Introduction
Breast cancer is a common, worldwide malignant tumor
of women. The number of female breast cancer patients in China
remains stubbornly high, and the mortality rate is also relatively
high; moreover, the occurrence of this disease increasingly tends
to affect the young population. Due to the occult feature of breast
cancer, most patients are at an advanced stage when they are
diagnosed. Therefore, how to accurately diagnose breast cancer at
an early stage without treatment delay for the treatment to improve
patient prognosis has become a hot issue in the diagnosis and
treatment of breast cancer in recent years (1). The technique of breast X-ray photography
is an important imaging examination method, which plays an
auxiliary role in the early diagnosis of breast cancer (2). Studies have shown that (3–5) related
cancer factors affect the biological behavior of tumor cells to a
certain extent, which is closely associated with the recurrence and
metastasis of cancer cells. Thus, this investigation focuses on
whether there is a correlation between the clinical features of
breast X-ray photography techniques and related cancer factors, how
to predict the biological behavior of breast cancer cells and
patients prognosis by using X-ray features in mammography combined
with related cancer factors. This study mainly aims to investigate
proliferating cell nuclear antigen (PCNA), proliferation-associated
nuclear antigen (Ki-67) and cyclooxygenase-2 (COX-2) expression in
breast invasive ductal carcinoma tissue, and to analyze the
correlations between these indexes and X-ray features in
mammography, in order to provide more valuable reference for the
growth, recurrence, metastasis and prognosis of breast cancer,
thereby benefiting clinical diagnosis and treatment in such
patients.
Materials and methods
General data
A total of 90 patients who were admitted to Huangshi
Central Hospital with confirmed breast invasive ductal carcinoma
from January 2014 to January 2016 were selected as the patient
group. All patients were women with single unilateral invasive
breast cancer lesions. Patients were aged from 29 to 73 years, with
an average of 56.7±1.6 years; for clinical staging, there were 60
cases in stages I+II and 30 cases in stages III+IV; for lymphatic
metastasis, there were 40 cases with metastasis and 50 cases
without metastasis; for tumor diameter, there were 33 cases with
the tumor size <2 cm and 57 cases with the size ≥2 cm; there
were 48 cases with cancer in the left breast and 42 cases with
disease in the right breast. The selected patients did not receive
any intervention therapy before operation, and breast invasive
ductal carcinoma was confirmed by pathology after operation
(6). Additionally, 40 patients who
had cancer-adjacent normal breast tissues being more than 5 cm far
away from cancer tissues confirmed by pathology were selected as
the normal control group. All patients were women, aged from 30 to
72 years, with an average of 56.2±1.4 years. This study was
approved by the Ethics Committee of Huangshi Central Hospital.
Signed written informed consents were obtained from all
participants before the study.
Methods
i) The expressions of PCNA, Ki-67 and COX-2 in
cancer-adjacent normal breast tissues and cancer tissues were
detected by immunohistochemical staining SP method. The above
staining procedures were operated, respectively according to the
instructions of EnVision kits (Biosharp, Hefei, China) of rabbit
polyclonal PCNA antibody (dilution, 1:100; cat. no. ab18197);
rabbit polyclonal Ki-67 antibody (dilution, 1:100; cat. no.
ab92742) and rabbit polyclonal COX-2 antibody (dilution, 1:100;
cat. no. ab52237) (all purchased from Abcam, Cambridge, MA, USA),
followed by observing staining results and ⅱ) patients were
routinely examined by full-field digital mammography (MAMMOMAT
3000; Siemens AG, Munich, Germany) before operation. Images were
jointly read and diagnostically analyzed by two radiologists who
were engaged in the X-ray diagnosis of breast cancer. The
occurrence of lump lesion of patients including burr,
calcification, asymmetric dense shadow and gland structure disorder
were mainly observed, and the above results were compared with the
corresponding pathological indexes for analysis.
Evaluation criteria
Using a semi-quantitative scoring method combined
with the Berry grading method (7):
the positive expression of PCNA, Ki-67 and COX-2 was stained in the
cytoplasm; the scores were recorded, respectively, according to the
positive cell rate and positive cell staining intensity; the
expression level was determined according to the staining degree
scores: staining the same as negative control, score 0; staining
light yellow, score 1; staining brown yellow, score 2; staining
reddish brown, score 3. The scores were recorded according to the
proportion of positive cells in the observed cells: positive cell
number ≤10%, score 1; ≥11 - ≤50% positive, score 2; ≥51 - ≤75%
positive, score 3; positive cell number >75%, score 4. The
product of two item scores: 0–3 scores (−), 4–5 scores (+), 6–7
scores (++), >8 scores (+++); the score ≤3 represented negative,
and the score >3 represented positive.
Statistical analysis
SPSS 20.0 software (IBM Corporation, Armonk, New
York, NY, USA) was used for statistical analysis. For enumeration
data, the Chi-square test was adopted for the comparison of
positive rate between groups; for measurement data, t-test was
utilized for the comparison between groups. Spearman correlation
test was used for correlation analysis, and the test level was
α=0.05.
