Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common malignant tumor in the world and its incidence is
increasing. It is the third most common cause of cancer-related
death among malignant tumors (1),
ranking second and sixth among tumor-related causes of death in
males and females, respectively (2).
Currently, surgery is the most common method of treatment of liver
cancer. However, the clinical symptoms of liver cancer are not
obvious and are often overlooked. When patients experience symptoms
such as weight loss, jaundice, abdominal mass and liver pain, the
vast majority have reached the advanced or terminal stage of the
disease. Although radiotherapy and chemotherapy have certain
effects, only 10% of patients are likely to undergo complete
hepatectomy. While 90% of patients are treated with radiotherapy,
chemotherapy, or radiofrequency ablation, it is difficult to
achieve the expected clinical results because of low efficacy and
high rate of severe side effects. Therefore, effective treatment of
liver cancer is severely lacking (3,4).
In traditional Chinese medicine, Chinese herbal
medicines have many advantages. Their active ingredients generally
have high efficiency and low toxicity. Scientists worldwide have
paid increasing attention to Chinese herbal medicines because of
their irreplaceable advantages. Studies have shown that Chinese
herbal medicine has significant advantages for the prolongation of
survival, prevention and treatment of liver cancer and metastasis
(5). In recent years, with studies on
the anticarcinogenic mechanisms of Chinese herbal medicine, more
active ingredients have been extracted. Puerarin (Pu) belongs to
the group of isoflavone flycoside compounds and is extracted from
the traditional Chinese medicine leguminous plants, Pueraria
thomsonii Benth and Pueraria lobata (Willd.) Ohwi. It
was reported that Pu has antitumor activity (6–8).
The aim of the present study was to investigate the
effect of Pu on the sensitivity of HepG2 human HCC cells to
chemotherapeutic drugs and its possible mechanism, to provide a
foundation for the treatment of HCC.
Materials and methods
Reagents
Pu (Aladdin Reagent Co. Ltd., Shanghai, China);
HepG2 cell line (Cell Bank of Chinese Academy of Sciences,
Shanghai, China); Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS) (HyClone Laboratories, Logan, UT, USA); primary
rabbit polyclonal GAPDH antibody (dilution, 1:1,000; cat. no.
10494-1-AP), rabbit polyclonal Bax antibody (dilution, 1:500; cat.
no. 50599-2-Ig), rabbit polyclonal Bcl-2 antibody (dilution, 1:500;
cat. no. 12789-1-AP) and mouse monoclonal HRP-conjugated secondary
antibody (dilution, 1:2,000; cat. no. HRP-66008) were all purchased
from Sanying Biotechnology Co. Ltd. (Wuhan, Hubei, China). Cell
lysis buffer, BCA protein concentration quantification kit, Annexin
V-FITC apoptosis detection kit (all from Biyuntian Biotechnology
Research Institute, Nantong, China);
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
dimethyl sulfoxide (DMSO) (Sigma, St. Louis, MO, USA).
Cell culture
HepG2 cells were cultured in an incubator at 37°C
and 5% CO2 in DMEM medium containing 100 U/ml
penicillin, 100 µg/ml streptomycin and 10% FBS. When cells were in
the logarithmic phase of growth, they were digested with trypsin
and single cell suspensions were prepared. Cells were grouped and
seeded in different culture plates or Petri dishes according to
experimental needs.
