Introduction
Multiple myeloma (MM) is a clonal plasma cell
malignancy that leads to an accumulation of plasma cells in the
bone marrow. Although the treatment response and survival rates of
MM patients have markedly improved over the last decade owing to
the broad use of novel agents, the majority of patients will
eventually develop resistant disease (1). As such, novel therapeutic options are
required by MM patients. Immunomodulatory drugs (IMiDs), including
lenalidomide and pomalidomide, are thalidomide analogues that have
efficacy in several hematological malignancies, including MM
(2,3).
Nevertheless, only 30% of patients respond to these drugs when used
as single agent, and the majority of patients will develop drug
resistance (4).
Although several mechanisms of action have been
proposed to explain the direct and indirect anti-myeloma effect of
IMiDs, such as antiangiogenic, pro-apoptotic, anti-proliferative
and immunomodulatory effects (5), the
precise molecular mechanisms and targets through which IMiDs exert
their effects remained unclear until 2010, when Ito et al
(6) identified cereblon (CRBN) as a
primary target of thalidomide teratogenicity by generating an E3
ubiquitin ligase complex with DDB1, CUL4 and Roc1. A further study
by Zhu et al (7) confirmed
that CRBN was also required for the anti-myeloma activity of
lenalidomide and pomalidomide.
Arsenic trioxide is a traditional Chinese medicine
that has been used therapeutically for ~2400 years (8); it is an effective treatment for acute
promyelocytic leukemia (APL) and can induce complete remission in
patients (9,10). Arsenic trioxide affects numerous
intracellular signal transduction pathways and causes multiple
alterations to cellular function, resulting in the induction of
apoptosis, the inhibition of growth and angiogenesis and the
promotion of differentiation in a wide variety of malignancies,
including MM (11). Thus, arsenic
trioxide may be an attractive therapeutic tool for overcoming the
resistance of myeloma cells to multiple agents.
To determine whether arsenic trioxide could
potentiate the sensitivity of multiple myeloma cells to
lenalidomide and to specify the mechanism by which this happens,
the present study investigated how arsenic trioxide affected CRBN
in MM U266 and RPMI8226 cell lines. Arsenic trioxide upregulates
the transcription and protein level of CRBN, the anti-myeloma
target of lenalidomide, and thus potentiates sensitivity of
multiple myeloma cells to lenalidomide, which sets the stage for
potential clinical trials of combination therapy to improve patient
outcome in MM.
Materials and methods
Reagents
Lenalidomide (CC-5013) was purchased from Selleck
Chemicals (Houston, TX, USA) and dissolved in dimethyl sulfoxide to
10 mM. Arsenic trioxide was obtained from Heilongjiang Harbin
Medical University Pharmaceutical Co., Ltd. (Harbin, China) at a
concentration of 5 mM. Drugs were diluted in culture medium prior
to use at the indicated working concentration.
Cell culture, and assessment of cell
viability, apoptosis and cell cycle
Human MM U266 and RPMI8226 cell lines (American Type
Culture Collection, Manassas, VA, USA) were cultured in RPMI 1640
medium supplemented with 10% fetal bovine serum, 100 U/ml
penicillin, 100 µg/ml streptomycin, and 2 mM L-glutamine (all from
Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in a 5%
CO2 humidified atmosphere. Analysis of cell viability
was performed using Cell Counting kit-8 (CCK-8; Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China), according to
the manufacturers instructions. The combination index (CI) of
arsenic trioxide and lenalidomide was calculated using CompuSyn 1.0
software (ComboSyn, Inc., Paramus, NJ, USA) based on the method
outlined by Chou (12) to determine
the existence of synergism; a CI value less than 1.0 indicates
synergism and a value less than 0.1 indicates strong synergism. The
position of cells in the cell cycle was analyzed using propidium
iodide staining by Cell Cycle and Apoptosis Analysis kit (Beyotime
Institute of Biotechnology, Jiangsu, China), according to the
manufacturers instructions, followed by analysis on BD FACSCanto II
(BD Biosciences, San Jose, CA, USA). Apoptosis was quantified using
the Annexin V-FITC/Propidium Iodide Apoptosis Detection kit
(Nanjing KeyGen Biotech Co., Ltd., Nanjing, China), according to
the manufacturers instructions, followed by analysis using a BD
FACSCanto II flow cytometer (BD Biosciences, San Jose, CA,
USA).
