Introduction
Cervical cancer is the second most common malignancy
in women (1) and has a significant
impact on their health and lives (2).
According to the World Health Organization, 266,000 women worldwide
die of cervical cancer annually. Cervical cancer is the most common
cancer among women in 45 different countries; these include
countries in sub-Saharan Africa, Asia, including India, and certain
Central and South American countries (3). A variety of factors contribute to
cervical cancer occurrence and development; for example, the
high-risk human papilloma virus (4,5). Cervical
cancer susceptibility is also increased by host genetic factors
(6). Additionally, there are other
biological molecules that are correlated with the risk of cervical
cancer, such as forkhead box protein P3 (7).
C-type lectins, a large superfamily of proteins,
have been reported to serve important roles in immunity, cell death
and tumorigenesis (8–11). Dendritic cell-specific intercellular
adhesion molecule-grabbing nonintegrin-related protein (DC-SIGN) is
a membrane-bound member of the C-type lectin superfamily that is
expressed in dendritic cells, monocyte-derived dendritic cells and
specialized macrophages in vitro (12). The genes encoding DC-SIGN and its
homologue DC-SIGN-related protein (DC-SIGNR) are located on human
chromosome 19p13.3, and belong to a subfamily in the lectin gene
cluster along with cluster of differentiation (CD)23 and liver and
lymph node sinusoidal endothelial cell C-type lectin (LSECtin)
(13–15). DC-SIGN is also expressed in the
endothelial cells of the hepatic sinusoid and lymphatic sinus
(16). DC-SIGN expression is
associated with colorectal cancer (17) and non-Hodgkin's lymphoma (NHL)
(18); thus DC-SIGN may be useful in
a clinical setting (18).
Since DC-SIGNR and DC-SIGN share a 77% structural
similarity (19), their function may
be similar. DC-SIGNR is highly expressed in endothelial cells in
the placenta, liver and lymph nodes (20), but is expressed at lower levels in NHL
and lung cancer (18,21). In addition, a previous study reported
that serum concentrations of DC-SIGNR were higher in brain
metastatic compared with in non-metastatic lung cancer (21). However, an association between
DC-SIGNR and cervical cancer has not been reported, to the best of
our knowledge. In the present study, the tissue expression of
DC-SIGNR and levels of serum (s)DC-SIGNR in patients with cervical
cancer were investigated in order to determine whether sDC-SIGNR
could be used as biomarker for the detection of cervical cancer.
Additionally, the association between the expression of DC-SIGNR
and squamous cell carcinoma antigen (SCC-Ag), the serum marker
typically used for the clinical monitoring of cervical cancer, was
measured (22).
Materials and methods
Clinical samples
Cervical tissue samples from 25 patients with
cervical cancer, 14 patients with cervical intraepithelial
neoplasia (CIN) and 15 patients with cervical polyps were obtained
from the Second Affiliated Hospital of Dalian Medical University
(Dailan, China) between July 2007 and September 2015. Additionally,
10 lymph node tissue samples from healthy individuals were obtained
from the Second Affiliated Hospital of Dalian Medical University
between August 2009 and May 2015. The lymph node tissue was used as
a positive control for DC-SIGNR expression, while the cervical
polyp tissue was used as a negative control. The clinical data for
these subjects are summarized in Table
I.
 | Table I.Clinical data of patients with
cervical cancer, cervical intraepithelial neoplasia and cervical
polyps whose tissue samples were included in the
immunohistochemical analysis. |
Table I.
Clinical data of patients with
cervical cancer, cervical intraepithelial neoplasia and cervical
polyps whose tissue samples were included in the
immunohistochemical analysis.
| Clinical data | Cervical cancer
(n=25), n (%) | CIN (n=14), n
(%) | Cervical polyp
(n=15), n (%) |
|---|
| Age |
|
|
|
|
≥50 | 15 (60) | 4
(29) | 6
(40) |
|
<50 | 10 (40) | 10 (71) | 9
(60) |
| SCC-Ag (ng/ml) |
|
|
|
|
≥2.5 | 10 (40) | 0 (0) | 0 (0) |
|
<2.5 | 6
(24) | 5
(36) | 1 (7) |
| No data
available | 9
(36) | 9
(64) | 14 (93) |
| Mean density |
|
|
|
|
≥0.000124498 | 23 (92) | 9
(64) | 9
(60) |
|
<0.000124498 | 2 (8) | 5
(36) | 6
(40) |
Serum samples from 84 patients with cervical cancer
were obtained between November 2012 and November 2015 from the
Second Affiliated Hospital of Dalian Medical University and stored
at −80°C until required. The mean age of the 84 patients was 50.38
years old and the range was 17–74 years. Clinical staging of
cervical cancer patients was performed according to the
International Federation of Gynecology and Obstetrics stage
(23). Clinical parameters including
age, clinical stage, concentrations of lactate dehydrogenase (LDH),
alkaline phosphatase (ALP), SCC-Ag, serum carcinoembryonic antigen
(CEA), carbohydrate antigens (CAs)-199, −153 and −125, and T cell
subsets were acquired from hospitalization records. The data are
illustrated in Table II. The control
group was composed of 69 healthy blood donor volunteers, which were
obtained from the Second Affiliated Hospital of Dalian Medical
University between June 2013 and September 2015. The mean age of
the control group volunteers was 52 years old, with a range of
23–74 years. Each healthy volunteer was submitted to a routine
physical examination, and all of the results were in the normal
range. Patients and healthy individuals with severe infections,
known allergies and cachexia were excluded from the present study.