MTT assay
Cells were divided into the experimental and control
groups. HepG2 cells in the logarithmic growth phase were collected
and seeded in 96-well plates at a concentration of
1×104/ml, with 200 µl per well. After 24 h, the
experimental group was divided into 12 subgroups according to
different treatments: Four groups treated with Pu alone (final
concentrations of 25, 50, 100 and 125 µM, respectively), four
groups treated with cisplatin (CDDP) alone (final concentrations of
2.5, 5, 10, and 20 µM, respectively) and four groups treated with
the combined drugs (final concentrations of 25 µM Pu + 2.5 µg/ml
CDDP, 50 µM Pu + 5 µg/ml CDDP, 100 µM Pu + 10 µg/ml CDDP and 125 µM
Pu + 20 µg/ml CDDP, respectively). Each condition was repeated five
times. Cells in the control group were cultured in the same culture
medium without treatment. After 48 h, the culture medium was
discarded and cells were washed three times with phosphate-buffered
saline (PBS). The cells were then incubated with 100 µl of MTT (5
mg/ml) for 4 h and 100 µl of DMSO was added to each well with
shaking in the dark for 10 min. The absorbance value (OD) at 570 nm
was measured using a microplate reader (Thermo Fisher Scientific,
New York, NY, USA). The rate of inhibition was calculated according
to the following formula: Inhibitory rate (%) = (OD value of the
control group - OD value of the experimental group/OD value of the
control group) × 100%.
Morphological observation
The cells were divided into four groups: The control
group, Pu group (100 µM), CDDP group (10 µg/ml) and Pu (100 µM) +
CDDP (10 µg/ml) group. After the cells were treated accordingly,
morphological changes were observed and recorded with an inverted
microscope (AZ100; Nikon, Tokyo, Japan).
Analysis of apoptosis
According to the results of the MTT assay, cells
were divided into four groups: The control group, Pu group (100
µM), CDDP group (10 µg/ml) and Pu (100 µM) + CDDP (10 µg/ml) group.
In the logarithmic growth phase, HepG2 cells were seeded in 6-well
plates. After cells adhered, they were treated accordingly for 24
h, washed three times with PBS, digested with trypsin and
centrifuged. All protocols were followed according to the
instructions of the kit. Cells were resuspended in 0.3 ml of
binding buffer, followed by addition of 5 µl of Annexin V and 5 µl
of PI. After incubation at room temperature for 15 min in the dark,
0.2 ml of binding buffer was added to each sample. Apoptosis was
detected by flow cytometry (Becton Dickinson, New York, NY,
USA).
Western blot analysis
Cells were treated in the same manner as for
morphological observation, harvested and lysed with lysis buffer.
The supernatant was collected after centrifugation at high speed
for 15 min. The protein concentration was determined using a BCA
kit. A total of 40 µg of protein was used for SDS-PAGE and the wet
transfer method was used for protein transfer to membranes.
Membranes were blocked in 10% skim milk, and incubated with primary
antibodies against Bcl-2, Bad and GAPDH (1:1,000) overnight at 4°C.
Next, secondary antibody (1:2,000) was added and membranes were
incubated at room temperature for 2 h. ECL was added to membranes,
blots were developed in a dark room and images were scanned and
recorded.
Statistical analysis
Data are presented as mean ± standard deviation.
Data were analyzed by SPSS 17.0 (IBM Corp., New York, NY, USA)
using one-way ANOVA. p<0.05 was considered statistically
significant.
Results
The effect of Pu combined with CDDP on
the proliferation of HepG2 cells
MTT assay was used to determine the effects of Pu
and CDDP, alone or in combination, on the proliferation of HepG2
cells (Table I). Pu and CDDP alone
inhibited the proliferation of HepG2 cells in a
concentration-dependent manner. The inhibitory effect increased
with increasing drug concentration. The inhibitory effect of Pu and
CDDP in combination on the proliferation of HepG2 cells was
significantly higher than that of the corresponding single drug
treatments (p<0.01). The doses of 100 µM Pu and 10 µg/ml CDDP
and 100 µM Pu + 10 µg/ml CDDP were used in the single drug groups
and combined drug group, respectively, for subsequent
experiments.
 | Table I.The effects of Pu and CDDP on HepG2
cell proliferation (mean ± SD). |
Table I.
