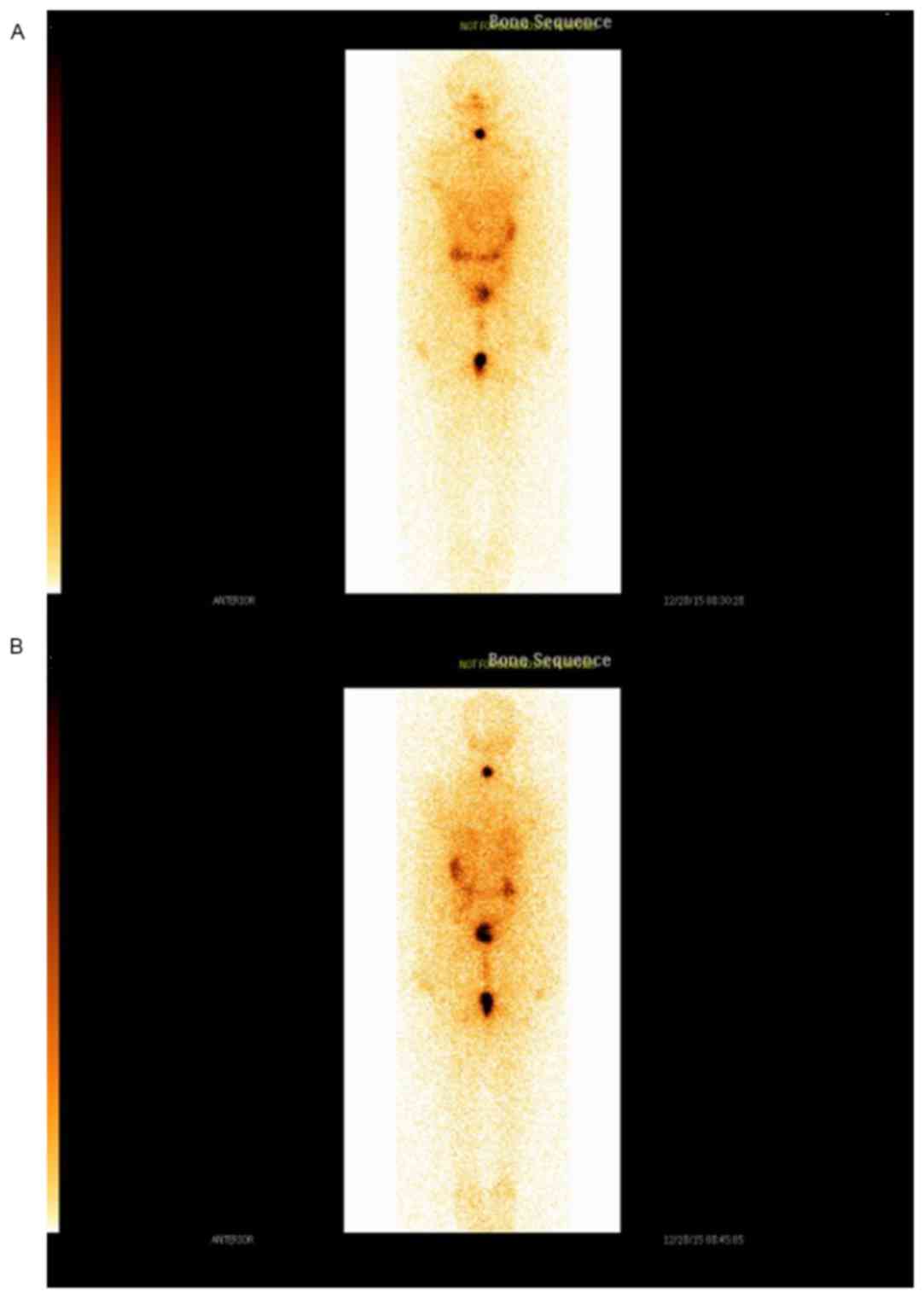
suggested possible malignancy. 99Tc scintigraphy
demonstrated diffused enlargement of the thyroid gland with
increased uptake (Fig. 2) and an
electrocardiogram revealed sinus tachycardia. A magnetic resonance
imaging scan of the pituitary gland excluded the presence of a
pituitary tumor or pituitary enlargement and X-ray of the left
wrist demonstrated normal bone age. The patient was prescribed with
a β-blocker (metoprolol succinate, 23.75 mg, once daily) to control
the tachycardia and after 2 weeks achieved the target heart rate
(between 70 and 80 beats/min). Subsequently, the patient was
hospitalized with a diagnosis of thyroid cancer and suspected RTH.
On 18 September 2015, the patient underwent total thyroidectomy and
lymph node dissection of the left side of the neck. Biopsy results
revealed multifocal PTCs (T1aN1bM0, using the tumor-node-metastasis
staging) with 1 papillary focus (1.0×0.7 cm) in the left thyroid
lobe, a micro-papillary carcinoma focus (0.6×0.5 cm) with HT and
nodular goiter in and around the right lobe, and HT with nodular
goiter in the isthmus. Lymph node metastases to the right and left
trachea and the esophagus were confirmed by histology results.