The present study was approved by the Research Ethics Committee of
Dalian Medical University and all participants provided written
informed consent prior to enrollment.
 | Table II.sDC-SIGNR levels according to age,
cancer stage and levels of serum SCC-Ag. |
Table II.
sDC-SIGNR levels according to age,
cancer stage and levels of serum SCC-Ag.
| Clinical data | No. of patients
(%) | sDC-SIGNR (ng/ml),
median (range) | P
valuea |
|---|
| Age (n=84) |
|
| 0.7944 |
|
≥50 | 45 (54) | 115.7
(51.15–769.5) |
|
|
<50 | 39 (46) | 118.0
(29.64–247.2) |
|
| Cancer stage
(n=82) |
|
| 0.0336 |
|
I–II | 60 (73) | 125.9
(29.64–769.5) |
|
|
III–IV | 22 (27) | 99.3
(42.27–225.0) |
|
| SCC-Ag (n=54) |
|
| 0.0426 |
|
≥2.5 | 16 (30) | 148.3
(42.27–378.9) |
|
|
<2.5 | 38 (70) | 105.4
(29.64–271.3) |
|
Immunohistochemistry
Sections from paraffin-embedded blocks of each
tissue sample were incubated at 60°C for 30 min. The paraffin was
removed with xylene and the samples were rehydrated in a graded
ethanol series. Tissue sections (5 µm thick) were washed with PBS
and endogenous peroxidase activity was blocked with 3% hydrogen
peroxide diluted in double-distilled water for 10 min at room
temperature. Antigen retrieval was performed by heating the slides
in 0.01 mol/l citrate-buffered solution (pH 6.0) for 20 min in a
microwave oven. Following antigen retrieval, sections were washed
with PBS and blocked with goat serum (ZSGB-BIO, Beijing, China) for
20 min at the room temperature. An anti-DC-SIGNR rabbit polyclonal
antibody (cat. no. ab169783; dilution, 1:150; Abcam, Cambridge, UK)
was incubated with sections overnight at 4°C. The next day,
sections were washed 3 times in PBS (5 min/wash). Then, the
sections were incubated for 1 h at 37°C with a horseradish
peroxidase-labeled anti-rabbit secondary antibody (1:1,000) and
washed 3 times in PBS. The sections were developed by applying
3,3′-diaminobenzidine tetrahydrochloride (ZSGB-BIO) to the sections
and then counterstaining with hematoxylin. An Olympus BX51
microscope (Olympus Corporation, Tokyo, Japan) was used to capture
images of all sections. The mean density of the DC-SIGNR expression
was obtained using Image-Pro Plus software (version 6.0; Media
Cybernetics, Inc., Rockville, MD, USA).
ELISA
An anti-DC-SIGNR goat polyclonal antibody (cat. no.
sc-17261; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) was used
at a final concentration of 0.27 µg/ml to coat 96-well microplates
before incubating the plates overnight at 4°C. The next day, the
plates were washed 3 times with PBS containing 0.05% Tween-20
(PBST; pH 7.4), and the wells were blocked with 5% bovine serum
albumin (cat. no. Roche1073507801; BIOSHSARP, Inc., Hefei, China)
at 37°C for 1 h. Following 3 washes with PBST, 100 µl of serum from
patients with cervical cancer and healthy individuals were added to
the wells, using 100 µl PBS as a negative control. Each plate was
incubated at 37°C for 1 h, and then the wells were washed 3 times
with PBST before adding 100 µl anti-DC-SIGNR mouse monoclonal
antibody (cat. no. MAB16211; R&D Systems, Inc., Minneapolis,
MN, USA), diluted to a concentration of 0.5 µg/ml, to each well.
The plates were incubated at 37°C for 1.5 h, and then the wells
were washed 3 times with PBST. Following the PBS wash, 100 µl of a
peroxidase-conjugated goat anti-mouse antibody (cat. no. ZB-2305;
dilution, 1:1,500; ZSGB-BIO) was added and the plates were
incubated for 60 min at 37°C. The plates were washed 3 times with
PBST and 3,3′,5,5′-tetramethylbenzidine (Tiangen Biotech Co., Ltd.,
Beijing, China) was added to each well prior to incubation at 37°C
for 30 min. Finally, 2 mol/l H2SO4 was added
to stop the reaction. The optical density (OD) was measured on a
microplate reader at a wavelength of 450 nm. The sDC-SIGNR
concentration in each sample was calculated by comparing the OD
values with a standard curve.
Statistical analysis
A non-parametric Mann-Whitney U test was used to
determine the statistical significance between groups in the
immunohistochemical and ELISA analyses. A non-parametric Spearman's
rank correlation coefficient test was performed to measure the
correlation of sDC-SIGNR with SCC-Ag levels and other clinical
parameters. Receiver operating characteristic (ROC) curve analysis
was applied to assess the diagnostic value of sDC-SIGNR in cervical
cancer. All statistical analyses were performed using GraphPad
Prism 5 software (version 5; GraphPad Software, Inc., La Jolla, CA,
USA).