The effects of Pu and CDDP on HepG2
cell proliferation (mean ± SD).
| Concentration |
|
|---|
|
|
|---|
| Pu (µM) | CDDP (µg/ml) | Inhibitory rate
(%) |
|---|
| 0 | 0 | 0 |
| 25 | 0 | 13.20a |
| 50 | 0 | 27.95a |
| 100 | 0 | 31.87a |
| 125 | 0 | 46.91a |
| 0 | 2.5 | 7.85a |
| 0 | 5 | 17.35a |
| 0 | 10 | 28.71a |
| 0 | 20 | 39.29a |
| 25 | 2.5 | 27.08a,b |
| 50 | 5 | 47.25a,b |
| 100 | 10 | 60.61a,b |
| 125 | 20 | 90.29a,b |
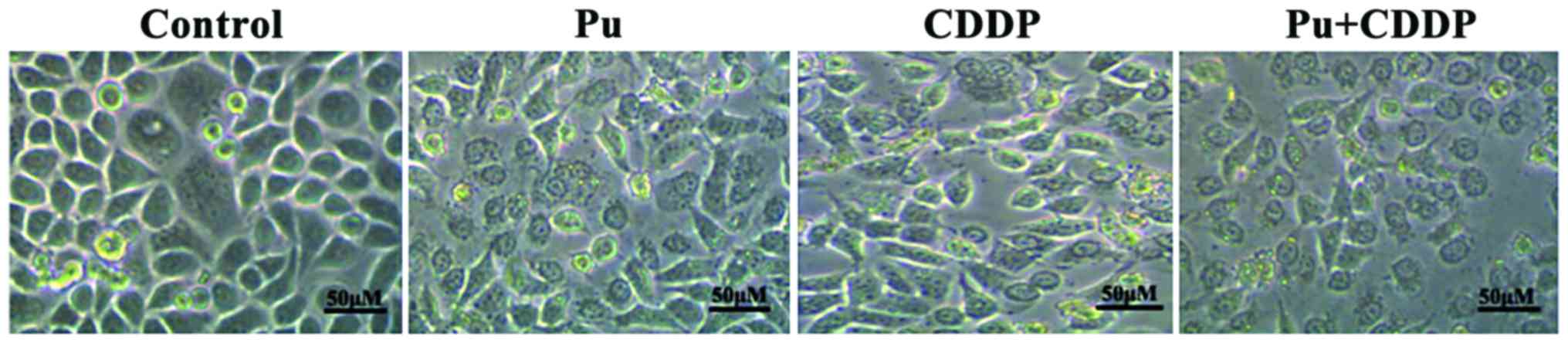
The effect of Pu combined with CDDP on
the morphology of HepG2 cells
Except for the control group, cells in the treatment
groups were treated with Pu (100 µM), CDDP (10 µg/ml) and Pu (100
µM) + CDDP (10 µg/ml), respectively (Fig.
1). After 48 h, cell morphology in the treatment groups changed
significantly compared with the control group, especially in the
combined treatment group. These changes included cell shrinkage,
reduction of cell adherence, decrease in cell number and increase
in the number of dead cells.
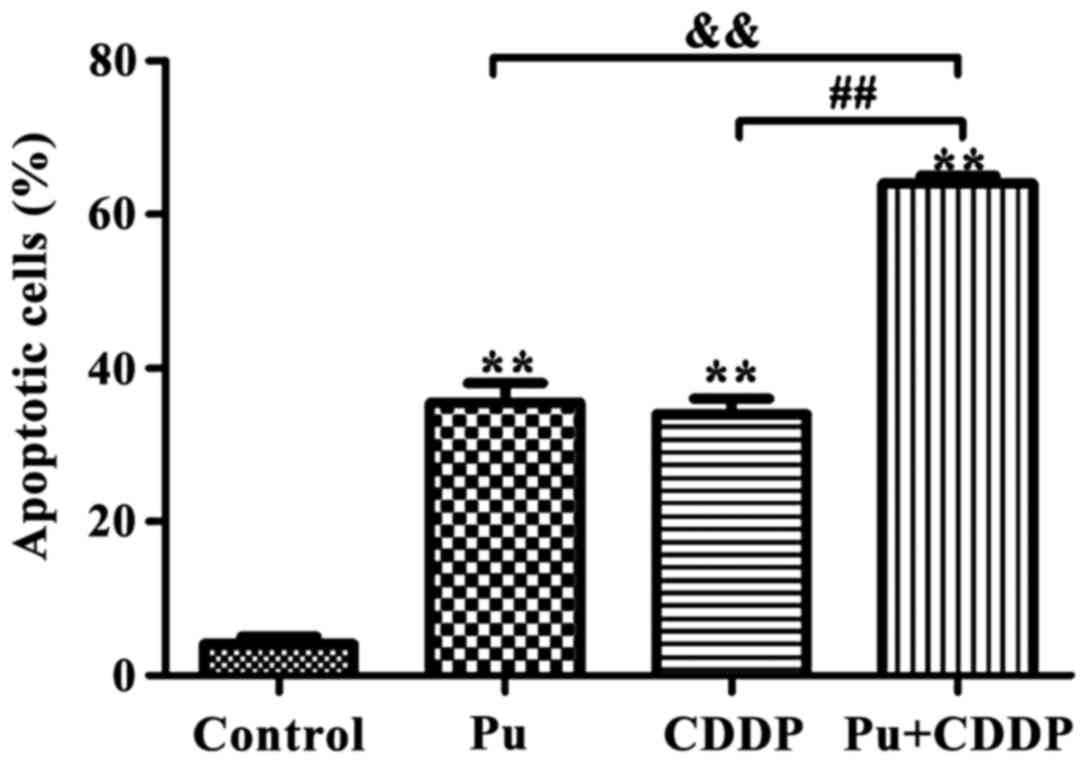
The effect of Pu combined with CDDP on
