Introduction
Esophageal carcinoma is a common malignant tumor
that ranks 8th in the world and 4th in China. In China, there are
approximately 259,000 new cases annually, and the number of deaths
are 211,000 cases every year which accounts for more than 50% of
cases globally (1). The pathological
type was mainly squamous cell carcinoma, and most patients had
arrived at the advanced stage before diagnosis. Surgical resection
mostly occurs in stage I to IIA; nevertheless, the local recurrence
rate reaches 40–60% and the 5-year overall survival rate was only
30% (2).
To the best of our knowledge, there is currently no
standard treatment paradigm for esophageal carcinoma in stage IIB
to IV. Conventional radiotherapy and chemotherapy after operation
were palliative therapies, while radiotherapy and chemotherapy
prior to surgery (or neoadjuvant therapy) may improve the surgical
resection rate, survival time and reduce the recurrence rate
(3). However, only a few studies have
been conducted on a small scale using different radiotherapy and
chemotherapy regimens, yielding inconsistent results, which bring
great challenges for clinical treatments (4,5). At the
same time, screening of the groups benefiting mostly is also
difficult. According to retrospective conclusion, this study
examined improvement of survival time and the effects of
neoadjuvant chemotherapy combined with radiotherapy on treating
patients with advanced esophageal carcinoma.
Patients and methods
Patients
We retrospectively summarized patients diagnosed
with esophageal carcinoma (squamous cell carcinoma) in the First
Affiliated Hospital of Soochow University from January, 2012 to
January, 2015. Inclusion criteria for patients were, age 18–70,
functional status, and KPS scores >69. It was the first
diagnosis, initial treatment for the selected patients. The
patients met the 2002 6th edition international standard of TNM
staging of esophageal carcinoma (stage IIB to IV), excluding
esophageal fistula and viscera metastasis. The blood biochemistry
was WBC ≥4.0×109/l, PLT ≥100×109/l and HGB
≥100 g/l, with normal functions of liver and kidney and complete
clinical data. Exclusion standards were patients with thoracic
injury, surgical history and other diseases that need to combine
with radiotherapy, chemotherapy or surgery to treat, patients with
tracheoesophageal fistula confirmed by fiberoptic bronchoscope or
upper digestive tract radiography, patients with diseases combined
with second primary malignant tumor (excluding patients who had
recovered from skin and cervical cancer in situ over 5
years), patients with diseases combined with major underlying
diseases who did not tolerate radiotherapy and chemotherapy at a
certain dose, patients withdrawn from treatments, and cases with
poor compliance. This study was approved by the Ethics Committee of
our hospital and written informed consent was obtained of the
patients or their families.
Finally 43 patients were selected who were
administered neoadjuvant chemotherapy combined with radiotherapy,
designated as the combination group. According to gender and tumor
staging, the nearest neighbor matching was carried out, 86 patients
(1:2) were selected who received neoadjuvant chemotherapy as the
simple (standard) group and 129 patients (1:3) who underwent
surgery as the surgical group. Baseline information is shown in
Table I.
 | Table I.Comparison of baseline information in
three groups. |
Table I.
Comparison of baseline information in
three groups.
| Group | Cases | Male/female | Age (years) | IIB | III | IV | Upper thoracic | Middle | Lower |
|---|
| Combination | 43 | 25/18 | 42.3±6.7 | 19 | 17 | 7 | 6 | 20 | 17 |
| Simple | 86 | 48/38 | 43.5±6.6 | 36 | 36 | 14 | 12 | 42 | 32 |
| Surgical | 129 | 78/51 | 44.2±6.5 | 60 | 51 | 18 | 13 | 62 | 54 |
| F-value
(χ2) |
| 0.463 | 0.524 |
| 0.560 |
|
| 1.120 |
|
| P-value |
| 0.793 | 0.821 |
| 0.967 |
|
| 0.891 |
|
Treatment methods
In the combination group, there were 28 cases that
received neoadjuvant chemotherapy, with 60–80 mg/m2 of
cisplatin (DDP) and 500–1,000 mg/m2 of 5-fluorouracil
(5-FU) added from day 1 to 5. The days 21–28 were taken as a cycle,
to perform 2 to 3 cycles. There were 6 cases with 80
mg/m2 of nedaplatin (NDP), 200 mg/day of calcium
folinate (CF) added from day 1 to 5 and 1,000 mg/day of tegafur
(FT)-207 added from day 1 to 5. The 21st day was taken as a cycle
to perform 2 cycles. There were 3 cases of ECF regimen
(pharmorubicin + DDP + 5-FU), 3 cases with paclitaxel and DDP or
carboplatin and 3 cases of MIC regimen (mitomycin + ifosfamide +
DDP).