Results
DC-SIGNR mean density is significantly
increased in cervical cancer tissue compared with CIN and cervical
polyp tissue
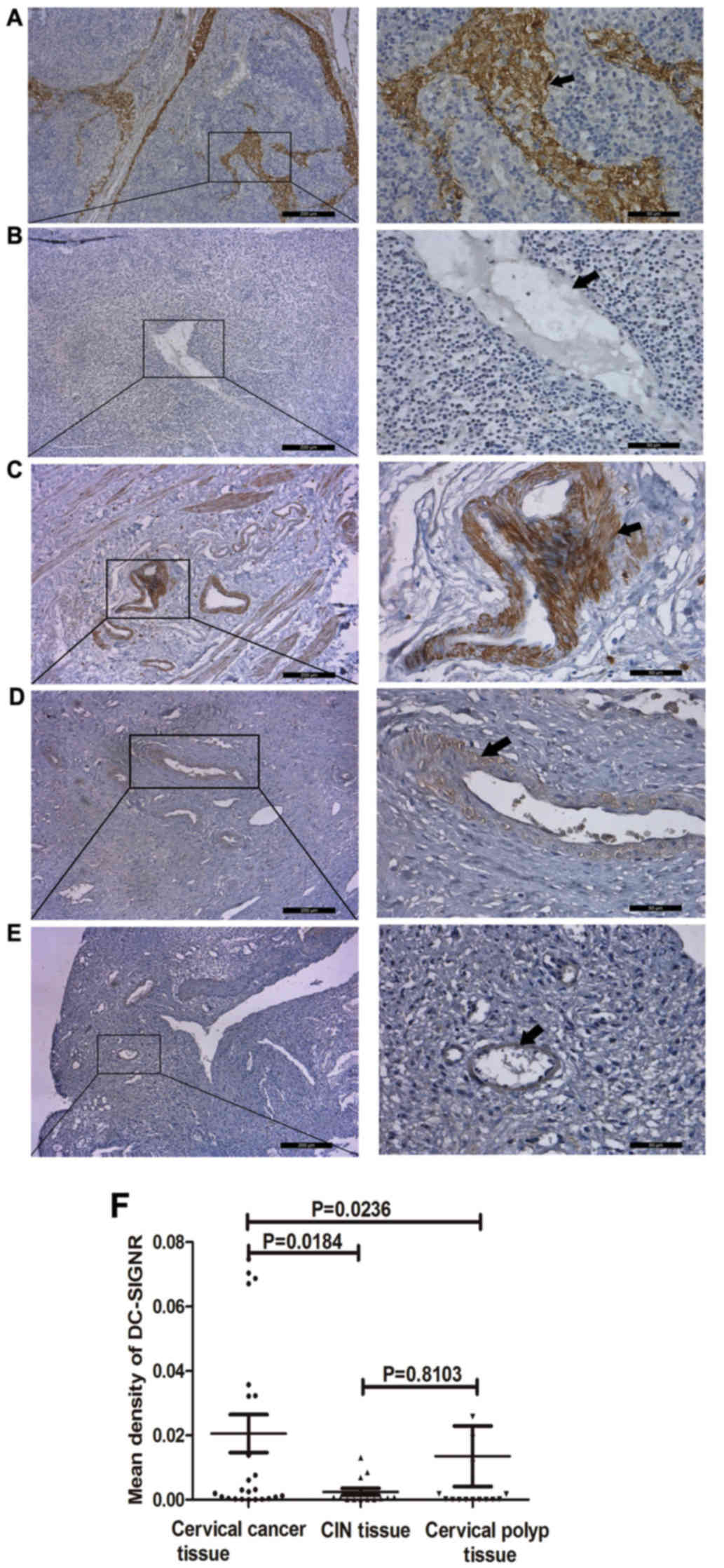
Immunohistochemical staining was used to explore
whether DC-SIGNR is overexpressed in cervical cancer (Fig. 1). Previous studies have demonstrated
that DC-SIGNR is expressed in normal lymph nodes (24), therefore normal lymph node tissue was
included as a positive control (Fig.
1A). Staining of cervical tissue samples from 25 patients with
cervical cancer, 14 patients with CIN and 15 patients with cervical
polyps revealed that DC-SIGNR was overexpressed in cervical cancer
tissue (Fig. 1C). The mean density of
DC-SIGNR in cervical cancer tissue was significantly higher
compared with CIN and cervical polyp tissue (P=0.0184 and P=0.0236,
respectively; Fig. 1F); however,
there was no significant difference between the mean density of
DC-SIGNR expression in CIN and cervical polyp tissue (P=0.8103;
Fig. 1F). Additionally, the mean
density of DC-SIGNR expression positively correlated with serum
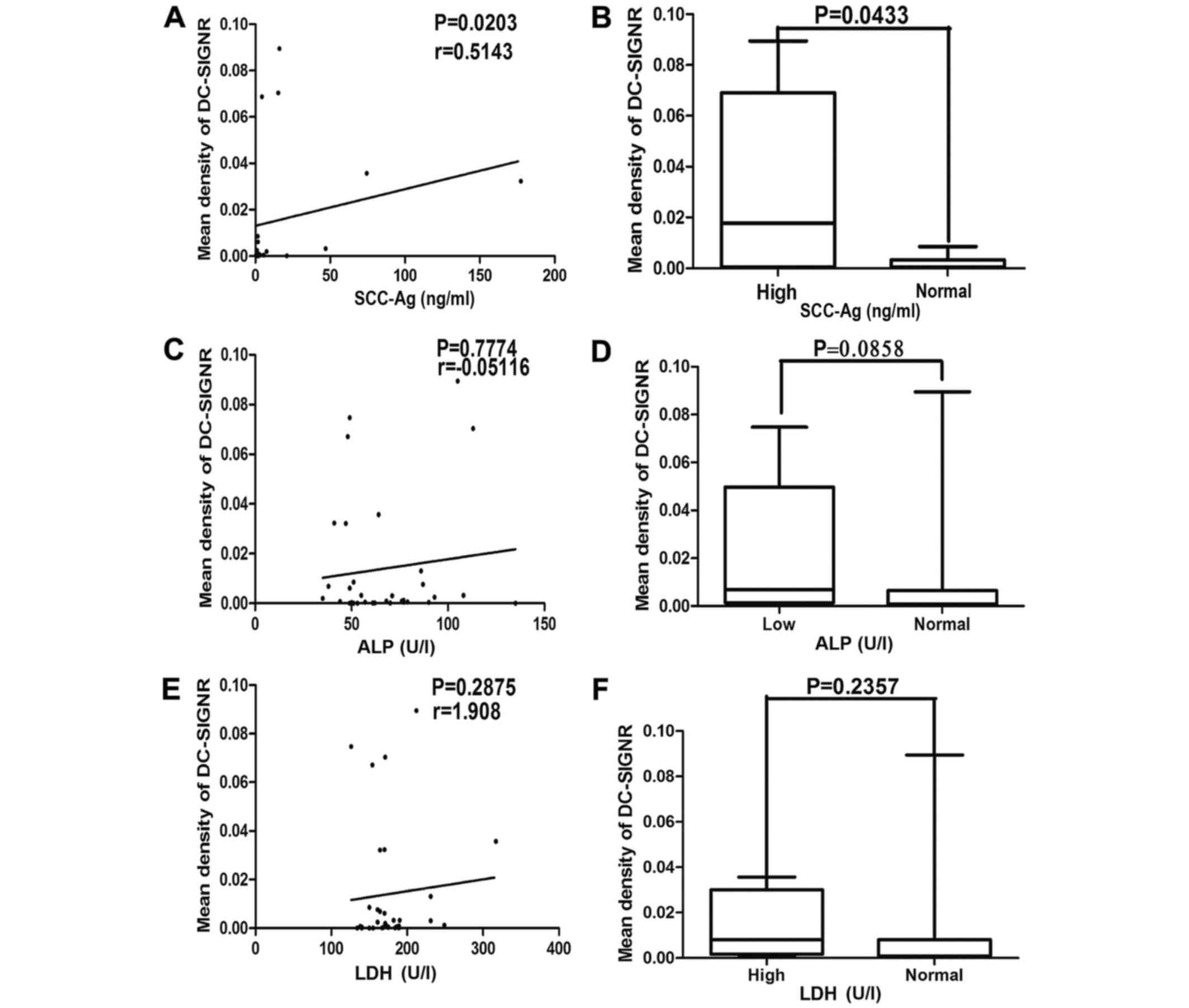
levels of SCC-Ag (r=0.5143; P=0.0203; Fig. 2A). Furthermore, the mean density of
DC-SIGNR was significantly increased in samples with serum SCC-Ag
levels higher compared with the normal range (P=0.0433; Fig. 2B). However, there was no correlation
between serum LDH (Fig. 2C and D),
ALP (Fig. 2E and F), or CD3, 4, and 8
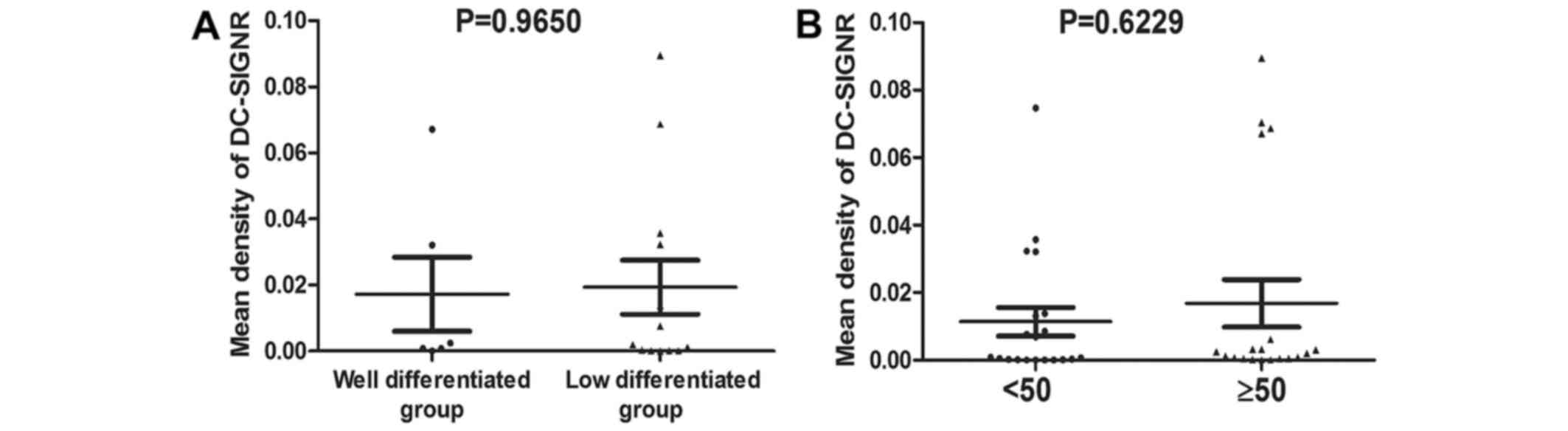
