Introduction
Arsenic trioxide (As2O3) is a
common drug in traditional Chinese medicine (1). Since the remarkable therapeutic effect
of As2O3 on acute promyelocytic leukemia
(APL) was recognized (2), an
increasing number of researchers have reported its potential
anticancer activity in solid tumors, including hepatocellular
carcinoma (3,4), and pancreatic (5), prostate (6) and cervical cancer (7). However, the effect of
As2O3 on lung cancer, which is the leading
cause of cancer mortality worldwide (8), has been poorly explored. Furthermore,
the majority of studies on As2O3 anticancer
effects were performed on cancer cell lines, and only a few in
vivo studies were reported (9–12). Among
the known mechanisms of the anticancer action of
As2O3, anti-angiogenesis is an important
characteristic of As2O3. Several studies have
reported that As2O3 could influence tumor
angiogenesis (6,13,14), but
the underlying mechanism remains unclear.
Angiogenesis, the process of new blood vessel
formation, is the key step in solid tumor development; it is
necessary in tumor growth, invasion and metastasis (15). According to the classical theory of
tumor angiogenesis, tumors obtain nutrients and oxygen by diffusion
in the early stage, but when the tumor size becomes larger,
diffusion can no longer meet the tumor's requirement of oxygen and
nutrients; thus, the angiogenesis process is initiated (16). The angiogenesis process in solid
tumors can be summarized as follows: Continuing growth of a tumor
promotes the so-called ‘angiogenic switch’ in the microenvironment,
which initiates the angiogenic process (17,18).
Matrix metalloproteinases (MMPs) induce the degradation and
remodeling of the extracellular matrix (ECM) (19–21), and
endothelial cells migrate through the remodeled ECM, induced by
platelet-derived growth factor (PDGF) and chemokines (22,23). Due
to the role of vascular endothelial growth factor (VEGF) and
fibroblast growth factor (FGF), endothelial cells greatly
proliferate (24–26). Meanwhile, tube-like structure
formation and vascular function are achieved by delta like
canonical Notch ligand 4 (Dll4)/Notch-1 signaling (27–30). Tumor
angiogenesis is a complex process that includes multiple stages and
is regulated by numerous signaling molecules that interact with one
another (18). Currently, inhibition
of angiogenesis has become an important target in the treatment of
solid tumors, including lung cancer (31,32). Each
stage of the angiogenic process and the relevant signal factors
involved in the whole process of angiogenesis may become potential
therapeutic targets. Several anti-angiogenic agents have been
approved by the Food and Drug Administration (FDA) for cancer
treatment, such as bevacizumab, a humanized anti-VEGF-A monoclonal
antibody, and the tyrosine kinase inhibitors sorafenib and
sunitinib, targeting VEGF receptors (VEGFRs) (31,32). These
drugs may inhibit the proliferation of endothelial cells without
influencing other stages of angiogenesis.
Our group has previously demonstrated that
As2O3 exhibits anti-lung cancer activity by
inhibiting angiogenesis (33). It was
also demonstrated that As2O3 could reduce
malignant pleural effusion caused by the pleural metastasis of lung
cancer by downregulating nuclear factor-κB, tumor necrosis factor-α
and VEGF-A (34). In the present
study, the antitumor activity and anti-angiogenic effect of
As2O3 were demonstrated on both non-small
cell lung cancer (NSCLC) and small cell lung cancer (SCLC) in
vivo. The present study also revealed that
As2O3 disrupted multiple stages of
angiogenesis, including endothelial cell migration, proliferation
and tube formation. In addition, a series of key signaling
molecules involved in multiple stages of angiogenesis were
identified to be regulated by As2O3. It is
expected that these results would provide a basis for the
application of As2O3 in lung cancer
treatment.
Materials and methods
Cell culture
The human NSCLC cell line A549 was obtained from the
Cell Bank of Chinese Academy of Sciences (Shanghai, China). The
human SCLC cell line NCI-H446 and human umbilical vein endothelial
cells (HUVECs), used to determine the effect of
As2O3 on tumor angiogenesis, were obtained
from the American Type Culture Collection (Manassas, VA, USA). A549
and NCI-H446 cells were cultured in a mixture of RPMI 1640 medium
(HyClone; GE Healthcare Life Sciences, Logan, UT, USA), 10% fetal
bovine serum (FBS) (HyClone; GE Healthcare Life Sciences), 100 U/ml
penicillin and 100 µg/ml streptomycin. HUVECs were cultured in
Dulbecco's modified Eagle's medium (HyClone; GE Healthcare Life
Sciences) supplemented with 10% FBS and the same antibiotics as
described above. All the cell lines were incubated in a humidified
atmosphere of 5% CO2 at 37°C.
Xenograft models and drug
treatment
A total of 40 male nude mice, 5–6 weeks old and ~18
g in weight, were purchased from and raised in the Experimental
Animal Center of Second Military Medical University (Shanghai,
China). All mice were housed at 22°C, 12-h light/12-h dark cycle
and free access to clean water and food. A total of 0.2 ml A549 or
NCI-H446 cell suspension at a density of 5.0×107
cells/ml was injected subcutaneously into the right flank of the
mice. At 20 days post-injection, tumor volume reached ~100
mm3. Mice were then randomly divided into four groups,
and were treated with 2.5 or 5.0 mg/kg As2O3
(i.p.) (Beijing Shuanglu Pharmaceutical Co., Ltd., Beijing, China),
30 mg/kg sorafenib (p.o.) (LC Laboratories, Woburn, MA, USA) or
normal saline (NS) (i.p.) once daily for 10 days. Tumor volume was
calculated as 0.5xa2xb2, where a and b are
the largest and smallest lengths of the tumor, respectively. Animal
welfare and experimental procedures were carried out in accordance
with the Guide for the Care and Use of Laboratory Animals (Ministry
of Science and Technology of China) and the Experimental Animal
Ethical Care Guidelines of Second Military Medical University. The
animal study was approved by the Committee on Ethics of
Biomedicine, Second Military Medical University.