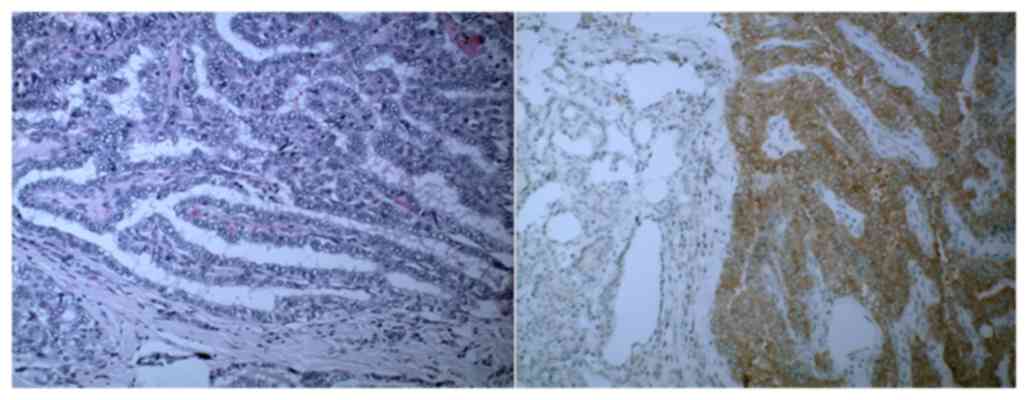
BRAFV600E mutation analysis demonstrated positivity
according to immunohistopathological results (Fig. 3).
DNA was isolated from peripheral blood leukocytes by
QIAamp Blood DNA Mini kit (Qiagen, Inc., Valencia, CA, USA) and all
10 exons of the THRB gene were amplified using the polymerase chain
reaction (PCR) and analyzed by automated fluorescence-based
sequencing. The primers of exon 1 to 10 of the THRB gene were
designed by Primer Premier 5.0 software (Premier Biosoft
International, Palo Alto, CA, USA) and the sequence information is
listed in Table III. The PCR
cycling condtions were as follows: Pre-denaturation at 95°C for 5
min, followed by 40 cycles of denaturation at 94°C for 30 sec,
annealing at 66–65°C for 30 sec and extending at 72°C for 30 sec,
Direct sequencing of the PCR products were performed by ABI 3500
sequencer (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
compared with reference sequences (NM_000461.4 and NG_009159.1;
https://www.ncbi.nlm.nih.gov/nuccore/NM_000461.4
and https://www.ncbi.nlm.nih.gov/nuccore/218156319,
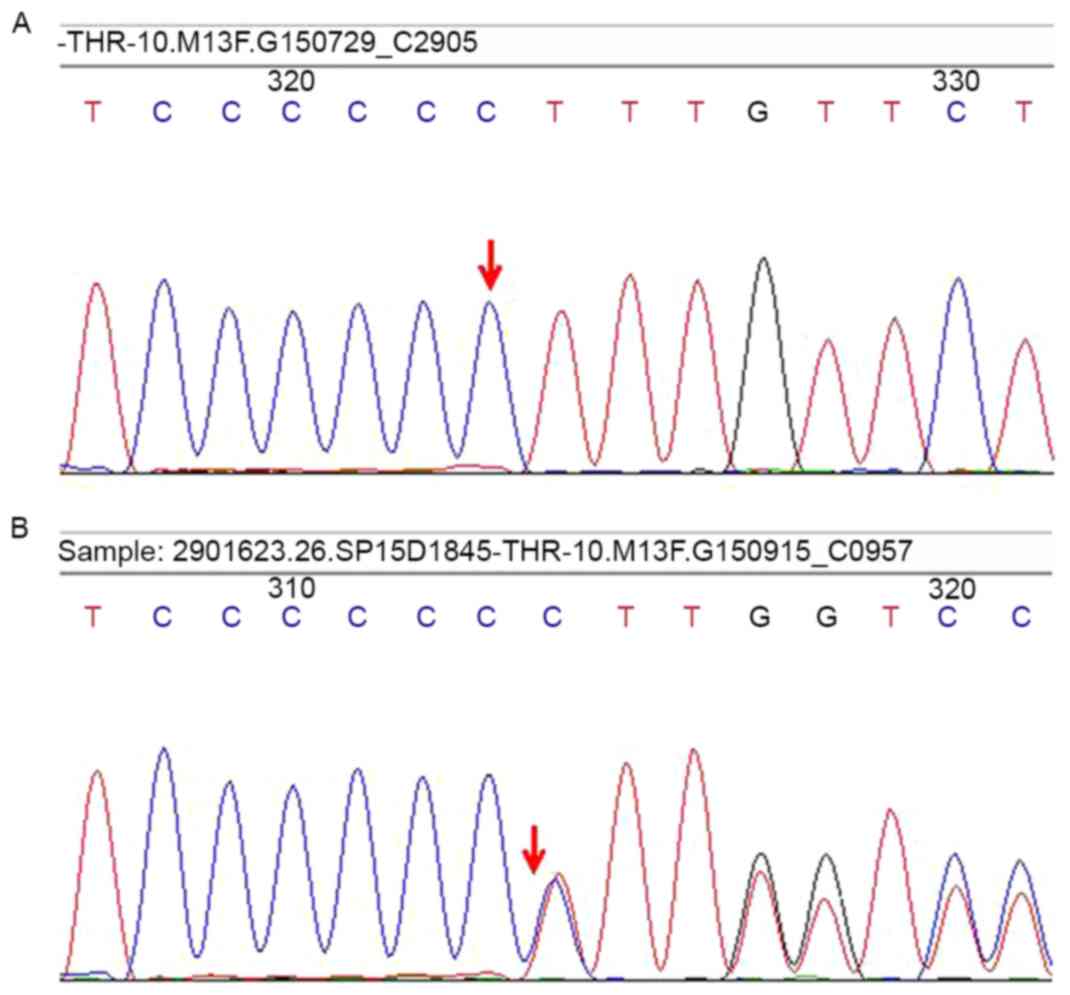
respectively). The results revealed a frameshift mutation in exon
10 of THRB (Leu454fs; c.1358dupC, Fig. 4), which has been identified in RTH
(20). Diagnosis of RTH, HT and PTC
was confirmed on the basis of laboratory and clinical results. The
thyroid function of the patient 1-week post-surgery revealed
slightly increased FT3 (6.84 pmol/l), increased
FT4 (26.84 pmol/l) and increased TSH (33.99 µIU/ml)
levels following treatment with levothyroxine (L-T4, 50
µg/day). The patient was subsequently prescribed with 100 µg/day
L-T4 and 1 month later thyroid function assays revealed
almost normal FT3 levels (5.23 pmol/l), slightly
increased FT4 (14.99 pmol/l) and increased TSH (>100
µIU/ml) levels. The levels of anti-TgAb and anti-TpoAb decreased