levels (data not shown) and the mean density of DC-SIGNR. There was
no significant difference between the mean density of DC-SIGNR in
cervical cancer samples that were well differentiated compared with
samples that exhibited low differentiation (P=0.9650; Fig. 3A) (25).
Additionally, there was no significant difference between the mean
density of DC-SIGNR in cervical cancer tissue from patients who
were ≥50 years old and patients who were <50 years old
(P=0.6229; Fig. 3B).
Levels of sDC-SIGNR are significantly
higher in patients with cervical cancer compared with healthy
individuals
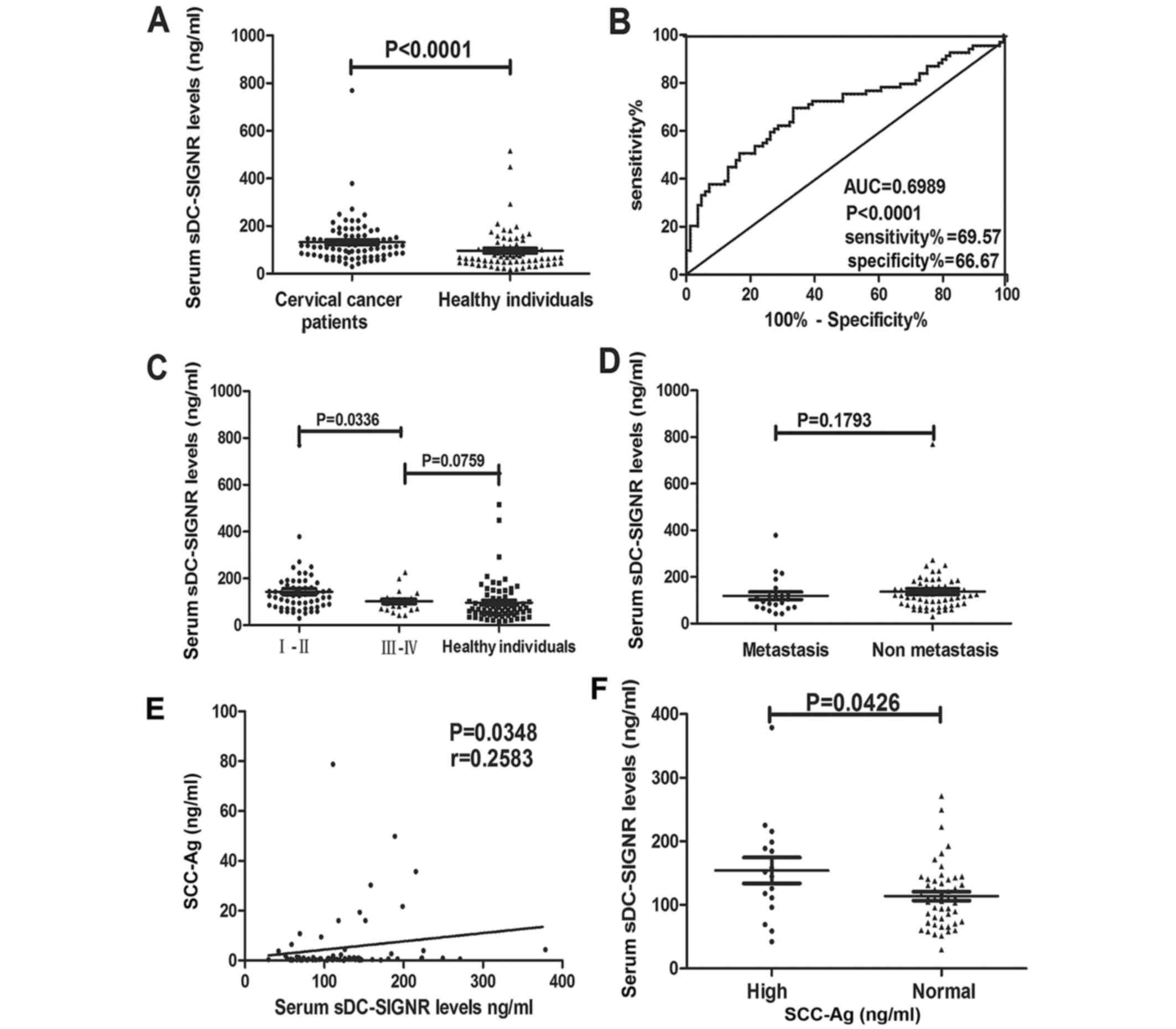
Using an ELISA, the sDC-SIGNR levels of 84 patients
with cervical cancer and 69 healthy individuals were compared.
Levels of sDC-SIGNR in patients with cervical cancer were
significantly higher compared with those in healthy individuals
(P<0.0001; Fig. 4A).
Concentrations of sDC-SIGNR ranged from 29.64–769.5 ng/ml (mean,
132.1 ng/ml) in patients with cervical cancer and from 16.76–515.8
ng/ml (mean, 96.28 ng/ml) in healthy individuals. A ROC curve is
frequently used to evaluate the power of a novel serum marker to
predict the presence of tumors. In the present study, a cut-off
value of 93.7 ng/ml for sDC-SIGNR was identified to predict the
presence of cervical cancer with 69.57% sensitivity and 66.67%
specificity (P<0.0001; Fig.
4B).
Levels of sDC-SIGNR are higher in
stage I and II cervical cancer compared with stage III and IV
cervical cancer
Excluding 2 patients with CIN III, levels of