Immunohistochemistry
Fresh tumor tissue samples were fixed in 4%
paraformaldehyde solution, embedded in paraffin and cut into
5-µm-thick sections. Sections were deparaffinized and blocked for
endogenous peroxidase ablation. Then, sections were incubated with
anti-cluster of differentiation CD31 primary antibody (1:75,
catalog no. AF3628, R&D Systems, Inc., Minneapolis, MN, USA)
overnight at 4°C and the secondary antibody (1:200, catalog no.
14-13-06, KPL, Inc., Gaithersburg, MD, USA) for 1 h at room
temperature. Sections were colored with 3,3′-diaminobenzidine
(Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) and
counterstained with hematoxylin to reveal the nuclei. The
quantification of microvessels was performed by counting the number
of positive CD31 signals under an inverted fluorescence microscope
in five random fields at ×200 magnification.
Cell proliferation assay
Cells (2×103 cells/well) were seeded in
triplicate in 96-well plates and incubated under the aforementioned
culture conditions. VEGF-A (10 ng/ml; Shanghai Biomart Technology
Co., Ltd., Shanghai, China) was added in the medium of HUVECs.
Cells were then treated with various concentrations (2.0 or 4.0 µM)
of As2O3 (Beijing Shuanglu Pharmaceutical
Co., Ltd., Beijing, China), 4.0 µM sorafenib (LC Laboratories,
Woburn, MA, USA) or NS. After additional 24 or 48 h, cell
proliferation was determined in triplicate, using a Cell Counting
Kit-8 (CCK-8) assay (Beyotime Institute of Biotechnology, Haimen,
China). The absorbance of each well at 450 nm was measured. The
results were expressed as contrast absorbance, considering the NS
group as control.
Wound-healing assay
Cells were seeded in 6-well plates and divided into
four groups: Control group, As2O3 2.0 µM
group, As2O3 4.0 µM group and sorafenib 4.0
µM group. When cells grew to confluency, a mechanical wound was
created by gently scratching the cells with a pipette tip (time 0
h). Images were captured after 24 and 48 h, and the wound healing
capacity was quantified by measuring the distance between the wound
edges. Experiments were carried out in triplicate wells from three
independent experiments.
Vascular tube formation assay in
vitro
Plates with 24 wells were firstly coated with
Matrigel (BD Biosciences, Franklin Lakes, NJ, USA). Unpolymerized
Matrigel was placed in the wells (300 µl/well) and allowed to
polymerize for 1 h at room temperature. HUVECs in 500 µl medium
were seeded onto the polymerized Matrigel at a density of
5×104 cells/well. VEGF-A (10 ng/ml; Shanghai Biomart
Technology Co., Ltd.) and basic FGF (bFGF) (10 ng/ml; Shanghai
Biomart Technology Co., Ltd.) were used as angiogenic stimuli
(35). After incubation at 37°C in 5%
CO2 for 18 h, images of tube formation were acquired
with an inverted phase-contrast light microscope (Olympus
Corporation, Tokyo, Japan) equipped with a microscope camera (Q
Imaging, Surrey, BC, Canada). The degree of tube formation was
quantified in five random fields from each well at ×40
magnification, using ImageJ software (version 1.48, National
Institutes of Health, Bethesda, MD, USA).
Western blotting
Total proteins were extracted from cells using
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China). Aliquots containing 20 µg protein
were used for western blotting. Proteins were separated by SDS-PAGE
(10% gel) and transferred to polyvinylidene fluoride membranes.
Membranes were blocked with a solution containing 5% nonfat milk
for 1 h, and then incubated with the corresponding primary
antibodies overnight at 4°C. Membranes were then washed three times
with TBS containing Tween-20 and incubated with the secondary
antibody (1:3,000, catalog no. ab6721, Abcam, Cambridge, UK) at
room temperature for 1 h. The protein bands were detected by
chemiluminescence (Western chemiluminescent horseradish peroxidase
substrate, catalog no. WBKLS0500, Merck KGaA, Darmstadt, Germany).
β-actin was used as an internal control. The following primary
antibodies were used all at 1:1,000 dilution: Anti-MMP-2 (catalog
no. ab86607, Abcam), anti-MMP-9 (catalog no. ab38898, Abcam),
anti-PDGF-BB (catalog no. ab23914, Abcam), anti-PDGF receptor
(PDGFR)-β (catalog no. ab111310, Abcam), anti-VEGF-A (catalog no.
ab46154, Abcam), anti-VEGFR-2 (catalog no. ab39256, Abcam),
anti-bFGF (catalog no. ab8880, Abcam), anti-FGF receptor (FGFR)-1
(catalog no. ab823, Abcam), anti-Dll4 (catalog no. ab7280, Abcam),
anti-Notch-1 (catalog no. ab27526, Abcam) and anti-β-actin (catalog
no. sc-47778, Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
Statistical analysis
Data were analyzed using SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA). The results are presented as means ±
standard deviation, as analyzed by one-way analysis of variance,
followed by Fisher's least significant difference t test. P<0.05
was considered to indicate a statistically significant
difference.