Results
Expression of PCNA, Ki-67 and COX-2 in
breast cancer tissues
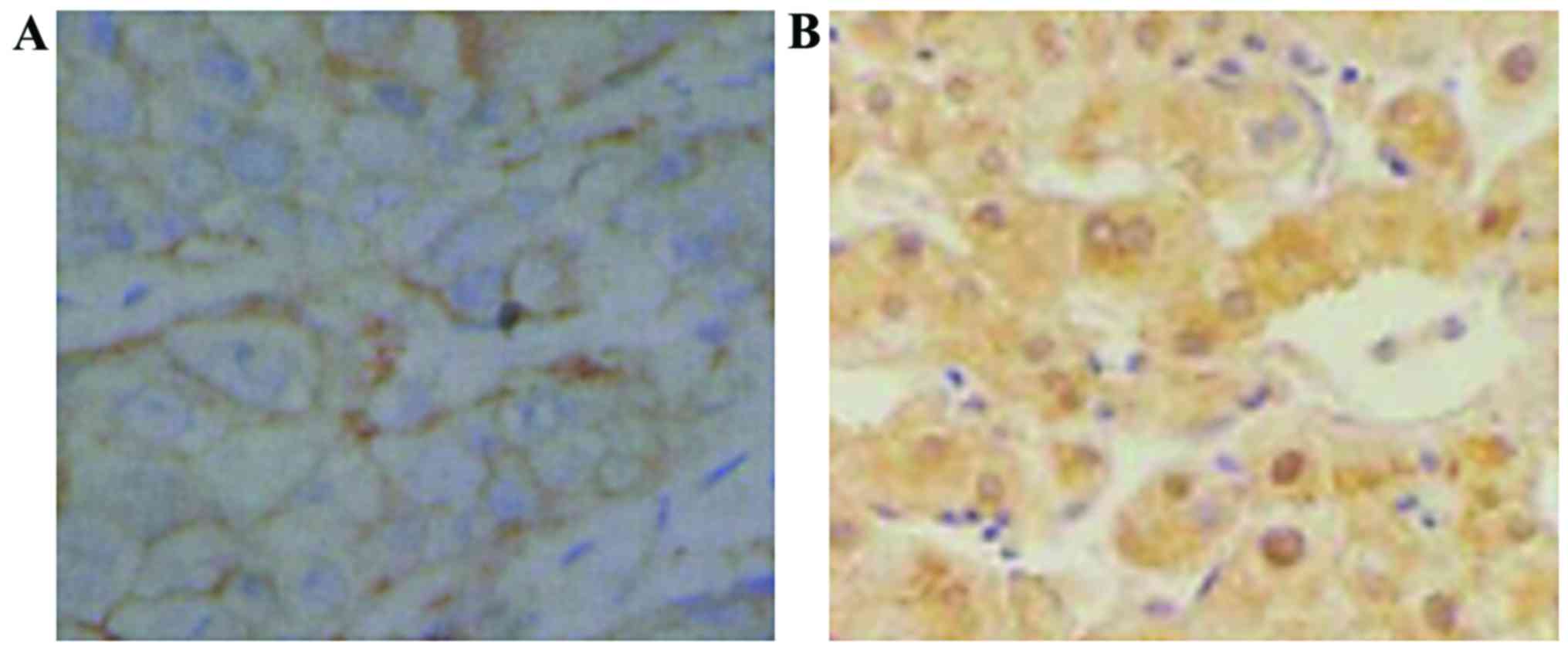
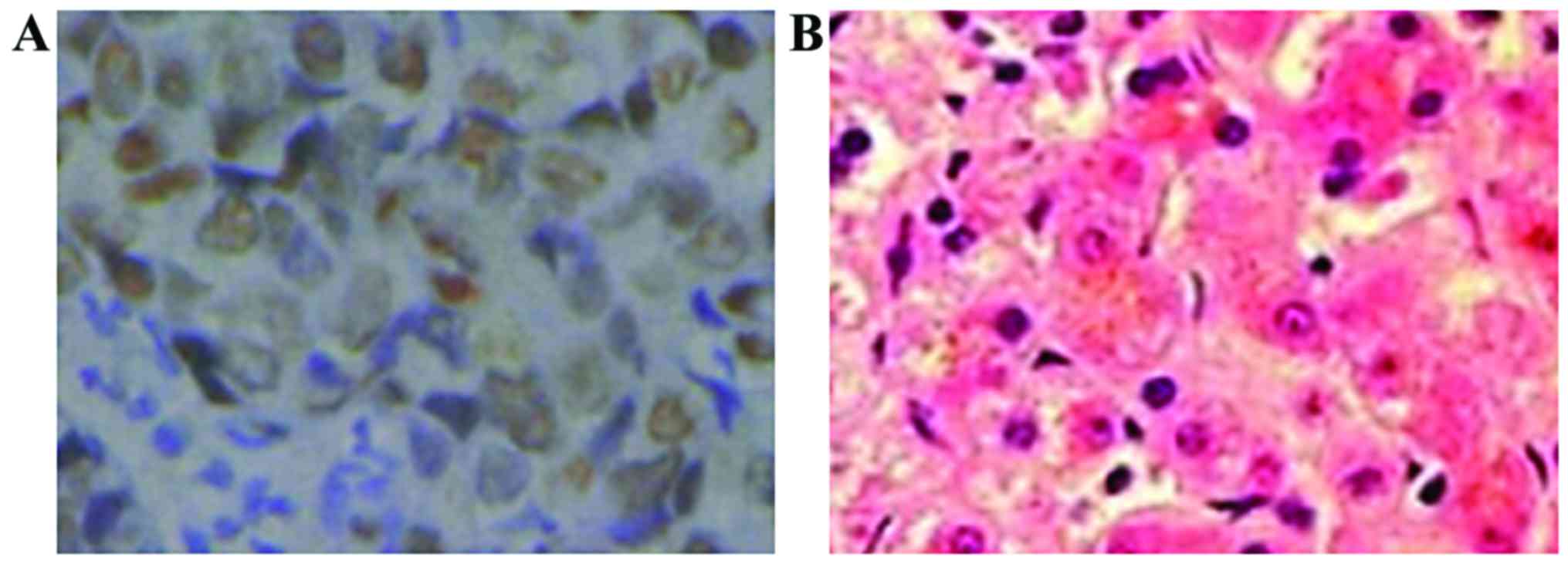
The positive expression products of PCNA, Ki-67 and
COX-2 were brown yellow particles, mainly located in the cytoplasm
(Figs. 1–3). The positive expression rate of PCNA in
cancer tissues of the patient group was 42.2%, which was
significantly higher than 2.5% in cancer-adjacent normal tissues of
the control group (p<0.05, Table
I). The positive expression rate of Ki-67 in cancer tissues of
the patient group was 45.6%, which was significantly higher than
2.5% in cancer-adjacent normal tissues of the control group
(p<0.05, Table II). The positive
expression rate of COX-2 in cancer tissues of the patient group was
51.1%, which was significantly higher than the 2.5% in
cancer-adjacent normal tissues of the control group (p<0.05,
Table III).
 | Table I.Expression of PCNA in cancer tissues
and cancer adjacent normal tissues (case). |
Table I.
Expression of PCNA in cancer tissues
and cancer adjacent normal tissues (case).
| Groups | n | + | ++ | +++ | Positive rate
(%) | − |
|---|
| Patient | 90 | 9 | 14 | 15 | 42.2 | 52 |
| Control | 40 | 1 | 0 | 0 | 2.5 | 39 |
| P-value |
|
|
|
|
| <0.05 |