Neoadjuvant radiotherapy was used to evaluate
tolerance during chemotherapy. Radiotherapy was employed at the
same time or chemotherapy interval or after chemotherapy and
BJ6B-400 6MV X was used as the linear accelerator by Elekta North
Institute. There were 30 cases of three dimensional conformal
radiation for 2 to 3 coplanar fields and 13 cases of emphatic
radiation. Two anterior oblique portal plus wedges were used for
cervical segment, and one anterior and two posterior fields for
middle-lower, with 2 Gy each time, once every day and 5 times every
week. The dosage range was 45–70 Gy/6 weeks, and the median dose
was 60 Gy/6 weeks.
In the simple group, there were 60 cases with DDP
and 5-FU, 12 cases with NDP, CF and FT-207, 5 cases with ECF, 7
cases with MIC and 2 other cases. Surgical methods were surgical
resection or palliative therapy of conventional open surgery.
The differences among median survival time, 1-year
survival rate, average biggest diameter of tumors, surgical
resection rate, margin negative rate, effective rate, recurrence
rate, quality of life (QOL) scores and the incidence of
complications related to neoadjuvant therapy, and screening of
factors that may influence survival outcomes were compared. The CT
scan was conducted for the measurement of tumor diameters,
pathological examinations of tumors for margin and NCI solid
tumor's effect evaluation criterion (RECIST 200 edition) for the
effective rate. The recurrence rate included recurrence in
situ and distant metastasis. Through the linear formula, the
original score of each item in QLQ-c30 scale was converted into
0–100. QOL score change by 5–10 was considered as slight, 10–20 as
obvious, and >20 as significant. The higher overall QOL scores
were associated with better quality of life. According to common
standards of toxicity of chemotherapy CTC 3.0 version and RTOCG
grading standards of acute radiation injury, the incidence of
complications included severe hematological complications (the
increase of neutrophils, anaemia, hemorrhage, bone marrow
transplantation), digestive and urinary systems (severe injuries of
liver and kidney), and radiation esophagitis. On the basis of
complication conditions, the symptomatic treatment was given or
evaluated whether or not there was need to stop treatment.
Statistical analyses
According to sequence, the EpiData 3.1 data
management software was used to input clinical data of every
selected patients, with two copies for two individuals
independently. SPSS 20.0 (Chicago, IL, USA) was used to analyze and
deal with data, and the mean ± standard deviation (SD) was used to
indicate quantitative data. One-way ANOVA was used for comparison
of several groups, and the independent sample t-test was employed
for comparisons between two groups. The cases or percentage was
used to indicate qualitative data, and the χ2 test was
used for comparisons within the group. The Kaplan-Meier (KM) method
(log-rank test) was used for median survival time, and Cox's
proportional hazards regression model (forward method) was used for
multifactor analysis. A difference of p<0.05 was considered as
statistically significant.