Results
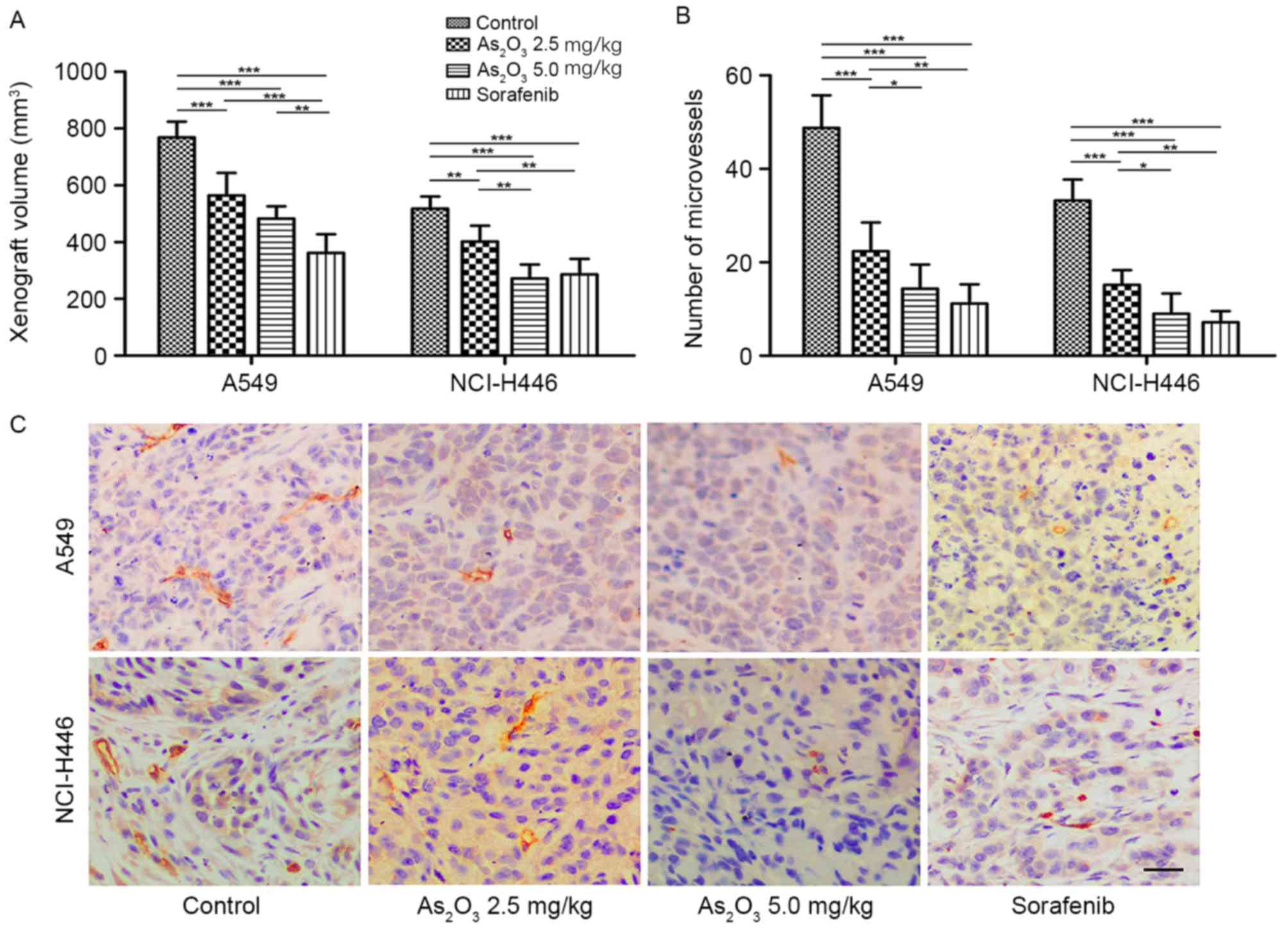
As2O3 inhibits
the growth of human lung cancer xenografts and tumor angiogenesis
in vivo
To determine the effect of
As2O3 on the growth of lung cancer, xenograft
tumor models were established using the NSCLC cell line A549 and
the SCLC cell line NCI-H446. When all nude mice developed tumors,
drug administration was performed for 10 continuous days. As
presented in Fig. 1A, the mean tumor
volumes in the As2O3 groups were
significantly smaller than those in the control groups at the end
of treatment, and tumor volumes in the 5.0 mg/kg
As2O3 group were smaller than those in the
2.5 mg/kg As2O3 group. These findings were
observed in both types of xenograft model. Sorafenib, an
anti-angiogenic, anti-tumor agent targeting VEGFR-2 (36), was used as a positive control. It was
observed that the inhibitory effect of sorafenib on tumor growth
was greater than that of 5.0 mg/kg As2O3 in
the A549 xenograft model, while it was similar to that of 5.0 mg/kg
As2O3 in the NCI-H446 xenograft model. These
results suggested that As2O3 had an
inhibitory effect on both NSCLC and SCLC tumor growth in a
dose-dependent manner.
Next, sections of xenografts were stained for CD31,
which was used primarily to demonstrate the presence of endothelial
cells, to detect the number and morphology of endothelial cells as
a measure of tumor angiogenesis (28). Representative images of
immunohistochemistry are presented in Fig. 1C, and the quantification of
microvessel numbers is presented in Fig.
1B. The mean microvessel number in the two
As2O3 groups was significantly lower than
that in the control group (P<0.001) in both types of xenograft
model. The morphology of microvessels in the
As2O3 groups was poorly developed compared
with the normal tube-like structure in the control group. The
microvessel number in the sorafenib group was also significantly
lower than that of the control group (P<0.001), but no
poorly-developed vascular structures were observed in the sorafenib
group. These data indicated that As2O3 could
inhibit tumor angiogenesis in NSCLC and SCLC in a dose-dependent
manner, but its mechanism may not be identical with that of
sorafenib. As2O3 may decrease the number of
blood vessels and delay the development of vascular structures,
whereas sorafenib may only decrease the number of blood
vessels.