markedly in comparison with pre-surgery amounts (6.9 and 173.3%,
respectively). The concentration of thyroglobulin was 0.46 (range,
1.6–59.9 ng/ml) and the patient exhibited no signs of
hyperthyroidism. Due to the unavailability of tri-iodothyronine
(T3), the L-T4 dosage was increased to 125
µg/day and combined with TH tablets (50 mg/day), derived from pig
thyroid gland (containing FT3 and FT4), and
bromocriptine (5 mg/day). After 1 month, thyroid function
assessment revealed slightly increased FT3 and
FT4 (6.23 and 14.47 pmol/l, respectively) and increased
TSH (46 µIU/ml) levels. 131I radio remnant ablation
therapy was administered at an oral dose of 104 mCi
Na131I. Subsequently, 4 days after radiotherapy, the
patient underwent a whole-body scan which detected a highly
radioactive region around the thyroid, but no other abnormal foci
of uptake in the rest of the body were identified (Fig. 5). Subsequently, L-T4
suppression therapy was continued and gradually increased up to 150
µg/day together with 3.75 mg/day bromocriptine and 50 mg/day TH
tablets. The latest thyroid function tests, 6 months post-surgery,
revealed that FT4 and TSH levels were slightly increased
with normal FT3 levels (15.97 pmol/l, 6.80 µIU/ml and
5.79 pmol/l, respectively) and the patient exhibited no signs of
thyrotoxicosis. Thyroid US demonstrated no signs of disease
persistence or relapse, and serum thyroglobulin and
carcinoembryonic antigen levels were within the normal range. The
patient remains under follow-up. All examined relatives (mother,
father and younger brother) exhibited normal thyroid function and
morphological features of thyroid US revealed normal results. The
patient's relatives were unwilling to undergo testing for the THRB
mutation.
 | Table III.Primer sequences of exon 1 to 10 of
TH receptor β gene. |
Table III.
Primer sequences of exon 1 to 10 of
TH receptor β gene.