Results
Comparison of survival time and
survival rate
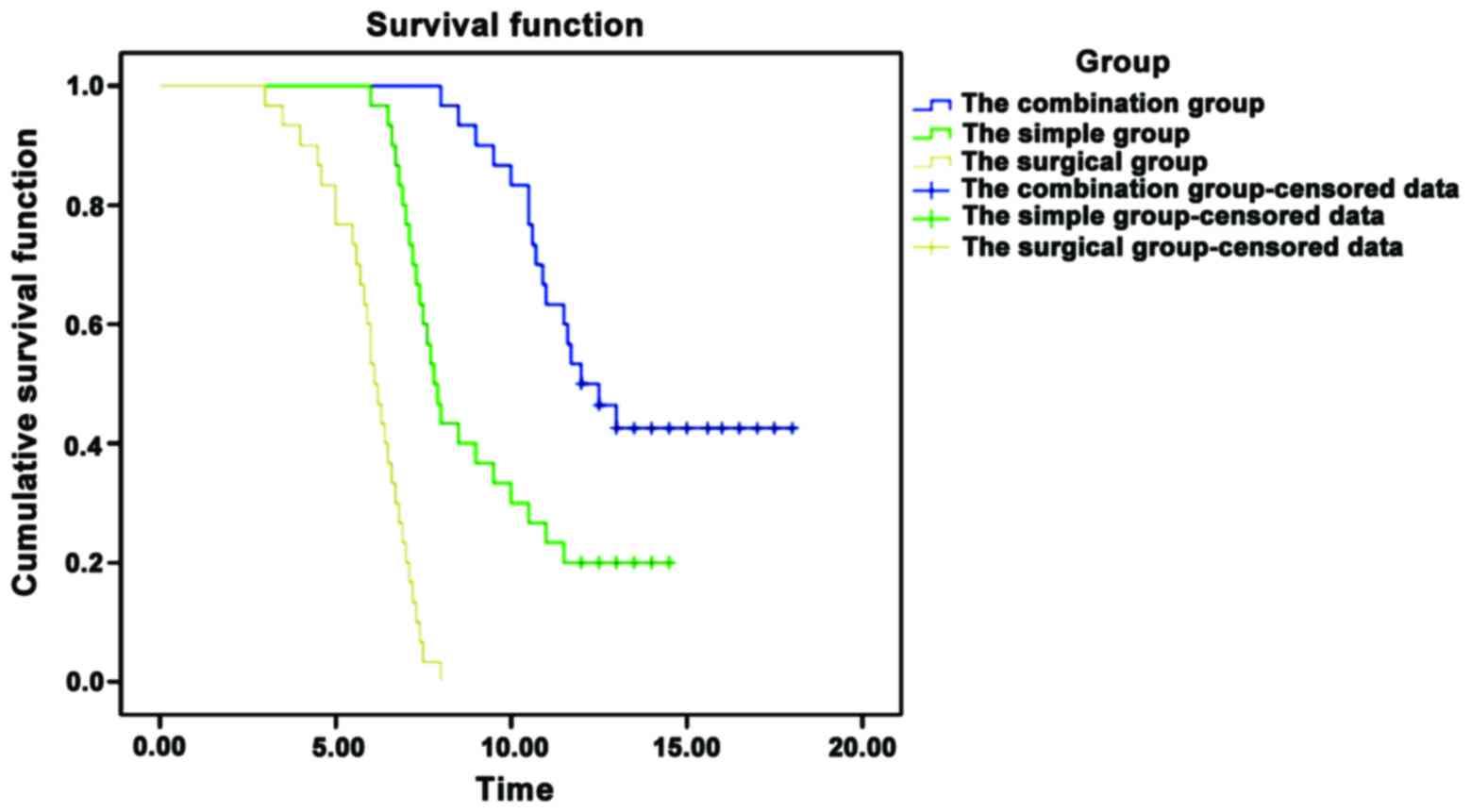
In the combination group, median survival time
significantly prolonged, and the 1-year survival rate improved
significantly (p<0.05), as shown in Table II and Fig.
1.
 | Table II.Comparison of survival time and
survival rate. |
Table II.
Comparison of survival time and
survival rate.
| Group | Median survival time
(months) | 95% CI | 1-year survival
rate |
|---|
| Combination
(n=43) | 12.0 | 10.224–13.776 | 23 (53.5%) |
| Simple (n=86) | 7.8 | 7.263–8.337 | 35 (40.7%) |
| Surgical (n=129) | 6.1 | 5.671–6.529 | 32 (24.8%) |
| χ2 | 94.079 |
| 13.600 |
| P-value | <0.001 |
| <0.001 |
Comparison of tumors diameter,
surgical resection rate and margin negative rate
In the combination group, the diameter of tumors
reduced significantly, whereas the surgical resection and margin
negative rates improved significantly (p<0.05), as shown in
Table III.
 | Table III.Comparison of the diameter of tumors,
the surgical resection rate and the margin negative rate. |
Table III.