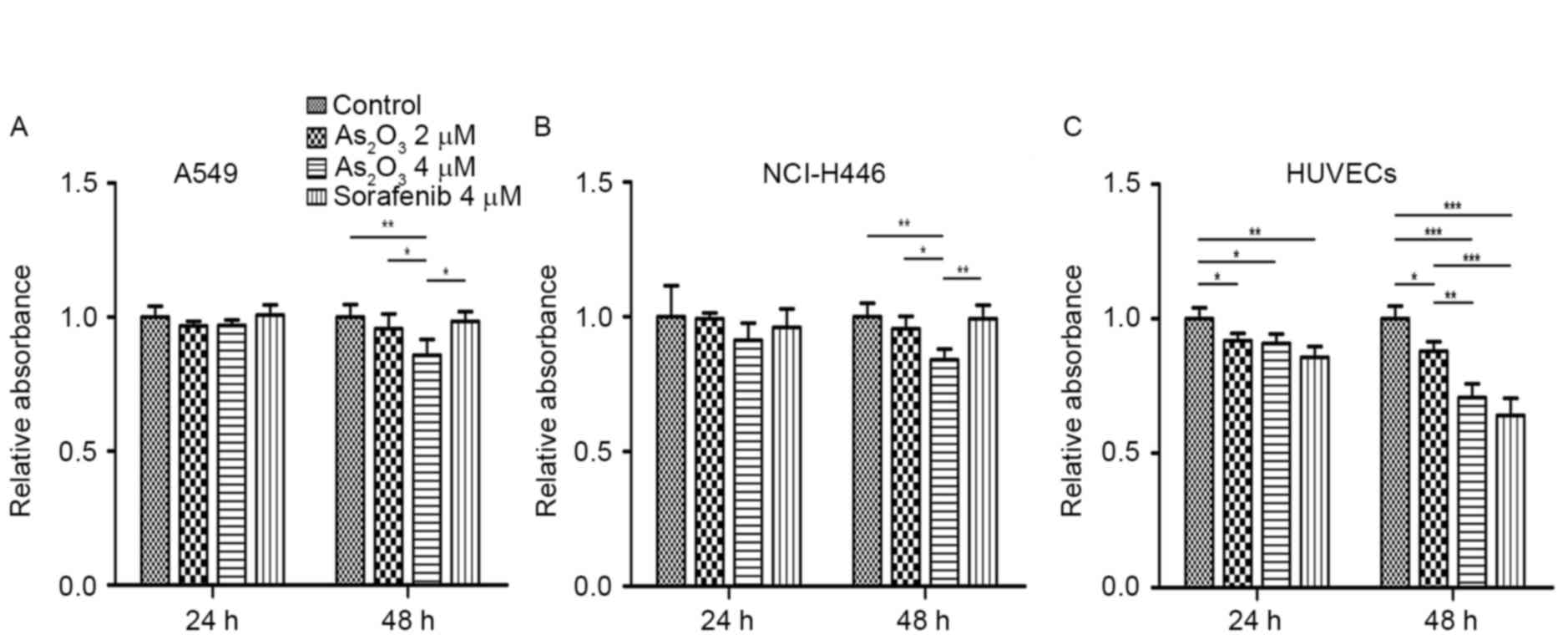
As2O3 inhibits
the proliferation of lung cancer cells and HUVECs
To further determine whether the anti-tumor effect
of As2O3 in vivo depended on its
anti-angiogenic effect or its direct cytotoxicity towards tumor
cells, its effect on the proliferation of lung cancer cells and
HUVECs was examined by CCK-8 assay. As presented in Fig. 2A and B, A549 and NCI-H446 cell
proliferation at 24 h exhibited no significant difference between
the groups, while at 48 h, cell proliferation in the
As2O3 groups was slightly lower than that in
the control and sorafenib groups. As2O3
significantly inhibited HUVEC proliferation compared with that of
the control group at both 24 and 48 h. The inhibitory effect of
sorafenib on HUVEC proliferation was also obvious (Fig. 2C). These results demonstrated that
direct cytotoxicity towards tumor cells was not the main factor in
the anti-lung cancer effect of As2O3, while
inhibition of vascular endothelial cell proliferation may be
important in this process.
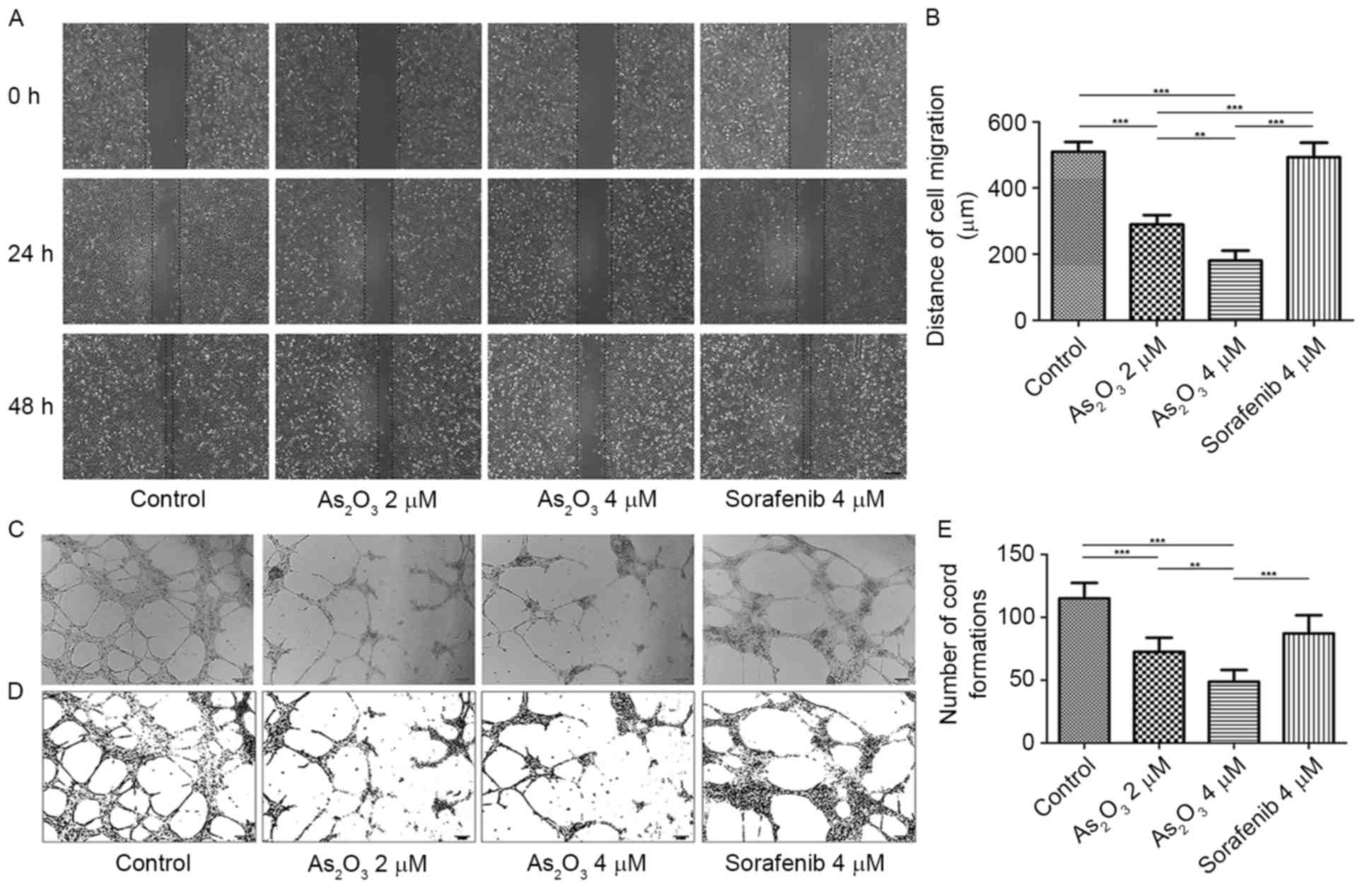
As2O3 disrupts
HUVEC migration and tube-like structure formation on Matrigel
To determine the effect of
As2O3 on the migration of HUVECs, a
wound-healing assay was performed. As presented in Fig. 3A, the wound-healing capacity of HUVECs
was diminished by As2O3 at 24 h after the
wounds were created, and at 48 h after scratching, this phenomenon
was more obvious. According to the quantitative comparison of cell
migration distances at 48 h (Fig.
3B), the distances of cell migration in the
As2O3 2 and 4 µM groups were 289.52±28.62 and
180.00±30.90 µm, respectively, which were significantly lower than
the cell migration distance in the control group (509.52±29.74 µm;
P<0.001), suggesting that the migration of HUVECs was inhibited
by As2O3 in a dose-dependent manner. However,
the distance of cell migration in the sorafenib group (493.33±43.74
µm) was not significantly different from that in the control group