Western blotting
Cells were harvested after 24 h incubation at 37°C
and lysed in radioimmunoprecipitation lysis buffer (Beyotime
Institute of Biotechnology) with 1% phenylmethanesulfonyl fluoride
(Pierce; Thermo Fisher Scientific, Inc.), according to the
manufacturers instructions. The BCA protein assay kit (Nanjing
KeyGen Biotech Co., Ltd.) was used to determine total protein
concentration. Proteins were separated on a 10% Tris-HCl
polyacrylamide gel and transferred to a polyvinylidene fluoride
membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
membrane was blocked for 1 h in PBS with 0.1% Tween 20 and 5%
non-fat dry milk, then incubated overnight at 4°C with antibodies
against CRBN (catalog no. H6-NBP1-91810; dilution, 1:1,000; rabbit
anti-human; Novus, Littleton, CO, USA), Ikaros family zinc finger
proteins 1 (IKZF1; catalog no. 23210002; dilution, 1:5,000; rabbit
anti-human; Novus), IKZF3 (catalog no. ab139408; dilution,
1:10,000; rabbit anti-human; Abcam, Cambridge, UK), and β-actin
(catalog no. NB600-501; dilution, 1:1,500; mouse anti-human;
Novus), followed by washing three times with Tris-buffered
saline-Tween (Beijing Solarbio Science and Technology Co., Ltd.,
Beijing, China). Membranes were subsequently incubated with IRDye
680-conjugated goat anti-rabbit IgG (catalog no. 926-32221;
dilution, 1:10,000; LI-COR Biosciences, Lincoln, NE, USA) and IRDye
680-conjugated goat anti-mouse IgG (catalog no. 926-32220;
dilution, 1:15,000; LI-COR Biosciences) for 1 h at room
temperature. Antigen-antibody complexes were detected using the
Odyssey Two-Color Infrared Imaging system (LI-COR Biosciences).
Densitometry was conducted using Image J software (version 2.1.4.7;
National Institutes of Health, Bethesda, MD, USA) to obtain
semi-quantitative data of each band.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA extraction from different cells was
performed using the RNAprep Pure Cell/Bacteria kit (TIANGEN Biotech
Co., Ltd., Beijing, China), according to the manufacturers
protocol. The RNA concentration was measured by absorbance of
ultraviolet light at a wavelength of 260 nm, using the
spectrophotometer Nanodrop 2000 (Thermo Fisher Scientific, Inc.),
and the integrity of the extracted total RNA was detected by 1%
agarose gel electrophoresis. Reverse transcription was performed
using a TIANScript RT kit (TIANGEN Biotech Co., Ltd.) with oligo-dT
primer, according to the manufacturers protocol. qPCR was performed
using SYBRGreen Real MasterMix (TIANGEN Biotech Co., Ltd.),
according to the manufacturers instructions. qPCR was conducted
using RealMasterMix/SYBR solution (9 µl, including 20X Mix SYBR
solution 1 µl and 2.5X RealMasterMix 8 µl; TIANGEN Biotech Co.,
Ltd.), primer (1 µl, 5 pmol/l), template DNA (2 µl), and sterile
water (7 µl). The reaction conditions for qPCR were 95°C for 2 min,
followed by 40 cycles of 95°C for 15 sec, 60°C for 30 sec and 68°C
for 50 sec. All reactions were performed in triplicate. Primers for
CRBN and GAPDH were obtained from Sangon Biotech Co., Ltd.