 | Table II.Expression of Ki-67 in cancer tissues
and cancer adjacent normal tissues (case). |
Table II.
Expression of Ki-67 in cancer tissues
and cancer adjacent normal tissues (case).
| Groups | n | + | ++ | +++ | Positive rate
(%) | − |
|---|
| Patient | 90 | 10 | 15 | 16 | 45.6 | 49 |
| Control | 40 | 1 | 0 | 0 | 2.5 | 39 |
| P-value |
|
|
|
|
| <0.05 |
 | Table III.Expression of COX-2 in cancer tissues
and cancer adjacent normal tissues (case). |
Table III.
Expression of COX-2 in cancer tissues
and cancer adjacent normal tissues (case).
| Groups | n | + | ++ | +++ | Positive rate
(%) | − |
| Patient | 90 | 12 | 16 | 18 | 51.1 | 44 |
| Control | 40 | 1 | 0 | 0 | 2.5 | 39 |
| P-value |
|
|
|
|
| <0.05 |
Relationships between PCNA, Ki-67 and
COX-2 and clinicopathological features of breast cancer
patients
PCNA, Ki-67 and COX-2 expression in cancer tissues
of the patient group was associated with clinical staging and
lymphatic metastasis (p<0.05), but had no correlation with age
and tumor size (p>0.05, Table
IV).
 | Table IV.Relationships between PCNA, Ki-67 and
COX-2 and clinicopathological features of breast cancer
patients. |
Table IV.
Relationships between PCNA, Ki-67 and
COX-2 and clinicopathological features of breast cancer
patients.