sDC-SIGNR were compared between 60 patients with stage I–II and 22
patients with III–IV cervical cancer, where stages I–II correspond
to earlier stages of disease progression and would therefore have a
better prognosis. Levels of sDC-SIGNR in patients with stage I and
II cervical cancer were significantly higher than in patients with
stage III and IV cervical cancer (P=0.0336; Fig. 4C). There was no statistical
significance between sDC-SIGNR levels in stage III and IV patients
compared with healthy individuals (P=0.0759; Fig. 4C). These results suggest that
sDC-SIGNR could be used as a biomarker for the early diagnosis of
cervical cancer. However, there was no significant difference in
sDC-SIGNR levels in patients with metastatic cervical cancer
compared with patients with non-metastatic cervical cancer
(P=0.1793; Fig. 4D).
Levels of sDC-SIGNR positively
correlate with serum levels of SCC-Ag
SCC-Ag serves as a prognostic marker for cervical
cancer (26); patients with cervical
cancer present with higher levels of serum SCC-Ag compared with
healthy individuals (27). Therefore,
the association between serum SCC-Ag and sDC-SIGNR levels were
analyzed in the group of patients with cervical cancer. Notably,
there was a positive correlation between the levels of serum SCC-Ag
and sDC-SIGNR (r=0.2583; P=0.0348; Fig.
4E). The levels of sDC-SIGNR were significantly higher in
samples with high serum SCC-Ag levels compared with samples with
normal serum SCC-Ag levels (P=0.0426; Fig. 4F).