(Shanghai, China). Primer sequences for the analyzed genes were as
follows: CRBN forward, 5-CAG TCT GCC GAC ATC ACA TAC-3 and reverse,
5-GCA CCA TAC TGA CTT CTT GAGGG-3; GAPDH forward, 5-AAG GTC GGA GTC
AAC GGATT-3 and reverse, 5-CTC CTG GAA GAT GGT GATGG-3. Expression
levels were calculated using the 2−ΔΔCq method (13).
Statistical analysis
All in vitro experiments were performed in
triplicate and repeated at least three times; a representative
experiment was selected for figures. Statistical significance of
differences observed in drug-treated vs. control cultures were
determined using Students t-test, with P<0.05 considered to
indicate a statistically significant difference. Statistical graphs
were produced using GraphPad Prism 6.0 (GraphPad Software, Inc., La
Jolla, CA, USA).
Results
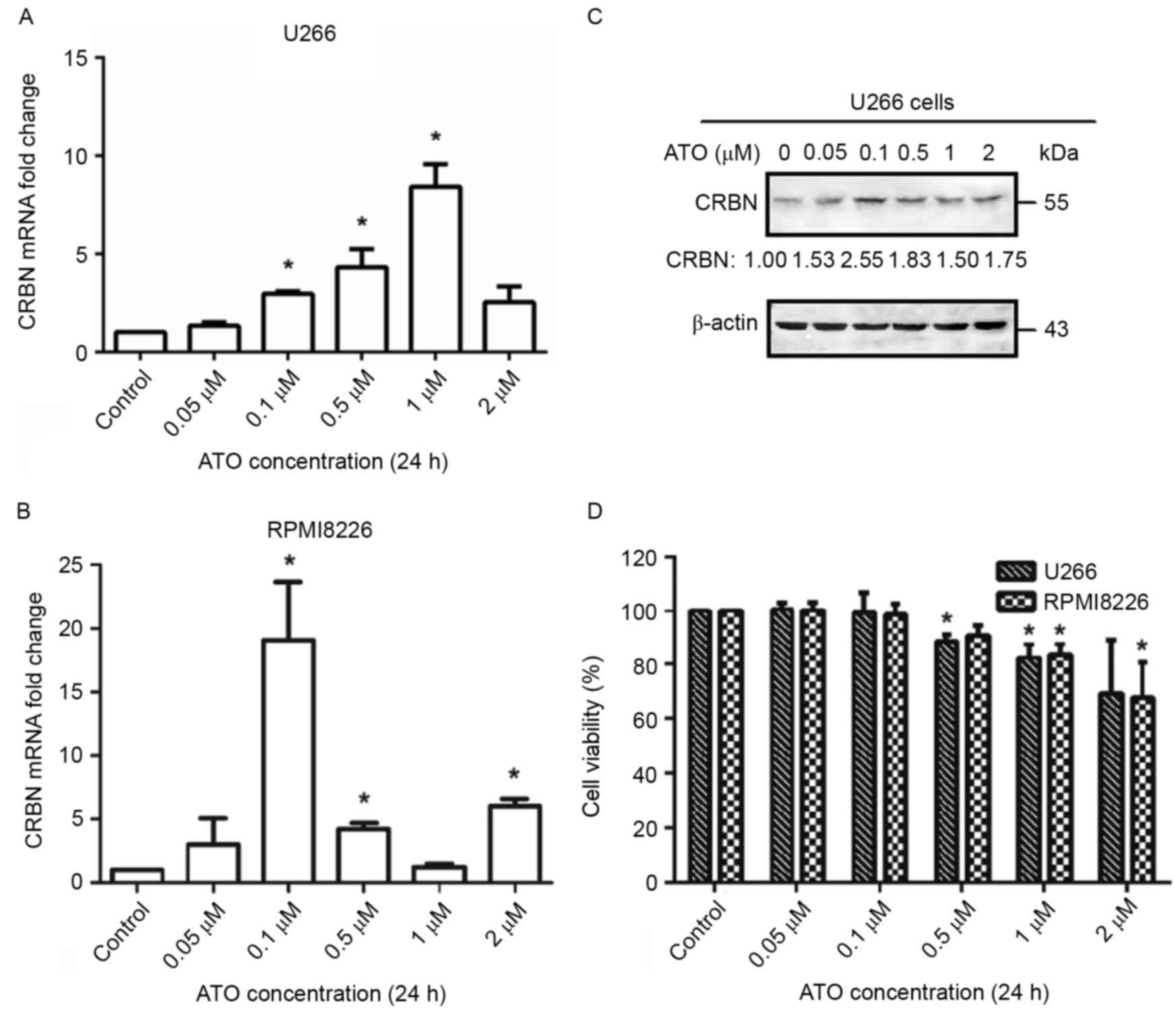
Low doses of arsenic trioxide
upregulates CRBN expression levels in MM cell lines U266 and
RPMI8226
As CRBN acts as the target of lenalidomide in MM
cells, the present study investigated whether arsenic trioxide
treatment results in the upregulation of CRBN. U266 and RPMI8226
cells were treated with varying concentrations of arsenic trioxide
in vitro for 24 h and detected the expression levels of CRBN
mRNA under different arsenic trioxide concentrations. As a result,
an increase in CRBN mRNA level was observed within a certain
arsenic trioxide concentration range (between 0.1 and 1 µM) both in