| Clinicopathological
feature | n | Positive case of
PCNA | P-value | Positive case of
Ki-67 | P-value | Positive case of
COX-2 | P-value |
| Age |
|
| >0.05 |
| >0.05 |
| >0.05 |
| ≤60
years | 46 | 20 |
| 21 |
| 24 |
|
| >60
years | 44 | 18 |
| 20 |
| 22 |
|
| Tumor size (cm) |
|
| >0.05 |
| >0.05 |
| >0.05 |
|
<2 | 33 | 19 |
| 19 |
| 22 |
|
| ≥2 | 57 | 19 |
| 22 |
| 24 |
|
| Clinical staging |
|
| <0.05 |
| <0.05 |
| <0.05 |
| I+II | 60 | 13 |
| 15 |
| 17 |
|
| III | 30 | 25 |
| 26 |
| 29 |
|
| Lymphatic
metastasis |
|
| <0.05 |
| <0.05 |
| <0.05 |
| Yes | 40 | 28 |
| 27 |
| 30 |
|
| No | 50 | 10 |
| 14 |
| 16 |
|
Digital mammography X-ray photography
features of the patient group
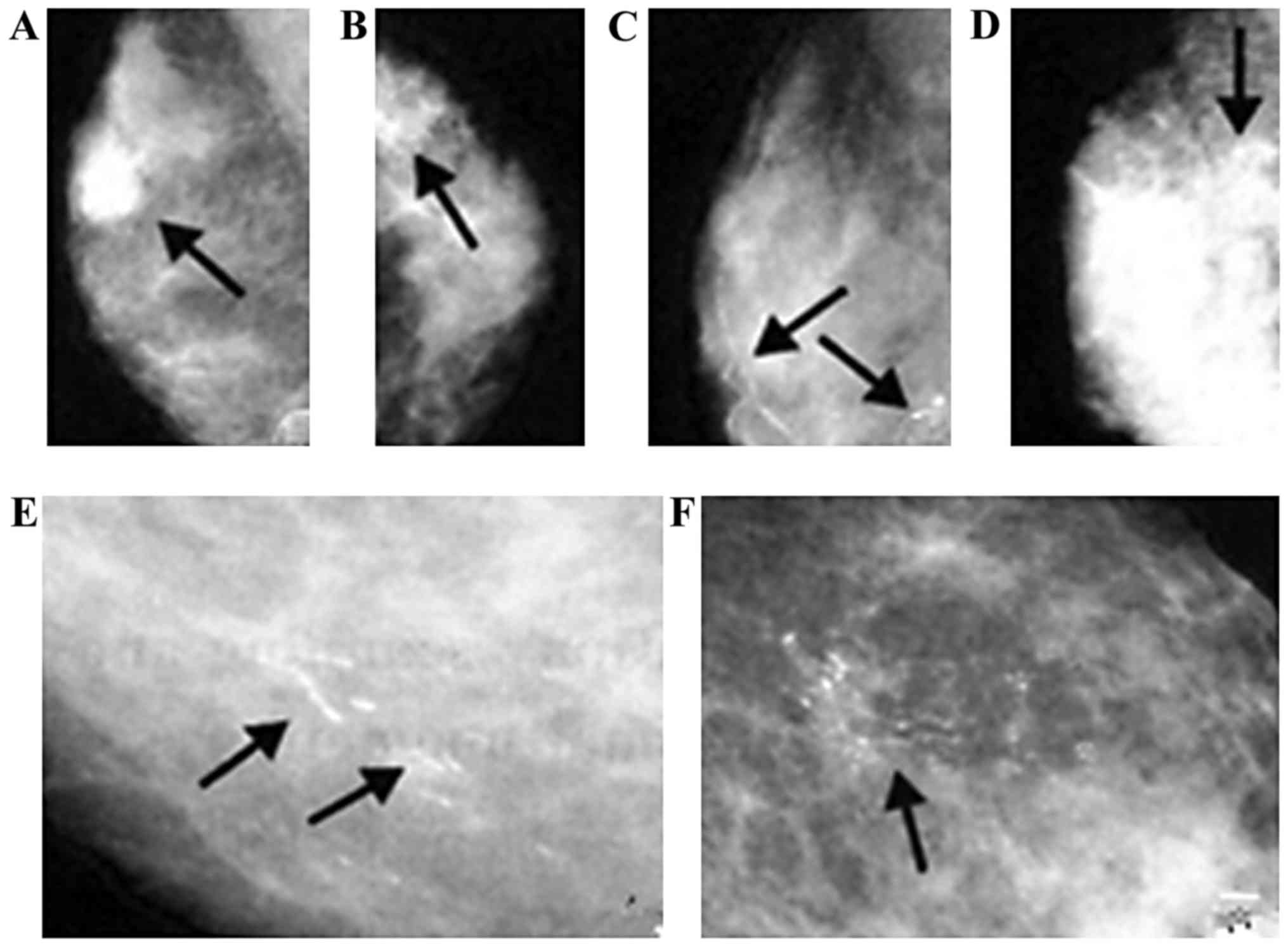
Among 90 cases in the patient group, 70 cases
manifested as lumps with different sizes; there were 8 cases of
mass shape calcification, 6 cases of cluster calcification, 14
cases of vascular calcification, 18 cases of sand-like
calcification, 14 cases of thick rod-shaped calcification and 20
cases of line like branching calcification (Fig. 4); there were 63 cases with
manifestations of architectural distortion, 41 cases with localized
density-increased shadow and 68 cases with skin and nipple
depression.
Relationships between X-ray
photography features and PCNA, Ki-67 and COX-2 expression in breast
invasive ductal carcinoma
PCNA, Ki-67 and COX-2 expression in cancer tissues
of the patient group had no correlation with the existence of lumps
and localized density-increased shadows (p>0.05), but were
associated with manifestations of architectural distortion,
calcification as well as skin and nipple depression (p<0.05,
Table V).
 | Table V.Relationships between X-ray
photography features and PCNA, Ki-67 and COX-2 expressions in
breast invasive ductal carcinoma. |
Table V.