There is no association between
sDC-SIGNR levels and the levels of serum CEA or CAs-199, −153 and
−125
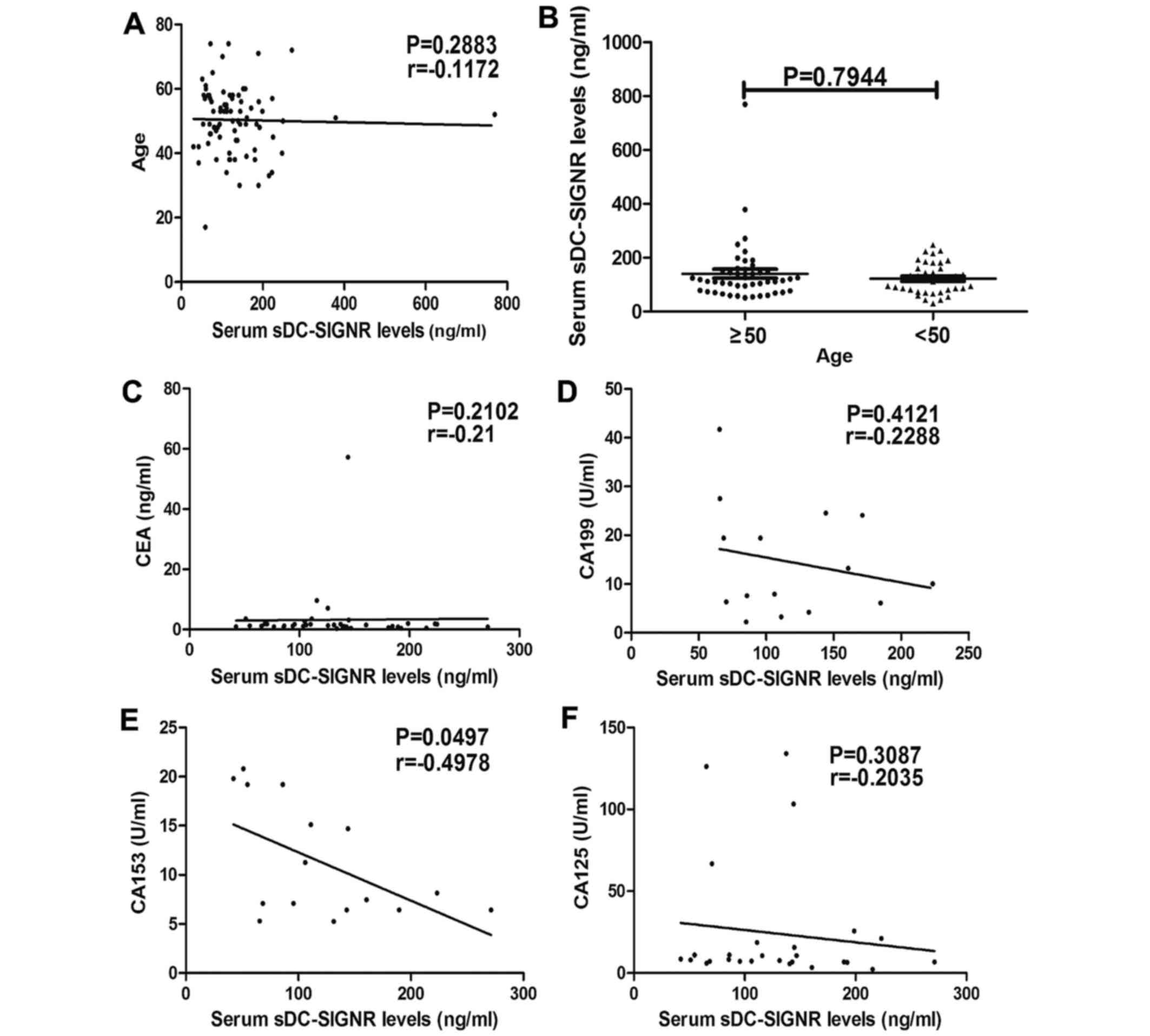
Levels of sDC-SIGNR were not associated with age
(r=−0.1172; P=0.2883; Fig. 5A), and
there was no statistically significant difference between sDC-SIGNR
levels in patients aged ≥50 and <50 (P=0.7944; Fig. 5B). There was no association between
sDC-SIGNR levels and the levels of serum CEA (r=−0.21; P=0.2102;
Fig. 5C), CA-199 (r=−0.2288;
P=0.4121; Fig. 5D), CA-153
(r=−0.4978; P=0.0497; Fig. 5E) and
CA-125 (r=−0.2035; P=0.3087; Fig.
5F).
Discussion
DC-SIGNR is a member of the C-type lectin family of
proteins. Previous research has focused on the association between
DC-SIGNR and viral infections, including human immunodeficiency
virus (HIV) (27,28) and hepatitis B (29). Previous studies have indicated that
DC-SIGNR is highly expressed in peripheral blood mononuclear cells
in patients with HIV-1 infection (30). However the role of DC-SIGNR in cancer,
particularly cervical cancer, remains unclear.
In order to study the clinical value of DC-SIGNR in
cervical cancer, the mean density of DC-SIGNR in the tissue of 25
patients with cervical cancer, 14 patients with CIN and 15 patients
with cervical polyps was determined by immunohistochemistry. The
mean density of DC-SIGNR was significantly higher in cervical
cancer tissue compared with CIN and cervical polyp tissue (P=0.0184
and P=0.0236, respectively). However, there was no significant
difference in the mean density between CIN and cervical polyp
tissue samples (P=0.8103). This result suggests that DC-SIGNR could
serve as a biomarker for the early diagnosis of cervical
cancer.
The rate of recurrence of cervical cancer in
patients is low; however, the sensitivity of the cancer to
radiotherapy and chemotherapy is significantly decreased in cases
where the cancer is recurrent (31).
SCC-Ag is a glycoprotein that belongs to the serpin family of
serine/cysteine proteinase inhibitors. SCC-Ag has been associated
with the prognosis of recurrent cervical squamous carcinoma
(32), and is of great significance
in the prognosis and clinical diagnosis of cervical cancer
(33–35). In the present study, a correlation
between the mean density of DC-SIGNR and the clinical serum SCC-Ag
concentration was observed (r=0.5143; P=0.0203). However, there was
no association between DC-SIGNR and other clinical markers, such as
CEA and CA-199, −125 and −153.
Due to the association between DC-SIGNR levels and
cervical cancer identified by immunohistochemistry, the levels of
sDC-SIGNR in patients with cervical cancer were analyzed by ELISA
and compared with the levels of sDC-SIGNR from healthy individuals.
The level of sDC-SIGNR was higher in patients with cervical cancer
compared with that in healthy individuals, and was significantly
positively correlated with SCC-Ag levels. Previous studies have
demonstrated that DC-SIGNR expression is lower in patients with NHL
and lung cancer compared with that in healthy individuals (21,36);
therefore DC-SIGNR may have a clinical application for the
diagnosis of a number of types of cancer. sDC-SIGNR levels in
patients with stage I and II cervical cancer were significantly
higher compared with those in patients with stage III and IV
cervical cancer. This result suggests that DC-SIGNR is likely to be
of most clinical value in the early diagnosis of cervical cancer.
There was no significant difference in sDC-SIGNR levels between
patients with stage III/IV cervical cancer and healthy individuals.
This result may be related to the decline of immune function in the
late stages of cancer.