U266 and in RPMI8226 (Fig. 1A and B).
For U266 cells, arsenic trioxide elevated CRBN expression levels by
2.96-fold at a treatment concentration of 0.1 µM, 4.31-fold at a
treatment concentration of 0.5 µM, and 8.41-fold at a treatment
concentration of 1 µM, compared with the control group (P<0.05).
In RPMI8226 cells, CRBN expression levels increased 19.07-fold upon
treatment with 0.1 µM arsenic trioxide (P<0.05). The results of
western blotting also demonstrated the upregulation of CRBN protein
levels upon treatment with the aforementioned concentrations of
arsenic trioxide, particularly at a concentration of 0.1 µM
(Fig. 1C). The inconsistency between
the expression of CRBN mRNA and protein may be due to the
difference in expression between the full length and spliced forms
of mRNA upon treatment with arsenic trioxide. As the change at
protein level was more important in a biological context, 0.1 µM
arsenic trioxide was chosen as the concentration to be used for the
following experiments. The initial effective concentration of
arsenic trioxide that increased CRBN mRNA and protein expression
levels was 0.1 µM, which was much lower than the quarter-maximal
inhibitory concentration (IC25) in both cell lines (~2
µM for each cell line) (Fig. 1D),
indicating that arsenic trioxide increases CRBN expression levels
by a different mechanism from the one through which is viability
inhibited or apoptosis driven.