Relationships between X-ray
photography features and PCNA, Ki-67 and COX-2 expressions in
breast invasive ductal carcinoma.
| Clinicopathological
feature | n | Positive case of
PCNA | P-value | Positive case of
Ki-67 | P-value | Positive case of
COX-2 | P-value |
|---|
| Lump |
|
| >0.05 |
| >0.05 |
| >0.05 |
|
Yes | 70 | 19 |
| 22 |
| 23 |
|
| No | 20 | 19 |
| 19 |
| 23 |
|
| Calcification |
|
| <0.05 |
| <0.05 |
| <0.05 |
|
Yes | 80 | 35 |
| 36 |
| 42 |
|
| No | 10 | 3 |
| 5 |
| 4 |
|
| Architectural
distortion |
|
| <0.05 |
| <0.05 |
| <0.05 |
|
Yes | 63 | 28 |
| 32 |
| 34 |
|
| No | 27 | 11 |
| 9 |
| 12 |
|
| Localized
density-increased shadow |
|
| >0.05 |
| >0.05 |
| >0.05 |
|
Yes | 41 | 18 |
| 22 |
| 23 |
|
| No | 49 | 20 |
| 19 |
| 23 |
|
| Skin and nipple
depression |
|
| <0.05 |
| <0.05 |
| <0.05 |
|
Yes | 68 | 31 |
| 36 |
| 37 |
|
| No | 21 | 7 |
| 5 |
| 9 |
|
Correlations among PCNA, Ki-67 and
COX-2 expression
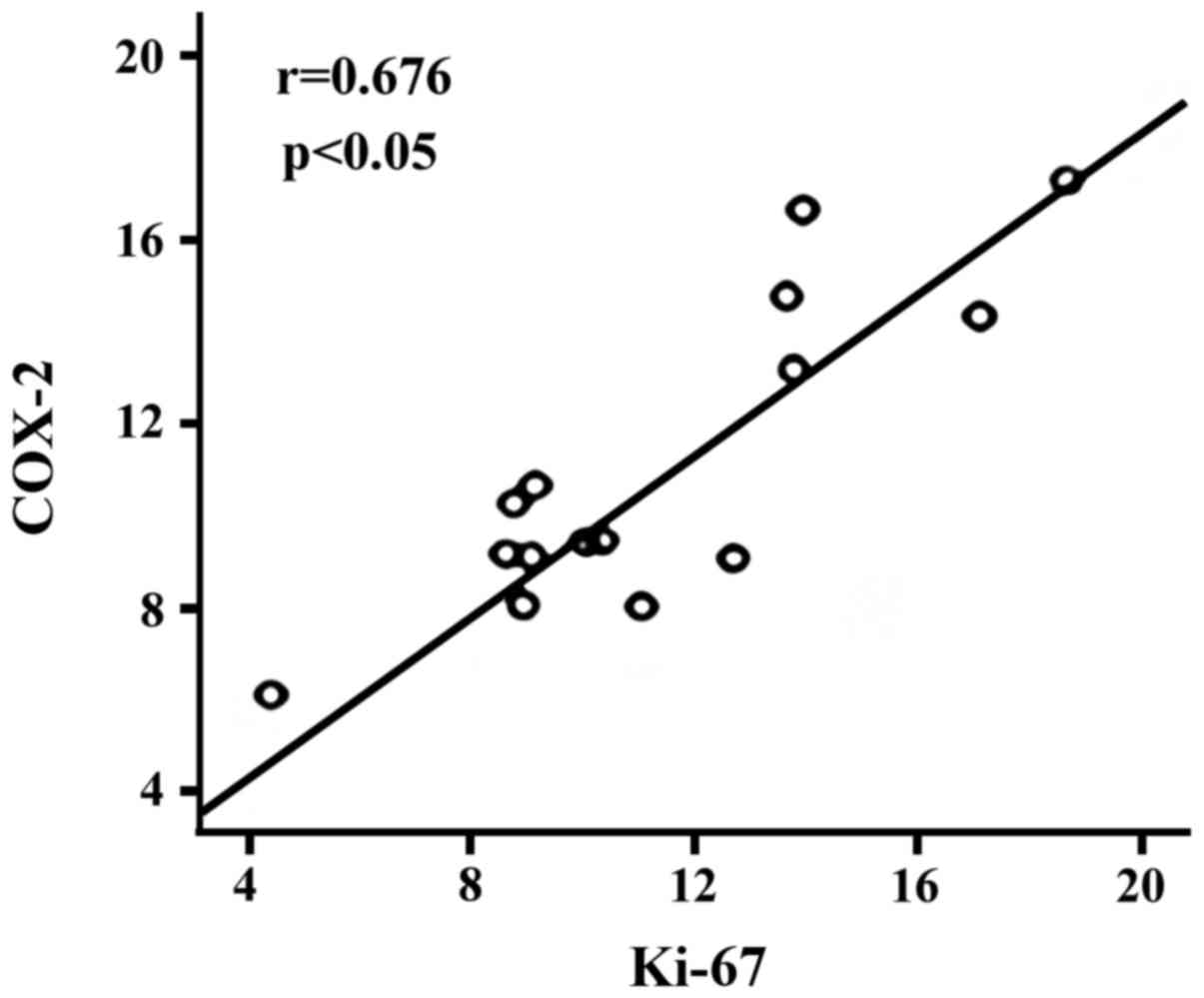
Spearman correlation analysis revealed that there
was significantly positive correlation between the expression of
PCNA and COX-2 in cancer tissues of the patient group (r=0.676,
p<0.05, Fig. 5); there was
significantly positive correlation between the expression of Ki-67
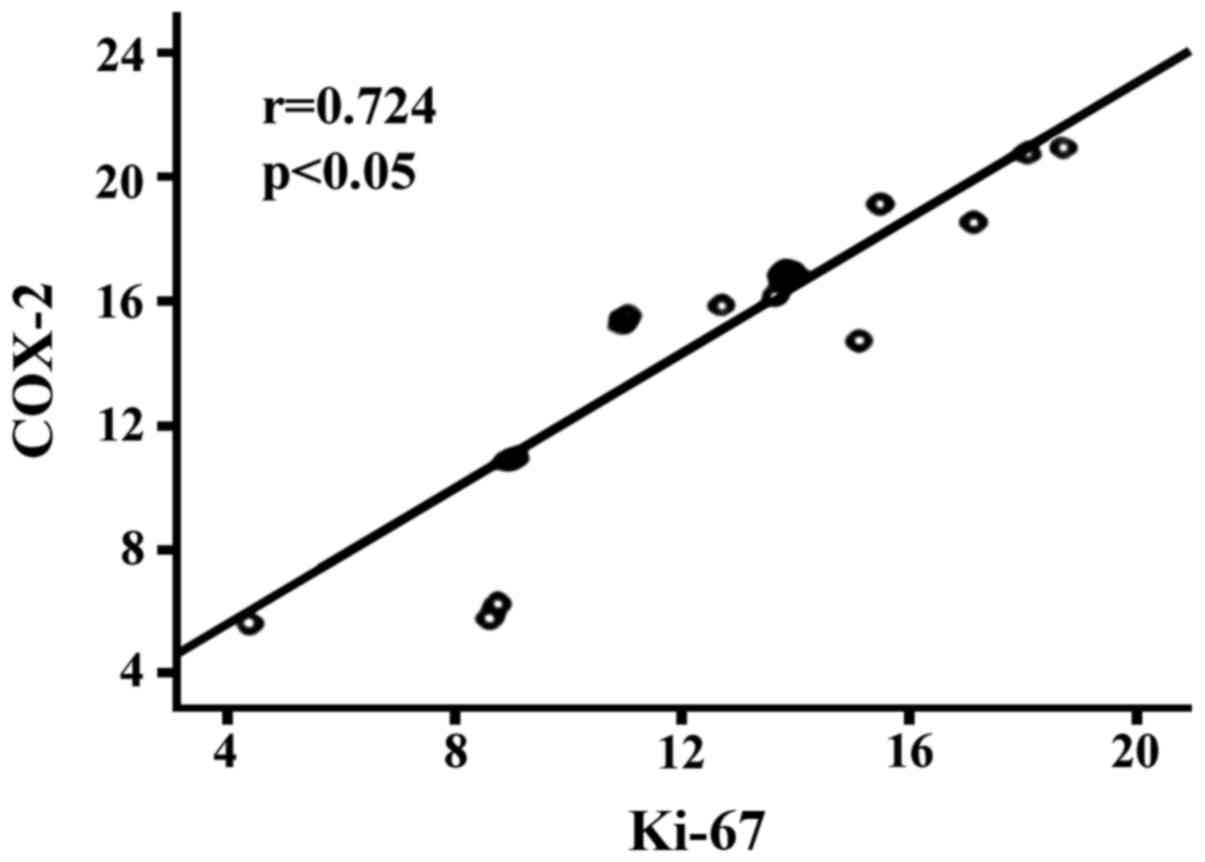
and COX-2 (r=0.724, p<0.05, Fig.
6); PCNA expression had no obvious correlation with the
expression of Ki-67 (p>0.05).
Discussion
The occurrence and development of breast cancer is
associated with multiple factors, especially for some
patho-biological factor indicators including HER-2 and P53
(1,8,9). With the
rapid development of modern biological techniques, many biological
factor indexes have been found (10,11). As
one kind of acidic protein in the nucleus, PCNA is an indispensable
coenzyme for eukaryotic cells to synthesize DNA. PCNA can be used
to measure cell proliferation activity; the higher the
proliferation activity is, the larger the tension between cells,
resulting in lower adhesion, which promotes tumor cells being
separated from primary lesions, and metastasizing (12). The results of this study indicated
that the positive expression rate of PCNA in cancer tissues of the
patient group was 42.2%, which was significantly higher than the
2.5% in the cancer-adjacent normal tissues of the control group
(p<0.05). PCNA expression in cancer tissues of the patient group
were associated with clinical staging and lymphatic metastasis
(p<0.05), but had no correlation with age and tumor size
(p>0.05), suggesting that PCNA expression is closely related to
the occurrence and development of breast cancer, which is
consistent with the conclusions of other international medical
researchers (13,14).