| Exon | Primer | Sequence | Size of product
(bp) |
|---|
| 1 | Forward |
5′-GCTGCGGCCGCCTCTCTTCGC-3′ | 420 |
|
| Reverse |
5′-GCCTCCGGGTTCTTGCGACGC-3′ |
|
| 2 | Forward |
5′-GAGTTTGAGGTTCACATTGAA-3′ | 541 |
|
| Reverse |
5′-AATACCTATAGAGTTCAACCT-3′ |
|
| 3 | Forward |
5′-ATTGCTAGCATAGGCATTGGC-3′ | 525 |
|
| Reverse |
5′-ATATATTTCAGTTAAGTACAGC-3′ |
|
| 4 | Forward |
5′-AAATTATCACAGATATATGACG-3′ | 418 |
|
| Reverse |
5′-GTGAGGATGCATCTTATATGAG-3′ |
|
| 5 | Forward |
5′-ACAACTTGCCTTCCAAAAGTGT-3′ | 492 |
|
| Reverse |
5′-GAAAAGCGACGCGCTAGTAAAG-3′ |
|
| 6 | Forward |
5′-GTGGGCCTATGTTAAGTCTAT-3′ | 370 |
|
| Reverse |
5′-TTGAATTTAACTTAACATTGC-3′ |
|
| 7 | Forward |
5′-AAGTGTGCCCAGTGTGAGCCAG-3′ | 458 |
|
| Reverse |
5′-TATCAGTAAAATGAGGCAATAAC-3′ |
|
| 8 | Forward |
5′-GATAAATAAAGCTCCCTTCAAC-3′ | 384 |
|
| Reverse |
5′-TAAATACAGAAAGTGGGAATC-3′ |
|
| 9 | Forward |
5′-CTTTGAGTATGAAATGGTTG-3′ | 502 |
|
| Reverse |
5′-TTAGCGCTAGAGAAGCAAAAG-3′ |
|
| 10 | Forward |
5′-TGGAGCACCAGAGTTCACC-3′ | 469 |
|
| Reverse |
5′-ACAAATGCAGCTAGCTAGAT-3′ |
|
All protocols followed were in accordance with the
national ethical standards previously approved by Local Ethical
Review Committees. The present case report was approved by the
Regional Ethical Review Board of General Hospital of Jinan Military
Command, China. Informed consent was obtained from the patient's
parents.
Discussion
RTH is the most common type of decreased sensitivity
to TH with an incidence of ~1/40,000 live births (21). RTH is caused by mutations of a number
of genes, including THRB, THRA and others involved with TH
transport and metabolism (4).
Clinical manifestations of RTH are heterogeneous (22); the most common signs include goiter
and sinus tachycardia, as identified in the present case report.
Distinct mutations of THRB have been studied in >3,000
individuals and ~1,000 families (23). In the present case report, a
frameshift mutation in exon 10 of THRB (Leu454fs;
c.1358dupC) was identified by gene sequencing. Among all reviewed
cases, including the present case study, only 1 revealed a mutation
in the TSH receptor (16) and all
remaining cases exhibited gene mutations within the three clusters
rich in CpG (24–26) between residues 310 and 353, 429 and
461 and 232 and 282 (clusters 1, 2 and 3, respectively) within the
ligand-binding domain of THRB.
THRB is the cellular homolog of the
transcriptionally inactive oncogene v-erbA, which may have an
influence on the development of cancer. In thyroidectomized tissues
of PTC, THRB1 mutations were identified in 93.8% of cases and no
mutations were detected in healthy euthyroid controls (27). In animal studies, the association of
THRB and thyroid carcinoma has been demonstrated (28,29) and
Kim et al (12) proposed that
the THRB mutation itself may also exert oncogenic effects.
Additionally, patients with PTC in RTH were all relatively young,
ranging between 9 and 63 years (mean, 35.1 years). Thus, thyroid US
evaluation and FNA may be performed in the follow-up of patients
with RTH in order to determine carcinogenesis.
Typically, anti-thyroid antibodies are negative in
RTH which eliminates autoimmunity in the etiology of this disorder
(30). HT is a common type of AITD
worldwide, whereas RTH is a rare condition and so the coexistence
of these two diseases is considered to be incidental (5). In the last decade, the coexistence of
RTH and AITD has become increasingly prevalent in single patients
(31,32) and also in families with RTH (33,34). Since
the TSH level is unsuppressed or abnormally increased, in
comparison with serum TH concentrations, a number of studies
hypothesized that chronic stimulation of TSH in RTH may activate
the intra-thyroidal lymphocytes, leading to thyroid damage and
autoimmune thyroiditis including HT (35). More recently, Barkoff et al
(6) revealed that patients with RTH
exhibit an increased risk of AITD, compared with unaffected
relatives, due to the THRB gene mutation which suggests that the
coexistence of HT and RTH may not be accidental. Furthermore, the
presence of HT and the resulting thyroid failure, caused by
destructive antibodies, may decrease serum TH levels, thereby
masking the cardinal features of RTH and leading to misdiagnosis
(33). Since HT is more common in
females than males (7), the ideal
approach may be to test for thyroid antibodies in females suspected
with RTH with close follow-ups in patients with HT and RTH.
HT is commonly observed following histological
examination of thyroidectomy specimens. The association between HT
and PTC was first proposed by Dailey et al (36) in 1955. Subsequently, the clinical
association of the two diseases has been extensively debated with a
number of studies confirming a relatively high incidence of PTC in
HT (37,38); however, other studies have identified
contradictory results (39).