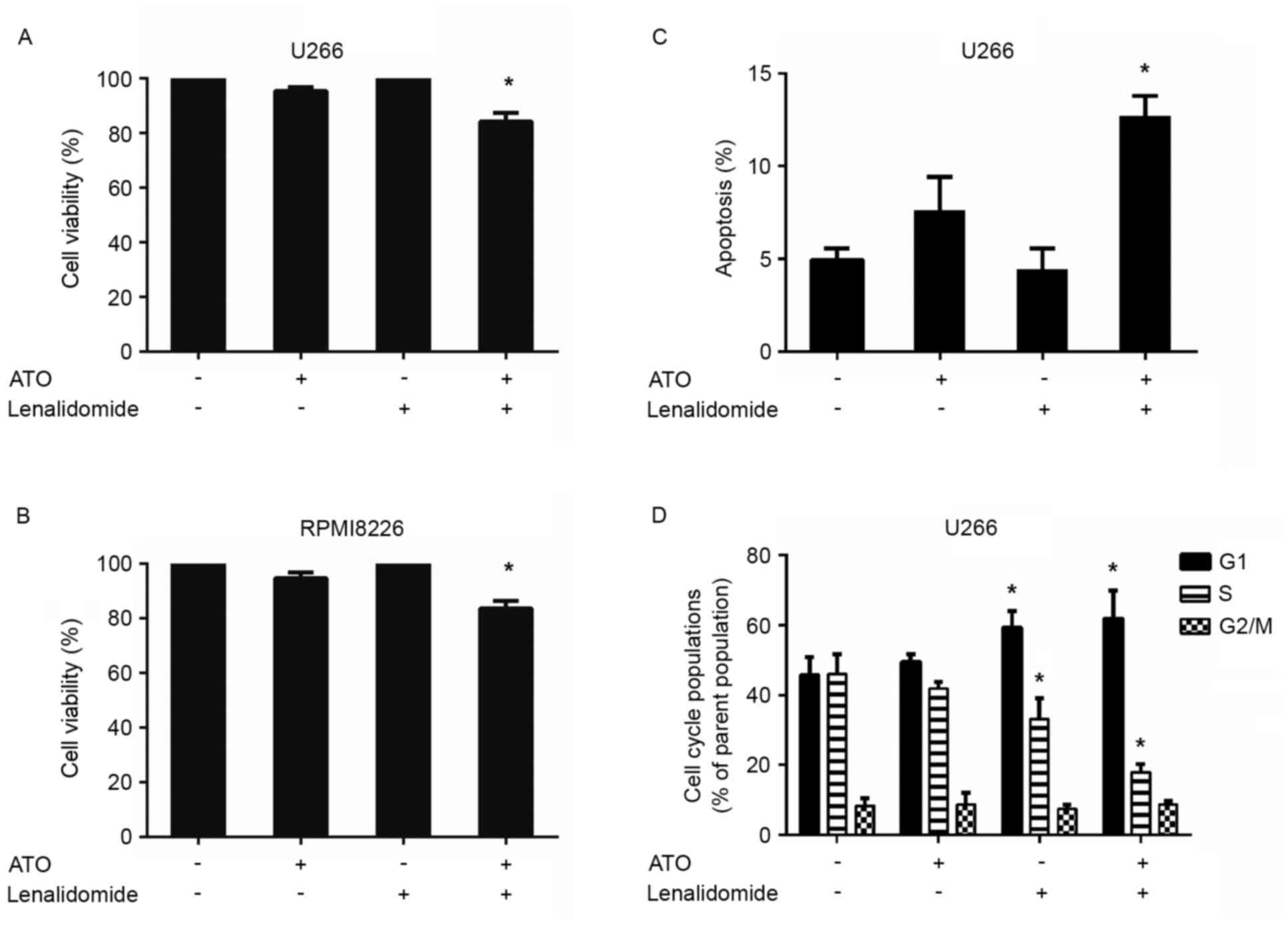
Combined low doses of arsenic trioxide
and lenalidomide trigger synergistic anti-MM activity
As arsenic trioxide could upregulate CRBN expression
levels in the MM U266 and RPMI8226 cell lines, whether pretreatment
of arsenic trioxide could result in increased sensitivity to
lenalidomide in these two cell lines was investigated. U266 and
RPMI8226 cells were pretreated with 0.1 µM arsenic trioxide for 24
h and lenalidomide was then added to a final concentration of 10 µM
for an additional 24 h, followed by assessment for cell viability
using CCK-8 assays. Arsenic trioxide and lenalidomide were used at
concentrations lower than the maximal cytotoxic concentration for
each cell line. A significant decrease in viability of both cell
lines was observed in response to the combination therapy
(P<0.05; n=3; Fig. 2A and B).
Treatment of U266 cells with low doses of arsenic trioxide (0.1 µM)
and lenalidomide (10 µM) triggered a 16% decrease in cell
viability, whereas only minimal viability inhibition was observed
using either of these agents alone at these low concentrations
(Fig. 2A). The same effect was also
observed in RPMI8226 cells: Treatment with low doses of the two
agents together (0.1 µM arsenic trioxide and 10 µM lenalidomide)
triggered a 17% decrease in cell viability, whereas only minimal
viability inhibition was observed using either of these agents
alone at these low concentrations (Fig.