Ki-67 is a kind of nuclear antigen, the quantity of
which generated from cell proliferation is closely associated with
mitosis (15). Previous studies have
confirmed that (16) Ki-67 is highly
expressed in gastric, prostate and liver cancer and other multiple
tumor cells, and there are close correlations between it and the
development and prognosis of the above tumors. This study revealed
that the positive expression rate of Ki-67 in cancer tissues of the
patient group was 45.6%, which was significantly higher than the
2.5% in the cancer adjacent normal tissues of the control group
(p<0.05). Ki-67 expression in cancer tissues of the patient
group were associated with clinical staging and lymphatic
metastasis (p<0.05), but had no correlation with age and tumor
size (p>0.05), suggesting that Ki-67 expression is closely
related to the clinical staging and lymphatic metastasis of breast
cancer, thus, the more severe the clinical staging is, the higher
the positive expression rate of Ki-67 will be.
As an important antigen that can promote malignant
tumor angiogenesis and cell proliferation, COX-2 greatly affects
prognosis of tumor patients (17).
COX-2 belongs to an inducible enzyme, which is not easily detected
in normal tissue, but under the stimulation of oncogenes and other
stimuli, its inducible expression can occur rapidly (18,19). This
study showed that the positive expression rate of COX-2 in cancer
tissues of the patient group was 51.1%, which was significantly
higher than the 2.5% in cancer-adjacent normal tissues of the
control group (p<0.05). COX-2 expression in cancer tissues of
the patient group was associated with clinical staging and
lymphatic metastasis (p<0.05), but had no correlation with age
and tumor size (p>0.05), suggesting that COX-2 can promote the
occurrence and development of breast tumors, which involves
inducing tumor cell proliferation, inhibiting tumor cell apoptosis,
suppressing anti-tumor immune response in vivo, promoting
malignant tumor angiogenesis and other multiple mechanisms.
Further analysis indicated that there was a
significantly positive correlation between the expression of PCNA
and COX-2 in cancer tissues of the patient group (r=0.676,
p<0.05); there was significantly positive correlation between
the expression of Ki-67 and COX-2 (r=0.724, p<0.05); PCNA
expression had no obvious correlation with the expression of Ki-67
(p>0.05). The results further demonstrated the relationships
among PCNA, Ki-67 and COX-2.
Breast X-ray mammography is characterized by simple
operation and low cost in examining benign and malignant breast
diseases, which also has a relatively high sensitivity and accuracy
in the detection of microcalcifications. The results of breast
X-ray photography for the 90 patients showed that the typical X-ray
in patients with breast cancer was manifested by lumps in different
sizes, malignant calcification, manifestations of architectural
distortion, localized density-increased shadow, skin and nipple
depression. The relationships between X-ray photography features
and PCNA, Ki-67 and COX-2 expression in breast invasive ductal
carcinoma were primarily investigated in this study, which showed
that PCNA, Ki-67 and COX-2 expression in cancer tissues of the
patient group had no correlation with the existences of lump and
localized density-increased shadow (p>0.05), but were associated
with manifestations of architectural distortion, calcification as
well as skin and nipple depression (p<0.05). Therefore, it can
be speculated that the infiltration of breast cancer with
manifestations of architectural distortion, calcification as well
as skin and nipple depression is stronger, and the malignancy
degree is also higher, which shows poor clinical prognosis.
In conclusion, expression of PCNA, Ki-67 and COX-2
is a significant predictor for the occurrence, invasion and
metastasis of breast invasive ductal carcinoma. There is a
correlation between PCNA, Ki-67 and COX-2 expression levels and
X-ray features in mammography in breast invasive ductal carcinoma.
The application of X-ray features in mammography can evaluate the
expression levels of PCNA, Ki-67 and COX-2 in tissues of breast
invasive ductal carcinoma, thereby prospectively predicting
biological behavior of these cancer cells and patients
prognosis.
References
|
1
|
Sawair F, Hassona Y, Irwin C, Stephenson
M, Hamilton P, Maxwell P, Gordon D, Leonard A and Napier S: p53,
Cyclin D1, p21 (WAF1) and Ki-67 (MIB1) expression at invasive
tumour fronts of oral squamous cell carcinomas and development of
local recurrence. Asian Pac J Cancer Prev. 17:1243–1249. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Choi BB and Shu KS: Metaplastic carcinoma
of the breast: Multimodality imaging and histopathologic
assessment. Acta Radiol. 53:5–11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nishimura R, Osako T, Okumura Y, Hayashi M
and Arima N: Clinical significance of Ki-67 in neoadjuvant
chemotherapy for primary breast cancer as a predictor for
chemosensitivity and for prognosis. Breast Cancer. 17:269–275.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bukholm IR, Bukholm G, Holm R and Nesland
JM: Association between histology grade, expression of HsMCM2, and
cyclin A in human invasive breast carcinomas. J Clin Pathol.