Comparison of the diameter of tumors,
the surgical resection rate and the margin negative rate.
| Group | Diameter of tumors
before treatment (cm) | Diameter of tumors
after treatment | Surgical resection
rate | Margin negative
rate |
|---|
| Combination
(n=43) | 5.6±1.3 | 3.3±0.9 | 36 (83.7) | 25 (69.4) |
| Simple (n=86) | 5.4±1.2 | 4.2±1.2 | 60 (69.8) | 30 (50.0) |
| Surgical (n=129) | 5.5±1.4 | – | 53 (41.1) | 19 (35.8) |
| F-value
(χ2) | 0.625 | 5.748a | 31.660 | 9.683 |
| P-value | 0.432 | 0.016 | <0.001 | 0.008 |
Comparison of effective rate and
recurrence rate
In the combination group, the total effective rate
improved significantly, and the recurrence rate decreased
significantly (p<0.05), as shown in Table IV.
 | Table IV.Comparison of effective rate and the
recurrence rate. |
Table IV.
Comparison of effective rate and the
recurrence rate.
| Group | Complete
remission | Partial
remission | Total effective | Local recurrence | Metastasis rate | Total recurrence
rate |
|---|
| Combination
(n=43) | 17 | 20 | 37 (86.0%) | 15 | 9 | 24
(55.8%) |
| Simple (n=86) | 22 | 38 | 60 (69.8%) | 30 | 26 | 56
(65.1%) |
| Surgical (n=129) | 25 | 46 | 71 (55.0%) | 44 | 59 | 103 (79.8%) |
| χ2 |
|
| 14.879 |
|
| 11.147 |
| P-value |
|
| <0.001 |
|
| 0.004 |
Comparison of QOL scores and the
incidence of complications
In the combination group, QOL scores improved
significantly (p<0.05). There were 4 cases that had
complications related to chemotherapy and 1 case that had
complications related to radiotherapy. The comparison of total
incidence of complications was not statistically significant
(p>0.05) as shown in Table V.
 | Table V.Comparison of QOL scores and the
incidence of complications. |
Table V.
Comparison of QOL scores and the
incidence of complications.
| Group | QOL scores | Incidence of
complications |
|---|
| Combination
(n=43) | 82.4±10.3 | 5 (11.6) |
| Simple (n=86) | 75.3±12.4 | 7 (8.1) |
| Surgical (n=129) | 62.6±13.5 | – |
| F-value
(χ2) | 6.527 | 0.413 |
| P-value | <0.001 | 0.520 |
Analysis of Coxs proportional hazard
regression model
The gender, age, tumor staging, tumor location, the
diameter of tumors (before or after neoadjuvant therapy),
therapeutic regimens (combination, simple, surgical), treatment
cycle, surgical resection rate, margin negative rate, effective
rate, recurrence rate, QOL scores and the incidence of
complications as arguments, and survival outcomes and time as
dependent variable, were included them in the model (Table VI).
 | Table VI.Analysis of Coxs proportional hazard
regression model. |
Table VI.
Analysis of Coxs proportional hazard
regression model.
| Factors | β | Wald | P-value | RR | 95% CI |
|---|
| Tumor staging | 0.125 | 10.425 | <0.001 | 3.953 | 2.320–5.203 |
| Tumor location | 0.323 |
6.635 | <0.001 | 1.524 | 0.867–2.326 |
| The diameter of
tumors after treatment | 0.426 |
9.567 | <0.001 | 2.746 | 1.867–3.402 |
| Therapeutic
regimens | 0.627 | 12.524 | <0.001 | 4.527 | 3.654–5.133 |
| Treatment
cycle | 0.824 |
5.926 |
0.013 | 1.935 | 1.130–2.534 |
| The margin negative
rate | 0.329 | 11.425 | <0.001 | 3.236 | 2.935–4.531 |
| The effective
rate | 0.565 | 12.203 | <0.001 | 2.568 | 2.132–3.439 |
Discussion
The theoretical advantages of neoadjuvant
chemotherapy lie in (6) decrease
tumor stage, reduce tumor volume, and increase surgical resection
rate; control and treatment of micro-metastases and decrease in the
recurrence rate; chemotherapeutics can reach tumor cells
sufficiently passing undamaged blood supply systems; evaluate in
vivo sensitivity to guide treatments after surgery. The
disadvantages may be postponement of surgical time of patients with
resectable esophageal cancer, thus missing the most suitable
surgical timing (7). The advantages
of neoadjuvant radiation includes accurate radiation, increase
surgical time of patients with esophageal cancer, increase
sensitivity of chemotherapeutic agents, increase dosage and
chemotherapy cycle of chemotherapeutics and increase surgical
resection rate, without enlarging damage of tissues around the
tumors (8). The disadvantages were
that radiation dose and regimens were inconsistent which can
influence treatment outcomes (9).