apoptotic rate of HepG2 cells
According to the results of the MTT assay, 100 µM Pu
and 10 µg/ml CDDP were selected to investigate whether Pu enhanced
the sensitivity of HepG2 cells to the chemotherapeutic drug, CDDP.
As shown in Fig. 2, the apoptotic
rates in the control, Pu, CDDP and combined drug groups were
3.92±0.42, 35.74±4.18, 32.93±3.68 and 62.03±7.65%, respectively.
Compared with Pu or CDDP alone, the apoptotic rate of the
combination group was significantly increased (p<0.01).
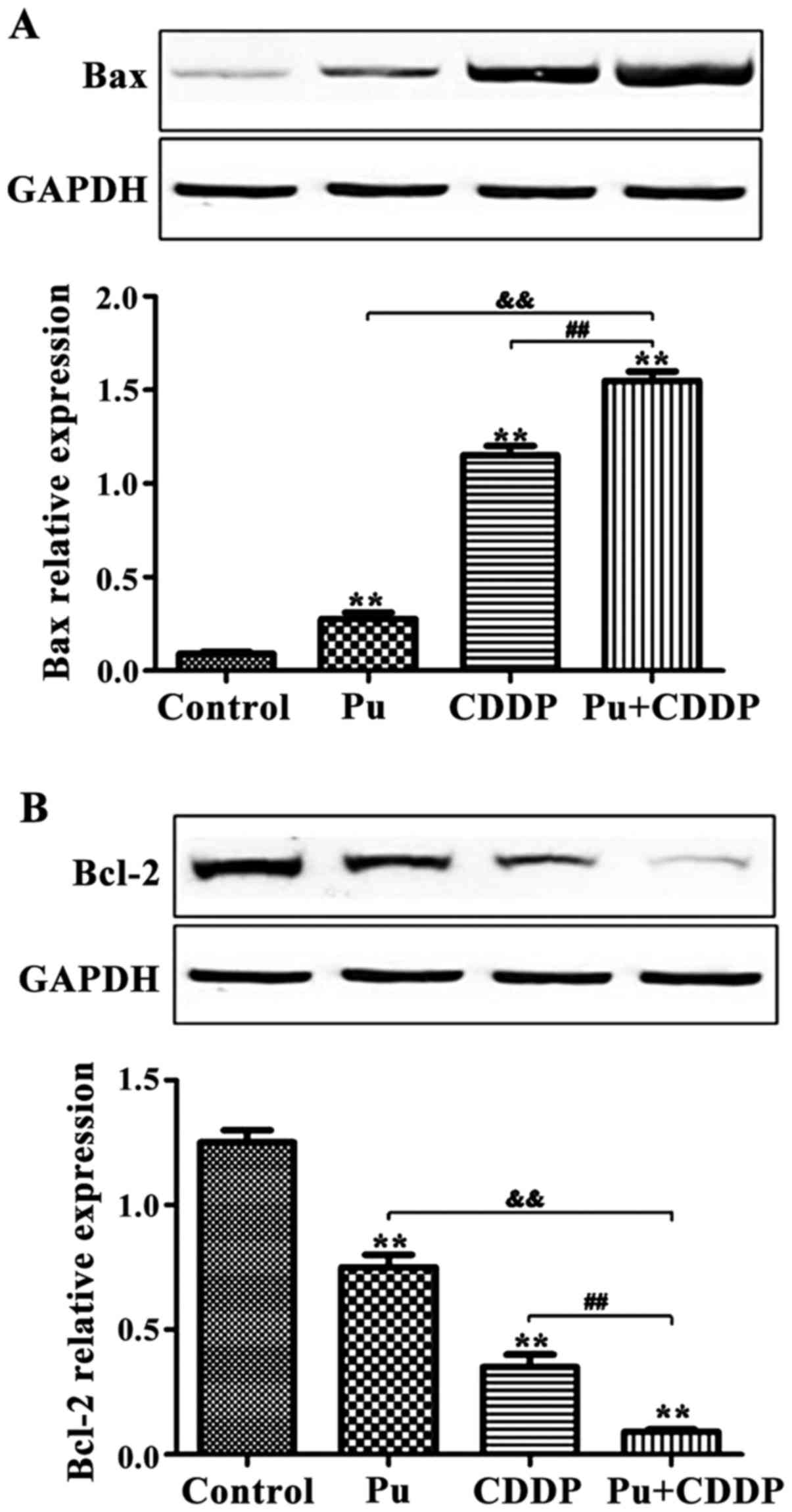
The effect of Pu combined with CDDP on
the expression of apoptosis-related proteins in HepG2 cells
Western blot analysis results are shown in Fig. 3. Compared with the control group, 100
µM Pu or 10 µg/ml CDDP alone induced Bax protein expression in
HepG2 cells. In contrast, Pu and CDDP in combination significantly
increased Bax protein expression (p<0.01). The expression of
Bcl-2 protein in HepG2 cells was downregulated by 100 µM Pu or 10
µg/ml CDDP alone and the inhibitory effect was more significant
when Pu was combined with CDDP (p<0.01).
Discussion
HCC is a common malignant tumor and has a high
mortality rate because of the lack of effective early diagnosis and
therapy (9). Liver cancer has various
causes, among which chronic liver inflammation caused by excessive
drinking, viral hepatitis and non-alcoholic liver fatty
degeneration is a key factor (10).
As a method of discovering new drugs, extracting natural compounds
from Chinese herbal medicines has been increasingly accepted and
employed. This not only results in acquiring better active
components, but also better control of the quality of Chinese
medicines. Therefore, extracting chemically active substances from
traditional Chinese medicine has been an area of strong interest in
antitumor studies. Pu belongs to the group of isoflavone flycoside
compounds and is one of the main active ingredients of leguminous
plants. It was approved for clinical use by the Ministry of Health
in 1993. Initially, it was primarily used for the treatment of
cerebrovascular disease and as the result of further study, Pu was
found to have a certain effect on cancer treatment (11).