(P>0.05), which suggested that, different from
As2O3, sorafenib did not affect the migration
of HUVECs.
Next, it was examined whether
As2O3 was able to disrupt endothelial network
formation by Matrigel assay. HUVECs were plated onto Matrigel in
the presence of VEGF-A and bFGF as angiogenic factors (35). Then, cells were treated with 2 or 4 µM
As2O3, 4 µM sorafenib, or NS as control for
18 h, and microphotographs were then obtained (Fig. 3C). Pictures of cords of
interconnecting cells were generated by ImageJ 1.48v software
(Fig. 3D), and the number of cord
formations was quantitatively analyzed (Fig. 3E). As presented in Fig. 3E, a significant decrease in tube
formation in the As2O3 groups was observed
(P<0.001), where normal tube structures were destroyed with
interrupted alignments and cords. Sorafenib also reduced the number
of cord formations (P<0.01), but the tube structures observed in
this group were as regular as those in the control group.
As2O3 inhibits
angiogenesis-associated factors in lung cancer cells and
HUVECs
Based on the aforementioned results,
As2O3 displayed effective anti-angiogenic
activity both in vivo and in vitro.
As2O3 could disrupt multiple stages of
angiogenesis, including endothelial cell migration, proliferation
and tube formation. The present study next examined
angiogenesis-associated factors involved in these stages at the
protein level by western blotting. As presented in Fig. 4A and B, As2O3
reduced the expression of MMP-2, MMP-9, PDGF-BB, VEGF-A, bFGF and
Dll4 in A549 and NCI-H446 cells. As2O3 also
reduced the expression of MMP-2, MMP-9, PDGFR-β, VEGFR-2, FGFR-1,
Dll4 and Notch-1 in HUVECs (Fig. 4C),
in a concentration-dependent manner. Sorafenib only downregulated
VEGFR-2 and PDGFR-β expression in HUVECs, but had no marked effect
on the expression of the other factors (Fig. 4C), verifying that sorafenib inhibited
endothelial cell proliferation mainly by targeting VEGF
signaling.