Although pathological diagnosis remains the gold
standard for the diagnosis of cervical cancer, serum tumor markers
could be important for the early diagnosis of cervical cancer,
particularly for high-risk screening. If cancer is detected at an
early stage, or even in the pre-malignant stage, physicians will
have a higher probability of successfully treating the patient
(37,38). The results from the present study
indicate that DC-SIGNR and sDC-SIGNR levels could be helpful for
the early diagnosis of cervical cancer.
C-type lectins are a large family of proteins that
serve roles in several biological processes. Various previous
studies have demonstrated that a number of C-type lectins
participate in tumor metastasis, for example LSECtin and L-selectin
(39,40). Although the specific role DC-SIGNR
serves in cervical cancer remains unclear, the results of the
present study indicate that DC-SIGNR and sDC-SIGNR levels can be
used to distinguish between patients with cervical cancer and
healthy individuals, and may allow for the early diagnosis of
cervical cancer. Further experiments are required to explore the
specific roles DC-SIGNR serves in cervical cancer.
Acknowledgements
The present study was supported by the Chinese State
Key Program in Basic Research (grant no. 2012CB822103), the Chinese
National Science Foundation Project (grant nos. 81372669 and
31270867), the Science and Technology Planning Project of Liaoning
Province (grant no. 2012225020) and the Chinese Ministry of Health
(grant no. W2012RQ23).
References
|
1
|
Parkin DM and Bray F: Chapter 2: The
burden of HPV-related cancers. Vaccine. 24 Suppl 3:3/11–25. 2006.
View Article : Google Scholar
|
|
2
|
Arbyn M, Castellsagué X, de Sanjosé S,
Bruni L, Saraiya M, Bray F and Ferlay J: Worldwide burden of
cervical cancer in 2008. Ann Oncol. 22:2675–2686. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
World Health Organization, . Comprehensive
Cervical Cancer Control: A Guide to Essential Practice. 2nd. World
Health Organization; Geneva, Switzerland: 2014
|
|
4
|
Mzarico E, Gómez-Roig MD, Guirado L,
Lorente N and Gonzalez-Bosquet E: Relationship between smoking, HPV
infection, and risk of cervical cancer. Eur J Gynaecol Oncol.
36:677–680. 2015.PubMed/NCBI
|
|
5
|
Ahmed HG, Bensumaidea SH and Ashankyty IM:
Frequency of human papillpoma virus (HPV) subtypes 31,33,35,39 and
45 among Yemeni women with cervical cancer. Infect Aqent Cancer.
10:292015. View Article : Google Scholar
|
|
6
|
Hildesheim A and Wanq SS: Host and viral
genetics and risk of cervical cancer: A review. Virus Res.
89:229–240. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Luo Q, Zhang S, Wei H, Pang X and Zhang H:
Roles of Foxp3 in the occurrence and development of cervical
cancer. Int J Clin Exp Pathol. 8:8717–8730. 2015.PubMed/NCBI
|
|
8
|
Dambuza IM and Brown GD: C-type lectins in
immunity: Recent developments. Curr Opin Immunol. 32:21–27. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Strenq-Ouwehand I, Unqer WW and Van Kooyk
Y: C-type lectin receptors for tumor eradication: Future
directions. Cancers (Basel). 3:3169–3188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Laskarin G, Redzovic A, Vlastelic I,
Haller H, Medancic SS, Solinas G and Rukavina D: Tumor-associated
glycoprotein (TAG-72) is a natural ligand for the C-type
lectin-like domain that induces anti-inflammatory orientation of
early pregnancy decidual CD1a+ dendritic cells. J Reprod Immunol.
88:12–23. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sancho D and e Sousa C Reis: Sensing of
cell death by myeloid C-type lectin receptors. Curr Opin Immunol.
25:46–52. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Geijtenbeek TB, van Vliet SJ, Enqering A,
't Hart BA and van Kooyk Y: Self-and nonself-recognition by C-type
lectins on dendritic cells. Annu Rev Immunol. 22:33–54. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Soilleux EJ, Barten R and Trowsdale J:
DC-SIGN; a related gene, DC-SIGNR; and CD23 from a cluster on
19p13. J Immunol. 165:2937–2942. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Martens JH, Kzhyshkowska J,
Falkowski-Hansen M, Schledzewski K, Gratchev A, Mansmann U,
Schmuttermaier C, Dippel E, Koenen W, Riedel F, et al: Differential
expression of a gene signature for scavenger/lectin receptors by
endothelial cells and macrophages in human lymph node sinuses, the
primary sites of regional metastasis. J Pathol. 208:574–589. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jianq Y, Zhanq C, Chen K, Chen Z, Sun Z,
Zhang Z, Ding D, Ren S and Zuo Y: The clinical significance of
DC-SIGN and DC-SIGNR, which are novel markers expressed in human
colon cancer. PLoS One. 9:e1147482014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bieijs DA, Geijtenbeek TB, Fiqdor CG and
van Kooyk Y: DC-SIGN and LFA-1: A battle for ligand. Trends
Immunol. 22:457–463. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu S, Bevier M, Huhn S, Sainz J, Lascorz
J, Pardini B, Naccarati A, Vodickova L, Novotny J, Hemminki K, et
al: Genetic variants in C-type lectin genes are associated with
colorectal cancer susceptibility and clinical outcome. Int J
Cancer. 133:2325–2333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ding D, Chen W, Zhang C, Chen Z, Jiang Y,
Yang Z, Jiang X, Zuo Y and Ren S: Low expression of dendritic
cell-specific intercellular adhesion molecule-3-grabbing
nonintegrin in non-Hodgkin lymphoma and a significant correlation
with β2-microglobulin. Med Oncol. 31:2022014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu W, Tang L, Zhang G, Wei H, Cui Y, Guo
L, Gou Z, Chen X, Jiang D, Zhu Y, et al: Characterization of a
novel C-type lectin-like gene, LSECtin: Demonstration of
carbohydrate binding and expression in sinusoidal endothelial cells