56:368–373. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wojnar A, Pula B, Piotrowska A, Jethon A,
Kujawa K, Kobierzycki C, Rys J, Podhorska-Okolow M and Dziegiel P:
Correlation of intensity of MT-I/II expression with Ki-67 and MCM-2
proteins in invasive ductal breast carcinoma. Anticancer Res.
31:3027–3033. 2011.PubMed/NCBI
|
|
6
|
Sankpal NV, Willman MW, Fleming TP,
Mayfield JD and Gillanders WE: Transcriptional repression of
epithelial cell adhesion molecule contributes to p53 control of
breast cancer invasion. Cancer Res. 69:753–757. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chae SW, Sohn JH, Kim DH, Choi YJ, Park
YL, Kim K, Cho YH, Pyo JS and Kim JH: Overexpressions of Cyclin B1,
cdc2, p16 and p53 in human breast cancer: The clinicopathologic
correlations and prognostic implications. Yonsei Med J. 52:445–453.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park H, Chang SK, Kim JY, Lee BM and Shin
HS: Risk factors for distant metastasis as a primary site of
treatment failure in early-stage breast cancer. Chonnam Med J.
50:96–101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Penault-Llorca F, André F, Sagan C,
Lacroix-Triki M, Denoux Y, Verriele V, Jacquemier J, Baranzelli MC,
Bibeau F, Antoine M, et al: Ki67 expression and docetaxel efficacy
in patients with estrogen receptor-positive breast cancer. J Clin
Oncol. 27:2809–2815. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lialiaris TS, Kouskoukis A, Georgiou G,
Tripsianis G, Fiska A, Giatromanolaki A, Chrisafi S, Sivridis E,
Vamvakopoulou DN, Soutopoulou DO, et al: Expression of 6 common
antigenic markers in invasive ductal breast carcinoma: Potential
clinical implications. Appl Immunohistochem Mol Morphol.
19:106–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wasielewski M, Elstrodt F, Klijn JG, Berns
EM and Schutte M: Thirteen new p53 gene mutants identified among 41
human breast cancer cell lines. Breast Cancer Res Treat. 99:97–101.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Juríková M, Danihel Ľ, Polák Š and Varga
I: Ki67, PCNA, and MCM proteins: Markers of proliferation in the
diagnosis of breast cancer. Acta Histochem. 118:544–552. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bouchbika Z, Benchakroun N, Taleb A,
Jouhadi H, Tawfiq N, Sahraoui S and Benider A: Association between
overexpression of Her-2 and other clinicopathologic prognostic
factors in breast cancer in Morocco. J Cancer Ther. 3:787–792.
2012. View Article : Google Scholar
|
|
14
|
Qu DW, Liu Y, Wang L, Xiong Y, Zhang CL
and Gao DS: Glial cell line-derived neurotrophic factor promotes
proliferation of neuroglioma cells by up-regulation of cyclins PCNA
and Ki-67. Eur Rev Med Pharmacol Sci. 19:2070–2075. 2015.PubMed/NCBI
|
|
15
|
Masubuchi T, Tada Y, Maruya S, Osamura Y,
Kamata SE, Miura K, Fushimi C, Takahashi H, Kawakita D, Kishimoto
S, et al: Clinicopathological significance of androgen receptor,
HER2, Ki-67 and EGFR expressions in salivary duct carcinoma. Int J
Clin Oncol. 20:35–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alshenawy HA: Evaluation of p16, human
papillomavirus capsid protein L1 and Ki-67 in cervical
intraepithelial lesions: Potential utility in diagnosis and
prognosis. Pathol Res Pract. 210:916–921. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mansourian M, Haghjooy-Javanmard S,
Eshraghi A, Vaseghi G, Hayatshahi A and Thomas J: Statins use and
risk of breast cancer recurrence and death: A systematic review and
meta-analysis of observational studies. J Pharm Pharm Sci.
19:72–81. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Di Francesco L, Dovizio M, Trenti A,
Marcantoni E, Moore A, Ogaora P, McCarthy C, Tacconelli S, Bruno A,
Alberti S, et al: Dysregulated post-transcriptional control of
COX-2 gene expression in gestational diabetic endothelial cells. Br
J Pharmacol. 172:4575–4587. 2015. View Article : Google Scholar
|
|
19
|
Kim HN, Kim DH, Kim EH, Lee MH, Kundu JK,
Na HK, Cha YN and Surh YJ: Sulforaphane inhibits phorbol
ester-stimulated IKK-NF-κB signaling and COX-2 expression in human
mammary epithelial cells by targeting NF-κB activating kinase and
ERK. Cancer Lett. 351:41–49. 2014. View Article : Google Scholar : PubMed/NCBI
|