2B). CI calculation confirmed the strong synergistic anti-MM
activity of these two agents, with a CI value <0.1 in both
MM-cell lines tested. These data demonstrate the synergistic
anti-MM activity of arsenic trioxide plus lenalidomide.
In order to gain insights into the mechanism by
which the decrease in cell viability of the combinations observed
in the CCK-8 assays, annexin V-FITC/propidium iodide apoptosis
detection was conducted using flow cytometry (Fig. 2C). The combination treatment induced
an apoptotic rate of 12.7%, which was higher than those in the
single agent group. The anti-proliferative activity of the
combination treatment was further analyzed by evaluating the
effects on the cell-cycle profile. The combination treatment of
arsenic trioxide and lenalidomide induced an increase in the
percentage of cells in G0/G1 phase, with a decrease in the
percentage of those in proliferative S and G2/M phases (Fig. 2D).
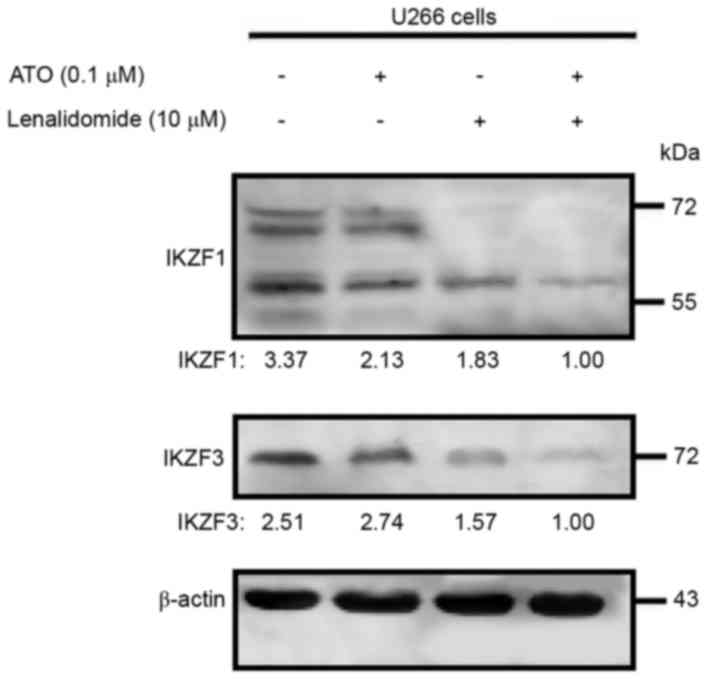
Arsenic trioxide plus lenalidomide
downregulates IKZF1 and IKZF3 expression levels
The ubiquitination and degradation of IKZF1 and
IKZF3 (downstream proteins of CRBN) is necessary for the
anti-myeloma effect of lenalidomide. As arsenic trioxide increased
the CRBN expression level, whether the enhanced anti-MM effect of
the combination therapy acted as the enhanced CRBN pathway was then
examined. Western blot analysis revealed that arsenic trioxide as a
single agent did not affect the expression level of IKZF1 or IKZF3
but, as anticipated, lenalidomide did downregulate expression of
the two proteins. Multiple IKZF1 bands were detected, presumably
due to alternative splicing (14).
The use of arsenic trioxide and lenalidomide in combination had a
significantly higher inhibitory effect on IKZF1 and IKZF3 protein
expression levels, compared with the effect of either agent alone
in the U266 cell line (Fig. 3), which
could be evidence that arsenic trioxide can act as a sensitizer
agent for the anti-myeloma agent lenalidomide.
Discussion
The present study revealed that low doses of arsenic
trioxide potentiate the sensitivity of MM cell lines to
lenalidomide by upregulating the expression of CRBN, the
anti-myeloma target of lenalidomide. The results of the present
study demonstrated that low doses of arsenic trioxide upregulated
CRBN mRNA level in U266 and RPMI8226 cell lines. In U266 cells,
arsenic trioxide increased CRBN in doses lower than the
IC25; a similar effect was also observed in RPMI8226
cells. CRBN protein expression levels validated the upregulation
effect of low dose arsenic trioxide. Genetic heterogeneity is a
hallmark of MM (15), which may
explain, at least in part, the differences observed in the effects
induced by arsenic trioxide between the two cell lines.