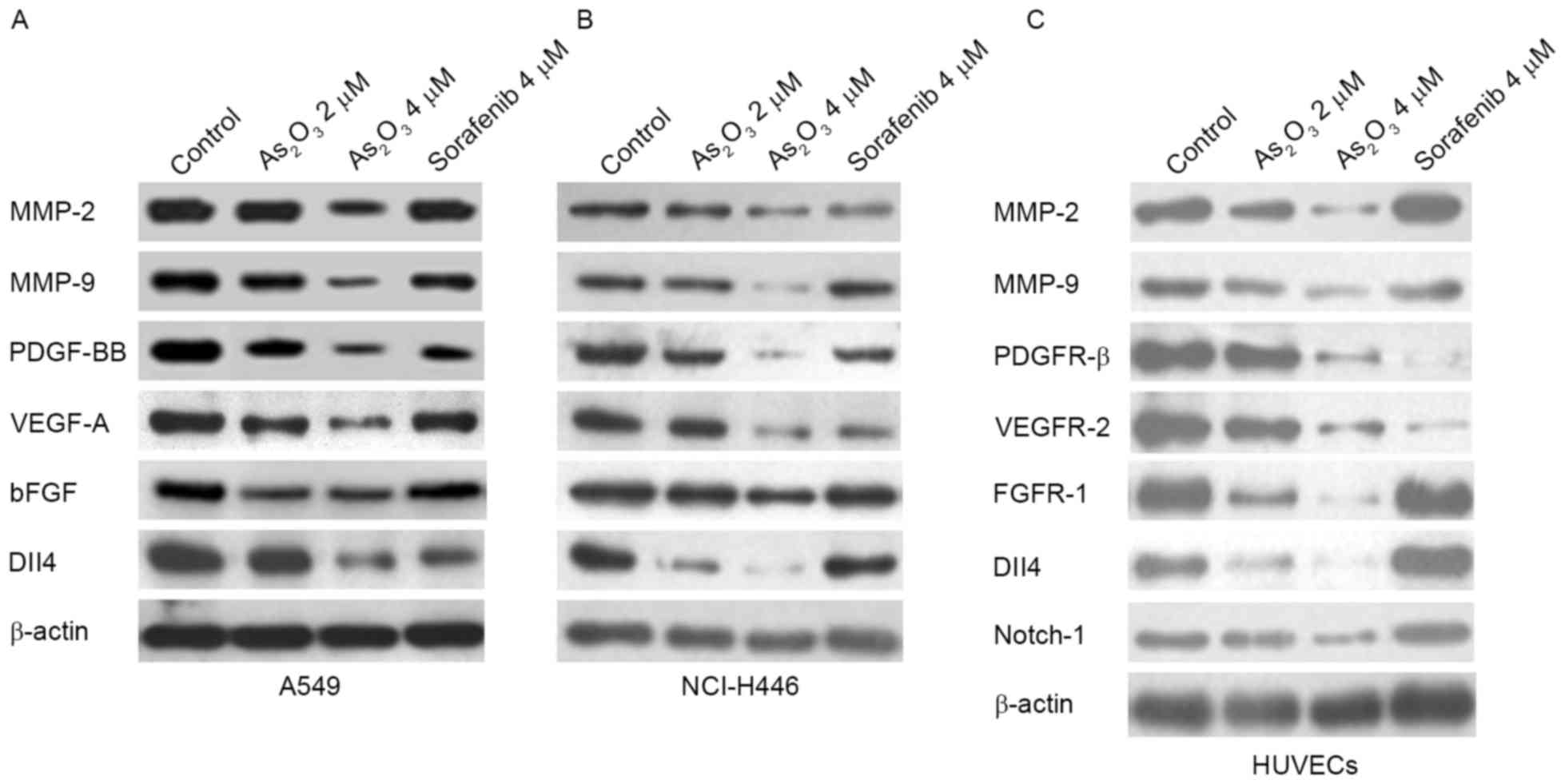 | Figure 4.As2O3 inhibits
angiogenesis-associated factors. As2O3
reduced MMP-2, MMP-9, PDGF-BB, VEGF-A, bFGF and Dll4 protein levels
in (A) A549 and (B) NCI-H446 cells. (C) As2O3
reduced MMP-2, MMP-9, PDGFR-β, VEGFR-2, FGFR-1, Dll4 and Notch-1
protein levels in HUVECs. The target protein levels were evaluated
by western blotting. HUVEC, human umbilical vein endothelial cell;
MMP, matrix metalloproteinase; PDGFR, platelet-derived growth
factor receptor; VEGFR, vascular endothelial growth factor; bFGF,
basic fibroblast growth factor; FGFR, fibroblast growth factor
receptor; Dll4, delta like canonical Notch ligand 4. |
Discussion
As2O3 was firstly introduced
as an effective agent with low toxicity for APL treatment by
Chinese researchers (37). Since its
approval by the FDA for use in leukemia therapy,
As2O3 has also been applied to numerous solid
tumors (38,39). However, the mechanism of its
anticancer activity in solid tumors is not yet fully
understood.
In the present study, in vivo experiments
demonstrated that As2O3 significantly
inhibited the growth of NSCLC and SCLC xenografts in a
dose-dependent manner. As2O3 also exhibited a
marked anti-angiogenic effect in animal models of lung cancer.
In vitro cell proliferation assays were conducted in the
present study, which revealed that the inhibitory effect of
As2O3 on NSCLC and SCLC cell growth was not
so remarkable as that observed in vivo. In addition,
As2O3 significantly suppressed endothelial
cell proliferation. These data suggested that anti-angiogenesis,
rather than direct cytotoxicity towards tumor cells, was the main
mechanism of the anticancer effect of
As2O3.
The anti-angiogenic effect of
As2O3 has been reported in various studies,
both in vivo and in vitro. A previous study reported
that 0.5 and 5.0 µM As2O3 caused inhibition
of VEGF in leukemic cells, and prevented capillary tube formation
in an endothelial cell-differentiation assay (40). It was also reported that
As2O3 delayed gastric cancer xenograft
growth, decreased microvessel density, and downregulated VEGFR-1
and VEGFR-2 expression (14). Besides
reducing the number of vessels, As2O3 also
influences the vascular morphology and function. Adding
As2O3 into the drinking water for mice
induced significant vascular remodeling, with increased sinusoidal
endothelial cell capillarization, resulting in decreased
permeability and transport function (41). In another study,
As2O3 (10 mg/kg i.p.) produced a preferential
vascular ‘shutdown’ in the tumor tissue in a model of
methylcholanthrene-induced fibrosarcoma in BALB/c mice, leading to
extensive necrosis in the central part of the tumor (42). In addition, 99mTc clearance
and 86Rb uptake in the tumor tissue were decreased,
suggesting declined tumor perfusion (42).
In the present study, As2O3
decreased the number of microvessels in lung cancer xenografts, and
it also inhibited HUVEC proliferation significantly at both 24 and
48 h. In addition, As2O3 reduced the
expression of VEGF-A and bFGF in lung cancer cells, as well as that
of VEGFR-2 and FGFR-1 in HUVECs. VEGF-A/VEGFR-2 and bFGF/FGFR-1 are
both potent stimulating factors of endothelial cell proliferation,
which have been previously validated (43,44).
The present results also indicated that
As2O3 disrupted endothelial cell migration,
and downregulated MMP-2, MMP-9 and PDGF-BB in lung cancer cells,
and PDGFR-β in HUVECs. MMPs such as MMP-2 and MMP-9 contribute to
basement membrane degradation and ECM remodeling, which allow
endothelial cell migration and sprouting (20). PDGF signaling promotes endothelial
cell migration through the remodeled ECM and the formation of new
blood vessels (45). The present
results confirmed that As2O3 could inhibit
this signaling pathway and thereby disturb HUVEC migration.