Recently, Koibuchi et al (40)
investigated three cases of children with PTC and HT; however, the
underlying molecular mechanism of the association between HT and
PTC remains unknown, with one study suggesting that increased
reactive oxygen species levels may contribute to the development of
PTC in HT (41). The
BRAFV600E mutation, identified in between 29 and 83% of
PTC cases (42) and considered an
early or initiating event in PTC, is typically in papillary
microcarcinoma and minute incidental cases (43). An identical BRAF V600E
mutation has been identified in solid cell nests in the thyroid and
adjacent PTC (44), indicating that
HT and PTC may be initiated by similar stem cell remnants, and may
be etiologically related (7). In
studied cases of RTH with PTC, BRAFV600E mutation
testing was carried out in two studies (17,19) in the
histological section and only one positive mutation was found
(17). To the best of our knowledge,
the present case report is the second patient to exhibit
BRAFV600E mutation in PTC with RTH. Since the
BRAFV600E mutation is rare in children and adolescents
with PTCs (45), and TSH suppression
therapy not always effective, using BRAFV600E mutation
tests in cases with PTC and RTH was hypothesized in the present
study, in order to identify the patients at risk of metastases and
patients with poor prognosis.
TSH is a growth factor for the thyroid gland and
nodules; however, whether it additionally serves a pathogenic role
in thyroid oncogenesis remains unclear. A previous study identified
that patients with increased serum TSH concentrations exhibited an
increased risk of developing thyroid malignancy (46). Within the normal range of TSH, a value
above the mean level for the general population is associated with
a markedly increased likelihood of thyroid cancer, compared with
TSH values below the mean (47). In a
retrospective study based on 637 medical records, Medenica et
al (48) revealed that patients
with increased serum TSH concentrations and/or AITD, exhibited an
increased risk of thyroid malignancy. Since RTH is characterized by
increased TH concentrations, accompanied by unsuppressed or
increased serum TSH levels, whether patients with RTH are at
increased risk of thyroid malignancy remains unknown (19). Owing to the low incidence of RTH and
the lack of specific symptoms associated with the disorder, RTH is
commonly misdiagnosed as hyperthyroidism or Grave's disease.
Patients who have been previously misdiagnosed and prescribed
anti-thyroid drugs (ATDs) may be at an increased risk of neoplasm
formation (30). In the present case
report, the patient with RTH was misdiagnosed with hyperthyroidism
and administered with MMI for ~8 months, and serum TSH levels were
increased to above the detection limit. It is hypothesized that
chronic HT and increased TSH stimulation caused by inappropriate
therapy may have contributed to the development of PTC in the
present case.
A literature review of 14 cases of differentiated
thyroid carcinoma DTC with RTH (Table
I) revealed that HT coexisted in only a 9-year-old girl from
Germany (15). Therefore, to the best
of our knowledge, the present case report is the first to reveal
PTC and HT with RTH in an Asian adolescent. Lymph node metastases
of the right and left trachea and esophagus were confirmed in the
present case, indicating a more aggressive thyroid malignancy in
this case when compared with the aforementioned 9-year-old female
patient. It was hypothesized that increased serum TSH levels in
patients with RTH and HT, who possess the BRAFV600E
mutation, may be a contributing factor for malignancy considering
the relatively young age of the present patient, the rare incidence
of the BRAF V600E mutation in children, HT coexistence,
increased serum TSH levels prior to and during treatment and
metastasis occurrence. TSH stimulation in RTH may be an important
growth factor for the thyroid gland and minute neoplastic
nodules.
Total thyroidectomy and post-operative
L-T4 suppression therapy is typically administered for
the treatment of patients with PTC as this therapy is considered to
prevent cancer relapse or progression. However, in a patient with
RTH and thyroidectomy, TSH suppression may not be achieved despite
increasing doses of L-T4. In the literature review of 14
cases, 3 patients (14,15,19) were
prescribed T3 or tri-iodothyroacetic acid (TRIAC), in
addition to L-T4 suppression therapy. Owing to the lack
of T3 or TRIAC, the dosage of L-T4 was
increased in combination with TH tablets and the dopaminergic agent
bromocriptine. To the best of our knowledge, only the study by
Unluturk et al (16)
demonstrated that bromocriptine in combination with L-T4
may be used to decrease serum TSH levels in a patient with RTH and
PTC. Previous studies have indicated that bromocriptine may inhibit
TSH secretion and additionally decrease the enlarged goiter in RTH
(49,50). In the present case, the TSH level
decreased under combination therapy of L-T4 and
bromocriptine in the patient with RTH and PTC, following surgery.
Considering the limited evidence for the use of bromocriptine in
RTH, additional studies are required to assess its value as a
treatment of RTH.