As the effect of elevated CRBN expression, whether
the upregulation of CRBN caused by arsenic trioxide results in
increased sensitivity of lenalidomide in MM cells was assessed. The
results of the present study demonstrated that combined low doses
of arsenic trioxide (0.1 µM) and lenalidomide (10 µM) triggered the
inhibition of viability in U266 and RPMI8226 cells, which was only
achievable at much higher doses of either agent alone. Apoptosis
analysis also demonstrated the synergetic effect in the combination
of these two drugs. Analysis of cell-cycle profiles confirmed that
the combination therapy induced an increase in the percentage of
cells in the G0/G1 phase, with a decrease in those in proliferative
phases (S and G2/M phases), which could contribute to the increased
inhibition of MM cell viability of this combination.
Previous studies found that the targets for
proteasomal degradation ubiquitinated by lenalidomide-bound CRBN
were identified as the B cell-specific transcription factors IKZF1
and IKZF3 (14,16). Analysis of myeloma cell lines
demonstrated that the loss of IKZF1 and IKZF3 expression is
necessary and sufficient for the therapeutic effect of
lenalidomide, through the downregulation of interferon regulatory
factor 4 (IRF4), and this is associated with its anti-myeloma
activity (14), suggesting a
mechanism of action for lenalidomide on MM cells. Previous studies
revealed that lenalidomide-bound CRBN acquires the ability to
target IKZF1 and IKZF3 for proteasomal degradation, and analysis of
myeloma cell lines demonstrating that the loss of IKZF1 and IKZF3
is necessary and sufficient for the therapeutic effect of
lenalidomide (14,16). The mechanism of the synergistic
mechanism of these two drugs was assessed through detection of the
expression levels of IKZF1 and IKZF3. The combination therapy of
arsenic trioxide and lenalidomide had a significantly greater
inhibitory effect on IKZF1 and IKZF3 protein expression levels,
compared with the effect of either agent alone in the U266 cell
line, whereas treatment with arsenic trioxide alone had no direct
effect on the expression of these two proteins. These data are
consistent with the findings of the present study, which found that
arsenic trioxide increases the expression of CRBN in MM cells and
the combination of low doses of arsenic trioxide and lenalidomide
has a synergistic effect on decreasing viability rate, while
increasing the apoptotic rate of MM cells. Myc proto-oncogene
protein (MYC) is known to be a major downstream target of
CRBN/IKZF1 signaling via IRF4 (7).
Although MYC often acts to promote cell viability by driving entry
into the cell cycle through the G1-S transition, the mechanism of
G0/G1 cell cycle arrest induced by the combination treatment may be
due to enhanced MYC downregulation, caused by the upstream
downregulation of IKZF1 by lenalidomide.
The findings of the present study reveal a novel
target for the traditional agent arsenic trioxide and provide the
potential value of this drug in the treatment of the plasma cell
disorder multiple myeloma. As the mechanism of the upregulation of
CRBN by arsenic trioxide remains unclear, future studies are
required to specify this mechanism, which could provide more
comprehensive understanding to arsenic trioxide and investigate
more therapeutic value. Since lenalidomide is increasingly used to
treat MM, the resistance to this IMiD is attracting a greater
degree of attention. The findings of the present study potentially
provide an efficient and economical way of working around
resistance to lenalidomide. However, since lenalidomide acts more
like an immunomodulatory drug, in vitro studies are not
sufficient and in vivo data are required to assess the
effect of the two drugs in combination in the future.
To summarize, the results of the present study
demonstrate the ability of arsenic trioxide to increase expression
of CRBN, the direct target of the anti-MM effect of lenalidomide,
and the potent in vitro anti-MM activity of arsenic trioxide
combined with lenalidomide at low doses. These findings provide the
framework for the next step of in vivo studies and, if these
studies are successful, for clinical trials of low-dose combination
of arsenic trioxide and lenalidomide to increase response, overcome
drug resistance, reduce side effects and improve patient outcome in
MM.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81172252 and
81500164), and the Capital of Clinical Characteristics and the
Applied Research Fund of China (grant no. Z131107002213146).