Furthermore, the present data revealed that
As2O3 influenced the morphology of
microvessels by inducing poorly developed vascular structures in
vivo. Tube formation assays on Matrigel were performed to
explore the effect of As2O3 on the number and
shape of newly formed microvessels upon stimulation with VEGF-A and
bFGF. It was observed that As2O3 reduced the
number of cord formations and destroyed the normal tube structures.
This effect helped to distinguish As2O3 from
the well-known angiogenesis-targeted inhibitor sorafenib. In
vivo, formation of vascular lumen structures is promoted by
Dll4/Notch signaling (46). It has
been reported that blockade of Dll4/Notch signaling induced
defective maturation of blood vessels with poor perfusion (28,47).
According to the present results, As2O3
reduced the level of Dll4 in lung cancer cells and HUVECs, and
reduced the level of Notch-1 in HUVECs. These findings implied that
As2O3 influenced the morphology and function
of new vessels in lung cancer, possibly by downregulating
Dll4/Notch signaling.
In summary, the present study demonstrated that
As2O3 could inhibit lung cancer xenograft
growth and tumor angiogenesis. It was also observed that
As2O3 could disrupt multiple stages of
angiogenesis, including endothelial cell migration, proliferation
and network formation, and could regulate the expression of the key
signaling molecules involved in these processes. The present
findings may provide the experimental basis to extend the
indications of As2O3 and to identify novel
therapeutic approaches for lung cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81172227, 81672929
and 81602618) and the Research Foundation of Shanghai Municipal
Education Commission (grant no. 12ZZ073).
References
|
1
|
Waxman S and Anderson KC: History of the
development of arsenic derivatives in cancer therapy. Oncologist.
6:(suppl 2). S3–S10. 2001. View Article : Google Scholar
|
|
2
|
Chen SJ, Zhou GB, Zhang XW, Mao JH, de Thé
H and Chen Z: From an old remedy to a magic bullet: Molecular
mechanisms underlying the therapeutic effects of arsenic in
fighting leukemia. Blood. 117:6425–6437. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang X, Jia S, Yang S and Yang Y, Yang T
and Yang Y: Arsenic trioxide induces G2/M arrest in hepatocellular
carcinoma cells by increasing the tumor suppressor PTEN expression.
J Cell Biochem. 113:3528–3535. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang X, Jiang F, Mu J, Ye X, Si L, Ning S,
Li Z and Li Y: Arsenic trioxide attenuates the invasion potential
of human liver cancer cells through the demethylation-activated
microRNA-491. Toxicol Lett. 227:75–83. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao JK, Wang LX, Long B, Ye XT, Su JN, Yin
XY, Zhou XX and Wang ZW: Arsenic Trioxide Inhibits Cell Growth and
Invasion via Down-Regulation of Skp2 in Pancreatic Cancer Cells.
Asian Pac J Cancer Prev. 16:3805–3810. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ji H, Li Y, Jiang F, Wang X, Zhang J, Shen
J and Yang X: Inhibition of transforming growth factor beta/SMAD
signal by MiR-155 is involved in arsenic trioxide-induced
anti-angiogenesis in prostate cancer. Cancer Sci. 105:1541–1549.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang H, Gao P and Zheng J: Arsenic
trioxide inhibits cell proliferation and human papillomavirus
oncogene expression in cervical cancer cells. Biochem Biophys Res
Commun. 451:556–561. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li H, Zhu X, Zhang Y, Xiang J and Chen H:
Arsenic trioxide exerts synergistic effects with cisplatin on
non-small cell lung cancer cells via apoptosis induction. J Exp
Clin Cancer Res. 28:1102009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Walker AM, Stevens JJ, Ndebele K and
Tchounwou PB: Arsenic trioxide modulates DNA synthesis and
apoptosis in lung carcinoma cells. Int J Environ Res Public Health.
7:1996–2007. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lam SK, Li YY, Zheng CY, Leung LL and Ho
JC: E2F1 downregulation by arsenic trioxide in lung adenocarcinoma.
Int J Oncol. 45:2033–2043. 2014.PubMed/NCBI
|
|
12
|
Zheng CY, Lam SK, Li YY, Fong BM, Mak JC
and Ho JC: Combination of arsenic trioxide and chemotherapy in
small cell lung cancer. Lung Cancer. 82:222–230. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang F, Wang X, Liu Q, Shen J, Li Z, Li Y
and Zhang J: Inhibition of TGF-β/SMAD3/NF-κB signaling by
microRNA-491 is involved in arsenic trioxide-induced
anti-angiogenesis in hepatocellular carcinoma cells. Toxicol Lett.
231:55–61. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiao YF, Wu DD, Liu SX, Chen X and Ren LF:
Effect of arsenic trioxide on vascular endothelial cell
proliferation and expression of vascular endothelial growth factor
receptors Flt-1 and KDR in gastric cancer in nude mice. World J
Gastroenterol. 13:6498–6505. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Aggarwal C, Somaiah N and Simon G:
Antiangiogenic agents in the management of non-small cell lung
cancer: Where do we stand now and where are we headed? Cancer Biol
Ther. 13:247–263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weis SM and Cheresh DA: Tumor
angiogenesis: Molecular pathways and therapeutic targets. Nat Med.
17:1359–1370. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bergers G, Brekken R, McMahon G, Vu TH,
Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z and
Hanahan D: Matrix metalloproteinase-9 triggers the angiogenic
switch during carcinogenesis. Nat Cell Biol. 2:737–744. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deryugina EI and Quigley JP: Pleiotropic
roles of matrix metalloproteinases in tumor angiogenesis:
Contrasting, overlapping and compensatory functions. Biochim
Biophys Acta. 1803:103–120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Humphries JD, Byron A and Humphries MJ:
Integrin ligands at a glance. J Cell Sci. 119:3901–3903. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sakurai T and Kudo M: Signaling pathways
governing tumor angiogenesis. Oncology. 81:(suppl 1). S24–S29.