A randomized study test reported by British Medical
Research Council (MRC) included 802 cases of patients with
resectable esophageal cancer, 400 cases in the neoadjuvant
chemotherapy with 2 cycles of DDP and 5-FU and surgical treatments
following, and 402 cases with simple surgery. The results showed
that in the neoadjuvant chemotherapy group, both median survival
time and 2-year survival rate improved, as reported (10). In another study, meta-analysis for
randomized tests with 11 neoadjuvant chemotherapy or radiation, and
the results manifested that in the neoadjuvant therapy group, the
2-year survival rate was higher than that in the simple surgical
group, and it increased by 4.4% (95% CI, 0.3–8.5) (11).
Another study included 440 cases with esophageal
cancer, which were randomly divided into the neoadjuvant
chemotherapy combined with surgery group and the simple surgical
group (5). The results showed that in
the combination group, the margin positive rate of surgery
decreased significantly (4% vs. 15%, p<0.01), but there were no
obvious differences among median time of these two (14.9 vs. 16.1
months, p=0.53), 1, 2 and 3-year survival rate (59 vs. 60%, 35 vs.
37%, 23 vs. 26%) disease-free survival rate, or the post-operative
complication rate. In another randomized trial, 2,051 patients with
esophageal cancer obtained neoadjuvant chemotherapy combined with
surgery group, there were no differences among 1 and 2-year
survival rate, 3 and 4-year survival rate had a tendency to
improve, and 5-year survival rate improved obviously (RR =1.44, 95%
CI =1.05–1.97, p=0.02) while comparing with the simple surgical
group (12). Total surgical resection
rate and pathological resection rate in these two groups were the
same.
The differences of study results may be related to
the number of samples, pathological types included in the groups
(the ratio of adenocarcinoma or squamous cell carcinoma),
chemotherapy regimens (DDP and 5-FU as basic regimens, also with
pharmorubicin, bleomycin, methotrexate, paclitaxel, fluconazole,
ifosfamide and other new drugs), and chemotherapy dose.
Neoadjuvant chemotherapy combined with radiation has
synergistic reactions, and many clinical studies also suggest that
neoadjuvant chemotherapy and radiation has more benefits for
patients with esophageal cancer compared with simple surgical
treatments. In a clinical non-controlled study with 69 cases of
patients with esophageal cancer in the stage II (13), all the patients received chemotherapy
with paclitaxel and DDP and hyperfraction radiotherapy
synchronizing twice/day (1.5 Gy once). The results manifested that
median survival time was 24 months, and 1, 2 and 3-year survival
rate was 75, 50 and 34%, respectively, which all increased. A
meta-analysis included 9 randomized controlled trial with 1,116
patients, which suggested that neoadjuvant chemotherapy combined
with radiation improves surgical resection rate, which reached 21%
complete remission and decreased the incidence rate of local
positions (14). Although the 1- and
2-year survival rate in the neoadjuvant chemotherapy combined with
radiation group was similar to those in the simple surgical group,
the 3-year survival rate improved. It also suggested that
synchronous neoadjuvant chemotherapy and radiation before operation
has more benefits than sequential chemoradiotherapy.
We found that in the combination group, median
survival rate prolonged, 1-year survival rate improved, the
diameter of tumors reduced, the surgical resection rate and the
margin negative rate improved, total effective rate improved, the
recurrence rate decreased, and QOL scores improved significantly.
Comparatively, the total incidence of complications was not
statistically significant. Independent risk factors that influence
survival outcomes and time include tumor stage, tumor location, the
diameter of tumors after neoadjuvant therapy, therapeutic regimens,
treatment cycle, the margin negative rate and effective rate. This
study is more close to real scenario, pathological type was
squamous cell carcinoma patients in the stage IIB to IV have low
surgical resection rate. Doctors and patients had more willingness
to utilize neoadjuvant therapy, compliance of treatment was high,
the regimens were more personal (more regimens and dose of
chemotherapy and radiation can be selected), and follow-up was
strict and information was complete. According to Cox's
proportional hazards regression model, and screening for risk
factors may influence survival outcomes.