Tests to identify a BRAF mutation, e.g. DNA
sequencing or PCR-based molecular assays, are not routinely applied
as they are costly and time-consuming. Recently, an
immunohistochemical technique was introduced into clinical
practice. The aforementioned technique identifies BRAF mutations
using the mouse anti-human BRAFV600E monoclonal antibody
VE1 and clinical information suggested a marked consistency between
this method and the BRAF mutation assessment in PTC1 and other BRAF
mutation-related cancers, including colon cancer (51,52).
Therefore, the use of BRAF immunohistochemistry in clinical
practice for BRAFV600E mutation detection is considered
to be useful due to its effectiveness, simplicity and economical
benefit. The limitations of the present study included small sample
size (only one patient), unknown lifestyle habits of the patient,
as well as a focus on only one region (Asian).
In conclusion, the present case report indicates
that RTH with increased or unsuppressed serum TSH concentrations
and positive anti-thyroid antibodies, suspect for HT, may be an
indication for thyroid carcinoma development. It is hypothesized
that, in clinical practice, treatment with ATDs which lead to
increased TSH, in addition to HT and RTH, may be avoided. Close
follow-up and genomic analysis should be performed in cases with
suspected RTH. Additional studies are required to disclose the
possible association of HT and PTC in RTH and explore the long-term
effects of medication.
References
|
1
|
Refetoff S: Resistance to thyroid hormone:
One of several defects causing reduced sensitivity to thyroid
hormone. Nat Clin Pract Endocrinol Metab. 4:12008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Refetoff S, DeWind LT and DeGroot LJ:
Familial syndrome combining deaf-mutism, stuppled epiphyses, goiter
and abnormally high PBI: Possible target organ refractoriness to
thyroid hormone. J Clin Endocrinol Metab. 27:279–294. 1967.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sakurai A, Takeda K, Ain K, Ceccarelli P,
Nakai A, Seino S, Bell GI, Refetoff S and DeGroot LJ: Generalized
resistance to thyroid hormone associated with a mutation in the
ligand-binding domain of the human thyroid hormone receptor beta.
Proc Natl Acad Sci USA. 86:pp. 8977–8981. 1989; View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Onigata K and Szinnai G: Resistance to
thyroid hormone. Endocr Dev. 26:118–129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lamberg BA, Rosengård S, Liewendahl K,
Saarinen P and Evered DC: Familial partial peripheral resistance to
thyroid hormones. Acta Endocrinol (Copenh). 87:303–312.
1978.PubMed/NCBI
|
|
6
|
Barkoff MS, Kocherginsky M, Anselmo J,
Weiss RE and Refetoff S: Autoimmunity in patients with resistance
to thyroid hormone. J Clin Endocrinol Metab. 95:3189–3193. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ahmed R, Al-Shaikh S and Akhtar M:
Hashimoto thyroiditis: A century later. Adv Anat Pathol.
19:181–186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Corrias A, Cassio A, Weber G, Mussa A,
Wasniewska M, Rapa A, Gastaldi R, Einaudi S, Baronio F, Vigone MC,
et al: Thyroid nodules and cancer in children and adolescents
affected by autoimmune thyroiditis. Arch Pediatr Adolesc Med.
162:526–531. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Skarpa V, Kousta E, Tertipi A, Anyfandakis
K, Vakaki M, Dolianiti M, Fotinou A and Papathanasiou A:
Epidemiological characteristics of children with autoimmune thyroid
disease. Hormones (Athens). 10:207–214. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taniyama M, Ishikawa N, Momotani N, Ito K
and Ban Y: Toxic multinodular goitre in a patient with generalized
resistance to thyroid hormone who harbours the R429Q mutation in
the thyroid hormone receptor beta gene. Clin Endocrinol (Oxf).
54:121–124. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Siristatidis C, Mastorakos G, Vitoratos N,
Gregoriou O, Iakovidou H, Salamalekis E and Creatsas G: Thyroid
hormone resistance and enlargement of the sella turcica during
pregnancy. Arch Gynecol Obstet. 269:152–155. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim HK, Kim D, Yoo EH, Lee JI, Jang HW,
Tan AH, Hur KY, Kim JH, Kim KW, Chung JH and Kim SW: A case of
resistance to thyroid hormone with thyroid cancer. J Korean Med
Sci. 25:1368–1371. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Paragliola RM, Lovicu RM, Locantore P,
Senes P, Concolino P, Capoluongo E, Pontecorvi A and Corsello SM:
Differentiated thyroid cancer in two patients with resistance to
thyroid hormone. Thyroid. 21:793–797. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sugita M, Harada H and Yamamoto T:
Perioperative management of a patient with thyroid hormone
resistance who underwent total thyroidectomy for thyroid cancer. J
Anesth. 26:595–597. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ramos-Prol A, Pérez-Lázaro M Antonia, del
Olmo-García M Isabel, León-de Zayas B, Moreno-Macián F, Navas-de
Solis S and Merino-Torres JF: Differentiated thyroid carcinoma in a
girl with resistance to thyroid hormone management with
triiodothyroacetic acid. J Pediatr Endocrinol Metab. 26:133–136.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Unluturk U, Sriphrapradang C, Erdoğan MF,
Emral R, Güldiken S, Refetoff S and Güllü S: Management of
differentiated thyroid cancer in the presence of resistance to
thyroid hormone and TSH-secreting adenomas: A report of four cases
and review of the literature. J Clin Endocrinol Metab.