2011. View Article : Google Scholar
|
|
23
|
Andrae J, Gallini R and Betsholtz C: Role
of platelet-derived growth factors in physiology and medicine.
Genes Dev. 22:1276–1312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lohela M, Bry M, Tammela T and Alitalo K:
VEGFs and receptors involved in angiogenesis versus
lymphangiogenesis. Curr Opin Cell Biol. 21:154–165. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cao Y, Cao R and Hedlund EM: R Regulation
of tumor angiogenesis and metastasis by FGF and PDGF signaling
pathways. J Mol Med (Berl). 86:785–789. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Korc M and Friesel RE: The role of
fibroblast growth factors in tumor growth. Curr Cancer Drug
Targets. 9:639–651. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kuhnert F, Kirshner JR and Thurston G:
Dll4-Notch signaling as a therapeutic target in tumor angiogenesis.
Vasc Cell. 3:202011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Noguera-Troise I, Daly C, Papadopoulos NJ,
Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD and Thurston
G: Blockade of Dll4 inhibits tumour growth by promoting
non-productive angiogenesis. Nature. 444:1032–1037. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ridgway J, Zhang G, Wu Y, Stawicki S,
Liang WC, Chanthery Y, Kowalski J, Watts RJ, Callahan C, Kasman I,
et al: Inhibition of Dll4 signaling inhibits tumour growth by
deregulating angiogenesis. Nature. 444:1083–1087. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Williams CK, Li JL, Murga M, Harris AL and
Tosato G: Up-regulation of the Notch ligand Delta like 4 inhibits
VEGF-induced endothelial cell function. Blood. 107:931–939. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ferrara N and Kerbel RS: Angiogenesis as a
therapeutic target. Nature. 438:967–974. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cooney MM, van Heeckeren W, Bhakta S,
Ortiz J and Remick SC: Drug insight: Vascular disrupting agents and
angiogenesis novel approaches for drug delivery. Nat Clin Pract
Oncol. 3:682–692. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang MH, Zang YS, Huang H, Chen K, Li B,
Sun GY and Zhao XW: Arsenic trioxide exerts anti-lung cancer
activity by inhibiting angiogenesis. Curr Cancer Drug Targets.
14:557–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xie SL, Yang MH, Chen K, Huang H, Zhao XW,
Zang YS and Li B: Efficacy of arsenic trioxide in the treatment of
malignant pleural effusion caused by pleural metastasis of lung
cancer. Cell Biochem Biophys. 71:1325–1333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cattaneo MG, Pola S, Dehò V, Sanguini AM
and Vicentini LM: Alprostadil suppresses angiogenesis in vitro and
in vivo in the murine Matrigel plug assay. Br J Pharmacol.
138:377–385. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Takahashi S: Vascular endothelial growth
factor (VEGF), VEGF receptors and their inhibitors for
antiangiogenic tumor therapy. Biol Pharm Bull. 34:1785–1788. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shen ZX, Chen GQ, Ni JH, Li XS, Xiong SM,
Qiu QY, Zhu J, Tang W, Sun GL, Yang KQ, et al: Use of arsenic
trioxide (As2O3) in the treatment of acute promyelocytic leukemia
(APL): II. Clinical efficacy and pharmacokinetics in relapsed
patients. Blood. 89:3354–3360. 1997.PubMed/NCBI
|
|
38
|
Ma Y, Wang J, Liu L, Zhu H, Chen X, Pan S,
Sun X and Jiang H: Genistein potentiates the effect of arsenic
trioxide against human hepatocellular carcinoma: Role of Akt and
nuclear factor-κB. Cancer Lett. 301:75–84. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guo W, Tang XD, Tang S and Yang Y:
Preliminary report of combination chemotherapy including Arsenic
trioxide for stage III osteosarcoma and Ewing sarcoma. Zhonghua Wai
Ke Za Zhi. 44:805–808. 2006.(In Chinese). PubMed/NCBI
|
|
40
|
Roboz GJ, Dias S, Lam G, Lane WJ, Soignet
SL, Warrell RP Jr and Rafii S: Arsenic trioxide induces dose- and
time-dependent apoptosis of endothelium and may exert an
antileukemic effect via inhibition of angiogenesis. Blood.
96:1525–1530. 2000.PubMed/NCBI
|
|
41
|
Straub AC, Stolz DB, Ross MA,
Hernández-Zavala A, Soucy NV, Klei LR and Barchowsky A: Arsenic
stimulates sinusoidal endothelial cell capillarization and vessel
remodeling in mouse liver. Hepatology. 45:205–212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lew YS, Brown SL, Griffin RJ, Song CW and
Kim JH: Arsenic trioxide causes selective necrosis in solid murine
tumors by vascular shutdown. Cancer Res. 59:6033–6037.
1999.PubMed/NCBI
|
|
43
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lindner V, Majack RA and Reidy MA: Basic
fibroblast growth factor stimulates endothelial regrowth and
proliferation in denuded arteries. J Clin Invest. 85:2004–2008.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang D, Huang HJ, Kazlauskas A and Cavenee
WK: Induction of vascular endothelial growth factor expression in
endothelial cells by platelet-derived growth factor through the
activation of phosphatidylinositol 3-kinase. Cancer Res.
59:1464–1472. 1999.PubMed/NCBI
|
|
46
|
Kume T: Novel insights into the
differential functions of Notch ligands in vascular formation. J
Angiogenes Res. 1:82009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Scehnet JS, Jiang W, Kumar SR, Krasnoperov
V, Trindade A, Benedito R, Djokovic D, Borges C, Ley EJ, Duarte A
and Gill PS: Inhibition of Dll4-mediated signaling induces
proliferation of immature vessels and results in poor tissue
perfusion. Blood. 109:4753–4760. 2007. View Article : Google Scholar : PubMed/NCBI
|