References
|
1
|
Avigan D and Rosenblatt J: Current
treatment for multiple myeloma. N Engl J Med. 371:961–962. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy
MQ, Hayman SR, Buadi FK, Zeldenrust SR, Dingli D, Russell SJ, Lust
JA, et al: Improved survival in multiple myeloma and the impact of
novel therapies. Blood. 111:2516–2520. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Richardson PG, Blood E, Mitsiades CS,
Jagannath S, Zeldenrust SR, Alsina M, Schlossman RL, Rajkumar SV,
Desikan KR, Hideshima T, et al: A randomized phase 2 study of
lenalidomide therapy for patients with relapsed or relapsed and
refractory multiple myeloma. Blood. 108:3458–3464. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kortuem KM, Zidich K, Schuster SR, Khan
ML, Jimenez-Zepeda VH, Mikhael JR, Fonseca R and Stewart AK:
Activity of 129 single-agent drugs in 228 Phase I and II clinical
trials in multiple myeloma. Clin Lymphoma Myeloma Leuk.
14:284–290.e5. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Quach H, Ritchie D, Stewart AK, Neeson P,
Harrison S, Smyth MJ and Prince HM: Mechanism of action of
immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia.
24:22–32. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ito T, Ando H, Suzuki T, Ogura T, Hotta K,
Imamura Y, Yamaguchi Y and Handa H: Identification of a primary
target of thalidomide teratogenicity. Science. 327:1345–1350. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu YX, Braggio E, Shi CX, Bruins LA,
Schmidt JE, Van Wier S, Chang XB, Bjorklund CC, Fonseca R,
Bergsagel PL, et al: Cereblon expression is required for the
antimyeloma activity of lenalidomide and pomalidomide. Blood.
118:4771–4779. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Douer D and Tallman MS: Arsenic trioxide:
New clinical experience with an old medication in hematologic
malignancies. J Clin Oncol. 23:2396–2410. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM,
Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, et al: Use of arsenic
trioxide (As2O3) in the treatment of acute promyelocytic leukemia
(APL): II. Clinical efficacy and pharmacokinetics in relapsed
patients. Blood. 89:3354–3360. 1997.PubMed/NCBI
|
|
10
|
Soignet SL, Maslak P, Wang ZG, Jhanwar S,
Calleja E, Dardashti LJ, Corso D, DeBlasio A, Gabrilove J,
Scheinberg DA, et al: Complete remission after treatment of acute
promyelocytic leukemia with arsenic trioxide. N Engl J Med.
339:1341–1348. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miller WH Jr, Schipper HM, Lee JS, Singer
J and Waxman S: Mechanisms of action of arsenic trioxide. Cancer
Res. 62:3893–3903. 2002.PubMed/NCBI
|
|
12
|
Chou TC: Theoretical basis, experimental
design, and computerized simulation of synergism and antagonism in
drug combination studies. Pharmacol Rev. 58:621–681. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu G, Middleton RE, Sun H, Naniong M, Ott
CJ, Mitsiades CS, Wong KK, Bradner JE and Kaelin WG Jr: The myeloma
drug lenalidomide promotes the cereblon-dependent destruction of
Ikaros proteins. Science. 343:305–309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bergsagel PL and Kuehl WM: Molecular
pathogenesis and a consequent classification of multiple myeloma. J
Clin Oncol. 23:6333–6338. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Krönke J, Udeshi ND, Narla A, Grauman P,
Hurst SN, McConkey M, Svinkina T, Heckl D, Comer E, Li X, et al:
Lenalidomide causes selective degradation of IKZF1 and IKZF3 in
multiple myeloma cells. Science. 343:301–305. 2014. View Article : Google Scholar : PubMed/NCBI
|