98:2210–2217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vinagre J, Borges F, Costa A, Alvelos MI,
Mazeto G, Sobrinho-Simões M and Soares P: Differentiated thyroid
cancer in patients with resistance to thyroid hormone syndrome. A
novel case and a review of the literature. Front Mol Biosci.
1:102014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aoyama M, Yamasaki S and Tsuyuguchi M: A
case of resistance to thyroid hormone diagnosed after total
thyroidectomy for thyroid cancer. J Med Invest. 62:268–271. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karakose M, Caliskan M, Arslan MS, Cakal
E, Yesilyurt A and Delibasi T: Thyroid hormone resistance in two
patients with papillary thyroid microcarcinoma and their BRAFV600E
mutation status. Arch Endocrinol Metab. 59:364–366. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cardoso LF, de Paula FJ and Maciel LM:
Resistance to thyroid hormone due to mutations in the THRB gene
impairs bone mass and affects calcium and phosphorus homeostasis.
Bone. 67:222–227. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lafranchi SH, Snyder DB, Sesser DE, Skeels
MR, Singh N, Brent GA and Nelson JC: Follow-up of newborns with
elevated screening T4 concentrations. J Pediatr. 143:296–301. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Refetoff S and Dumitrescu AM: Syndromes of
reduced sensitivity to thyroid hormone: Genetic defects in hormone
receptors, cell transporters and deiodination. Best Pract Res Clin
Endocrinol Metab. 21:277–305. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dumitrescu AM and Refetoff S: The
syndromes of reduced sensitivity to thyroid hormone. Biochim
Biophys Acta. 1830:3987–4003. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Weiss RE, Weinberg M and Refetoff S:
Identical mutations in unrelated families with generalized
resistance to thyroid hormone occur in cytosine-guanine-rich areas
of the thyroid hormone receptor beta gene. Analysis of 15 families.
J Clin Invest. 91:2408–2415. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Adams M, Matthews C, Collingwood TN, Tone
Y, Beck-Peccoz P and Chatterjee KK: Genetic analysis of 29 kindreds
with generalized and pituitary resistance to thyroid hormone.
Identification of thirteen novel mutations in the thyroid hormone
receptor beta gene. J Clin Invest. 94:506–515. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Collingwood TN, Wagner R, Matthews CH,
Clifton-Bligh RJ, Gurnell M, Rajanayagam O, Agostini M, Fletterick
RJ, Beck-Peccoz P, Reinhardt W, et al: A role for helix 3 of the
TRbeta ligand-binding domain in coactivator recruitment identified
by characterization of a third cluster of mutations in resistance
to thyroid hormone. EMBO J. 17:4760–4770. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Puzianowska-Kuznicka M, Krystyniak A,
Madej A, Cheng SY and Nauman J: Functionally impaired TR mutants
are present in thyroid papillary cancer. J Clin Endocrinol Metab.
87:1120–1128. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Suzuki H, Willingham MC and Cheng SY: Mice
with a mutation in the thyroid hormone receptor beta gene
spontaneously develop thyroid carcinoma: A mouse model of thyroid
carcinogenesis. Thyroid. 12:963–969. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ying H, Suzuki H, Furumoto H, Walker R,
Meltzer P, Willingham MC and Cheng SY: Alterations in genomic
profiles during tumor progression in a mouse model of follicular
thyroid carcinoma. Carcinogenesis. 24:1467–1479. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Agrawal NK, Goyal R, Rastogi A, Naik D and
Singh SK: Thyroid hormone resistance. Postgrad Med J. 84:473–477.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kammoun I, Bouzid C, Kandara H, Ben Salem
L, Turki Z and Ben Slama C: A Case of resistance to thyroid hormone
with Chronic thyroiditis: Discovery of a novel mutation (I54V).
Case Rep Endocrinol. 2011:5849302011.PubMed/NCBI
|
|
32
|
Sato H, Koike Y, Honma M, Yagame M and Ito
K: Evaluation of thyroid hormone action in a case of generalized
resistance to thyroid hormone with chronic thyroiditis: Discovery
of a novel heterozygous missense mutation (G347A). Endocr J.