In conclusion, employing neoadjuvant chemotherapy
combined with radiation can be used to treat advanced esophageal
cancer and can improve the median survival time and 1-year survival
rate, improve surgical resection rate and promote prognostic
survival.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ando N, Iizuka T, Ide H, Ishida K, Shinoda
M, Nishimaki T, Takiyama W, Watanabe H, Isono K, Aoyama N, et al:
Japan Clinical Oncology Group: Surgery plus chemotherapy compared
with surgery alone for localized squamous cell carcinoma of the
thoracic esophagus: a Japan Clinical Oncology Group Study -
JCOG9204. J Clin Oncol. 21:4592–4596. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boonstra JJ, Kok TC, Wijnhoven BP, van
Heijl M, van Berge Henegouwen MI, Ten Kate FJ, Siersema PD, Dinjens
WN, van Lanschot JJ, Tilanus HW, et al: Chemotherapy followed by
surgery versus surgery alone in patients with resectable
oesophageal squamous cell carcinoma: long-term results of a
randomized controlled trial. BMC Cancer. 11:1812011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Medical Research Council Oesophageal
Cancer Working Group, . Surgical resection with or without
preoperative chemotherapy in oesophageal cancer: a randomised
controlled trial. Lancet. 359:1727–1733. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kelsen DP, Ginsberg R, Pajak TF, Sheahan
DG, Gunderson L, Mortimer J, Estes N, Haller DG, Ajani J, Kocha W,
et al: Chemotherapy followed by surgery compared with surgery alone
for localized esophageal cancer. N Engl J Med. 339:1979–1984. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lv J, Cao XF, Zhu B, Ji L, Tao L and Wang
DD: Long-term efficacy of perioperative chemoradiotherapy on
esophageal squamous cell carcinoma. World J Gastroenterol.
16:1649–1654. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Metzger R, Bollschweiler E, Drebber U,
Mönig SP, Schröder W, Alakus H, Kocher M, Baldus SE and Hölscher
AH: Neoadjuvant chemoradiotherapy for esophageal cancer: impact on
extracapsular lymph node involvement. World J Gastroenterol.
16:1986–1992. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Campbell NP and Villaflor VM: Neoadjuvant
treatment of esophageal cancer. World J Gastroenterol.
16:3793–3803. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ajani JA, Barthel JS, Bentrem DJ, Damico
TA, Das P, Denlinger CS, Fuchs CS, Gerdes H, Glasgow RE, Hayman JA,
et al: National Comprehensive Cancer Network: Esophageal and
esophagogastric junction cancers. J Natl Compr Canc Netw.
9:830–887. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cunningham D, Allum WH, Stenning SP,
Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ,
Falk SJ, Iveson TJ, et al: MAGIC Trial Participants: Perioperative
chemotherapy versus surgery alone for resectable gastroesophageal
cancer. N Engl J Med. 355:11–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kaklamanos IG, Walker GR, Ferry K,
Franceschi D and Livingstone AS: Neoadjuvant treatment for
resectable cancer of the esophagus and the gastroesophageal
junction: a meta-analysis of randomized clinical trials. Ann Surg
Oncol. 10:754–761. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Malthaner R and Fenlon D: Preoperative
chemotherapy for resectable thoracic esophageal cancer. Cochrane
Database Syst Rev: CD001556. 2003. View Article : Google Scholar
|
|
13
|
Urba SG, Orringer MB, Ianettonni M, Hayman
JA and Satoru H: Concurrent cisplatin, paclitaxel, and radiotherapy
as preoperative treatment for patients with locoregional esophageal
carcinoma. Cancer. 98:2177–2183. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Urschel JD and Vasan H: A meta-analysis of
randomized controlled trials that compared neoadjuvant
chemoradiation and surgery to surgery alone for resectable
esophageal cancer. Am J Surg. 185:538–543. 2003. View Article : Google Scholar : PubMed/NCBI
|