of liver and lymph node. J Biol Chem. 279:18748–18758. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pöhlmann S, Soilleux EJ, Baribaud F,
Leslie GJ, Morris LS, Trowsdale J, Lee B, Coleman N and Doms RW:
DC-SIGNR, a DC-SIGN homologue expression in endothelial cells,
binds to human and simian immunodeficiency viruses and activates
infection in trans. Proc Natl Acad Sci USA. 98:pp. 2670–2675. 2001;
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu X, Zhanq H, Su L, Yang P, Xin Z, Zou
J, Ren S and Zuo Y: Low expression of dendritic cell-specific
intercellular adhesion molecule-grabbing nonintegrin-related
protein in lung cancer and significant correlations with brain
metastasis and natural killer cells. Mol Cell Biochem. 407:151–160.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Van de Lande J, Davelaar EM, von
Mensdorff-Pouilly S, Water TJ, Berkhof J, van Baal WM, Kenemans P
and Verheijen RH: SCC-Ag, lymph node metastases and sentinel node
procedure in early stage squamous cell cervical cancer. Gynecol
Oncol. 112:119–125. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rauch GM, Kaur H, Choi H, Ernst RD, Klopp
AH, Boonsirikamchai P, Westin SN and Marcal LP: Optimization of MR
imaging for pretreatment evaluation of patients with endometrial
and cervical cancer. Radiographics. 34:1082–1098. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pöhlmann S, Soilleux EJ, Baribaud F,
Leslie GJ, Morris LS, Trowsdale J, Lee B, Coleman N and Doms RW:
DC-SIGNR, a DC-SIGN homologue expressed in endothelial cell, binds
to human and simian immunodeficiency viruses and activates
infection in trans. Proc Natl Acad Sci USA. 98:pp. 2670–2675. 2001;
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yulin Li: Pathology. 8th. People's Medical
Publishing House; Beijing: 2013, (In Chinese).
|
|
26
|
Bashirova AA, Geijtenbeek TB, van
Duijnhoven GC, van Vliet SJ, Eilering JB, Martin MP, Wu L, Martin
TD, Viebig N, Knolle PA, et al: A dendritic cell-specific
intercellular adhesion molecule 3-grabbing nonintegrin
(DC-SIGN)-related protein is highly expressed on human liver
sinusoidal endothelial cells and promotes HIV-1 infection. J Exp
Med. 193:671–678. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mitchell DA, Fadden AJ and Drickamer K: A
novel mechanism of carbohydrate recognition by the C-type lectins
DC-SIGN and DC-SIGNR. Subunit organization and binding to
multivalent ligands. J Biol Chem. 276:28939–28945. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Op den Brouw ML, de Jonq MA, Ludwiq IS,
van der Molen RG, Janssen HL, Geijtenbeek TB and Woltman AM:
Branched oligosaccharide structures on HBV prevent interaction with
both DC-SIGN and L-SIGN. J Viral Hepat. 15:675–683. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Soilleux EJ: DC-SIGN (dendritic
cell-specific ICAM-grabbing non-integrin) and DC-SIGN-related
(DC-SIGNR): Friend or foe? Clin Sci (Lond). 104:437–446. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chaudhary O, Kumar S, Bala M, Singh J,
Hazarika A and Luthra K: Association of DC-SIGNR expression in
peripheral blood mononuclear cells with DC-SIGNR genotypes in HIV-1
infection. Viral Immunol. 28:472–475. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jeonq BK, Choi DH, Huh SJ, Park W, Bae DS
and Kim BG: The role of squamous cell carcinoma antigen as a
prognostic and predictive factor in carcinoma of uterine cervix.
Radiat Oncol J. 29:191–198. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Cui T, Du L, Xu X, Tian B, Sun T,
Han C, Zhao X and Jing J: The correlation between the serum
squamous carcinoma antigen and the prognosis of recurrent cervical
squamous carcinoma. J Clin Lab Anal. 31:2017. View Article : Google Scholar
|
|
33
|
Shimura K, Mabuchi S, Yokoi T, Sasano T,
Sawada K, Hamasaki T and Kimura T: Utility of serum squamous cell
carcinoma antigen levels at the time of recurrent cervical cancer
diagnosis in determining the opti mal treatment choice. J Gynecol
Oncol. 24:321–329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim BG: Squamous cell carcinoma antigen in
cervical cancer beyond. J Gynecol Oncol. 24:291–292. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li X, Zhou J, Huanq K, Tang F, Zhou H,
Wang S, Jia Y, Sun H, Ma D and Li S: The predictive value of serum
squamous cell carcinoma antigen in patients with cervical who
receive neoadjuvant chemotherapy followed by radical surgery: A
single-institute study. PLoS One. 10:e01223612015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Z, Chen K, Yan L, Yang Z, Zhu Z,
Chen C, Zeng J, Wei W, Qi X, Ren S and Zuo Y: Low expression of
dendritic cell-specific intercellular adhesion molecule-grabbing
nonintegrin-related protein in non-Hodgkin lymphoma and significant
correlations with lactic dehydrogenase and β2-microglobulin.
Biochem Cell Biol. 91:214–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wulfkuhle JD, Liotta LA and Petricoin EF:
Proteomic applications for the early detection of cancer. Nat Rev
Cancer. 3:267–275. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gdowski A, Ranjan AP, Mukerjee A and
Vishwanatha JK: Nanobiosensors: Role in cancer detection and
diagnosis. Adv Exp Med Biol. 807:33–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zuo Y, Ren S, Wanq M, Liu B, Yang J, Kuai
X, Lin C, Zhao D, Tang L and He F: Novel roles of liver sinusoidal
endothelial cell lectin in colon carcinoma cell adhesion, migration
and in-vivo metastasis to the liver. Gut. 62:1169–1678. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Qian F, Hanahan D and Weissman IL:
L-selectin can facilitate metastasis to lymph nodes in a transgenic
mouse model of carcinogenesis. Proc Natl Acad Sci USA. 98:pp.
3976–3981. 2001; View Article : Google Scholar : PubMed/NCBI
|