54:727–732. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aksoy DY, Gurlek A, Ringkananont U, Weiss
RE and Refetoff S: Resistance to thyroid hormone associated with
autoimmune thyroid disease in a Turkish family. J Endocrinol
Invest. 28:379–383. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sato H and Sakai H: A family showing
resistance to thyroid hormone associated with chronic thyroiditis
and its clinical features: A case report. Endocr J. 53:421–425.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gavin C, Meggison H and Ooi TC: Proposing
a causal link between thyroid hormone resistance and primary
autoimmune hypothyroidism. Med Hypotheses. 70:1024–1028. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dailey ME, Lindsay S and Skahen R:
Relation of thyroid neoplasms to Hashimoto disease of the thyroid
gland. AMA Arch Surg. 70:291–297. 1955. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim KW, Park YJ, Kim EH, Park SY, Park DJ,
Ahn SH, Park DJ, Jang HC and Cho BY: Elevated risk of papillary
thyroid cancer in Korean patients with Hashimoto's thyroiditis.
Head Neck. 33:691–695. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Repplinger D, Bargren A, Zhang YW, Adler
JT, Haymart M and Chen H: Is Hashimoto's thyroiditis a risk factor
for papillary thyroid cancer? J Surg Res. 150:49–52. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Matesa-Anic D, Matesa N, Dabelić N and
Kusić Z: Coexistence of papillary carcinoma and Hashimoto's
thyroiditis. Acta Clin Croat. 48:9–12. 2009.PubMed/NCBI
|
|
40
|
Koibuchi H, Omoto K, Fukushima N,
Toyotsuji T, Taniguchi N and Kawano M: Coexistence of papillary
thyroid cancer and Hashimoto thyroiditis in children: Report of 3
cases. J Ultrasound Med. 33:1299–1303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yi JW, Park JY, Sung JY, Kwak SH, Yu J,
Chang JH, Kim JH, Ha SY, Paik EK, Lee WS, et al: Genomic evidence
of reactive oxygen species elevation in papillary thyroid carcinoma
with Hashimoto thyroiditis. Endocr J. 62:857–877. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Trovisco V, Soares P, Preto A, Castro P,
Máximo V and Sobrinho-Simões M: Molecular genetics of papillary
thyroid carcinoma: Great expectations. Arq Bras Endocrinol Metabol.
51:643–653. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sobrinho-Simões M, Máximo V, Rocha AS,
Trovisco V, Castro P, Preto A, Lima J and Soares P: Intragenic
mutations in thyroid cancer. Endocrinol Metab Clin North Am.
37333–362. (viii)2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cameselle-Teijeiro J, Abdulkader I,
Pérez-Becerra R, Vázquez-Boquete A, Alberte-Lista L, Ruiz-Ponte C,
Forteza J and Sobrinho-Simões M: BRAF mutation in solid cell nest
hyperplasia associated with papillary thyroid carcinoma. A
precursor lesion? Hum Pathol. 40:1029–1035. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fugazzola L, Puxeddu E, Avenia N, Romei C,
Cirello V, Cavaliere A, Faviana P, Mannavola D, Moretti S, Rossi S,
et al: Correlation between B-RAFV600E mutation and
clinico-pathologic parameters in papillary thyroid carcinoma: Data
from a multicentric Italian study and review of the literature.
Endocr Relat Cancer. 13:455–464. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Boelaert K: The association between serum
TSH concentration and thyroid cancer. Endocr Relat Cancer.
16:1065–1072. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Haymart MR, Repplinger DJ, Leverson GE,
Elson DF, Sippel RS, Jaume JC and Chen H: Higher serum thyroid
stimulating hormone level in thyroid nodule patients is associated
with greater risks of differentiated thyroid cancer and advanced
tumor stage. J Clin Endocrinol Metab. 93:809–814. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Medenica S, Radojevic N, Stojkovic M,
Nedeljkovic-Beleslin B, Savic S, Ciric J, Trbojevic B and Zarkovic
M: Autoimmunity and thyrotropin level in developing thyroid
malignancy. Eur Rev Med Pharmacol Sci. 19:2824–2829.
2015.PubMed/NCBI
|
|
49
|
Dulgeroff AJ, Geffner ME, Koyal SN, Wong M
and Hershman JM: Bromocriptine and Triac therapy for
hyperthyroidism due to pituitary resistance to thyroid hormone. J
Clin Endocrinol Metab. 75:1071–1075. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ohzeki T, Hanaki K, Motozumi H, Ohtahara
H, Ishitani N, Urashima H, Tsukuda T, Shiraki K, Sasaki S, Nakamura
H, et al: Efficacy of bromocriptine administration for selective
pituitary resistance to thyroid hormone. Horm Res. 39:229–234.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Pyo JS, Sohn JH and Kang G: BRAF
Immunohistochemistry using Clone VE1 is strongly concordant with
BRAF(V600E) mutation test in papillary thyroid carcinoma. Endocr
Pathol. 26:211–217. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Roth RM, Hampel H, Arnold CA, Yearsley MM,
Marsh WL and Frankel WL: A modified Lynch syndrome screening
algorithm in colon cancer: BRAF immunohistochemistry is efficacious
and cost beneficial. Am J Clin Pathol. 143:336–343. 2015.
View Article : Google Scholar : PubMed/NCBI
|