Bcl-2 has been shown to inhibit apoptosis (12–14) and it
plays an important role in the mechanism of apoptosis. It can
protect cells from various causes of death, and improve cell
survival, thereby increasing the number of cells. In some tumor
cells, when Bcl-2 gene expression is upregulated, tumor cells are
prevented from dying, or have their survival prolonged (15), suggesting that the Bcl-2 gene is
closely related to the tumor. In contrast, Bax can promote
apoptosis. Bcl-2 and Bax belong to the same gene family, and Bax
binds the Bcl-2 protein, which can not only inhibit Bcl-2-mediated
apoptosis, but also directly promote cell apoptosis (16,17). The
BH1 and BH2 domains in the coding region of the Bax gene are highly
homologous to the Bcl-2 gene, which are important components
involved in the regulation of apoptosis. When Bcl-2 forms a
homodimer, it plays a role in inhibiting apoptosis. When Bax
protein expression increases and aggregates with Bcl-2 forming a
dimer, or Bax protein itself homodimerizes, it plays a role in
promoting apoptosis (11).
In this study, we found that Pu or CDDP alone
inhibited HepG2 cell proliferation and induced apoptosis. The
inhibitory effect on HepG2 proliferation was significantly greater
in the Pu and CDDP combination group compared with the same doses
in the single drug groups. The morphological changes such as
shrinkage, decreased cell adherence, reduced cell number and
increased cell death number were more obvious in the combined drug
group. The results of flow cytometry using Annexin V-PI double
staining showed that the combination of the two drugs resulted in a
significantly higher rate of apoptosis compared with the single
drug groups. Western blot analysis showed that compared with the
control group, Pu or CDDP alone induced the expression of Bax
protein in HepG2 cells. However, the expression of Bax protein was
more significant when Pu and CDDP were used in combination. Pu or
CDDP alone downregulated the expression of Bcl-2 protein in HepG2
cells. The inhibitory effect on the expression of Bcl-2 protein was
significant in the Pu and CDDP combination group compared with the
same doses in the single drug groups. The study by Xi et al
(18) indicated that some active
ingredients of traditional Chinese medicines can upregulate Bax
expression by downregulating Bcl-2 expression in tumor cells,
thereby inducing apoptosis of tumor cells. For example, carnosol
was shown to upregulate Bax expression and downregulate Bcl-2
expression by 34–53%. Neri et al (19) showed that the expression of Bax
protein was upregulated and the expression of Bcl-2 was
downregulated when epithelial tumor cells of the digestive tract
were abnormal, which was likely related to the formation of
digestive tract tumors. Their results were similar to those of our
study, further confirming that Pu increases the apoptosis of liver
cancer cells, likely by upregulating Bax and downregulating Bcl-2
protein expression.
In conclusion, this study demonstrated that Pu can
enhance the sensitivity of HepG2 cells to chemotherapeutic drugs
and induce the apoptosis of HepG2 cells. The mechanism is likely
related to the upregulation of Bax and downregulation of Bcl-2
protein expression.
References
|
1
|
Bruix J, Reig M and Sherman M:
Evidence-based diagnosis, staging, and treatment of patients with
hepatocellular carcinoma. Gastroenterology. 150:835–853. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sileri P, D'Ugo S, Benavoli D, Stolfi VM,
Palmieri G, Mele A and Gaspari AL: Metachronous splenic metastasis
from colonic carcinoma five-years after surgery: A case report and
literature review. South Med J. 102:733–735. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang MQ, Lin X, Li Y and Lu S: Irinotecan
as a second-line chemotherapy for small cell lung cancer: A
systemic analysis. Asian Pac J Cancer Prev. 16:1993–1995. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Phelps MA and Sparreboom A: Irinotecan
pharmacogenetics: A finished puzzle? J Clin Oncol. 32:2287–2289.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takeno S, Noguchi T, Kikuchi R, Uchida Y,
Yokoyama S and Müller W: Prognostic value of cyclin B1 in patients
with esophageal squamous cell carcinoma. Cancer. 94:2874–2881.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wei SY, Chen Y and Xu XY: Progress on the
pharmacological research of puerarin: A review. Chin J Nat Med.
12:407–414. 2014.PubMed/NCBI
|
|
7
|
Maji AK, Pandit S, Banerji P and Banerjee
D: Pueraria tuberosa: A review on its phytochemical and therapeutic
potential. Nat Prod Res. 28:2111–2127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang J, Yang ZR, Guo XF, Song J, Zhang JX,
Wang J and Dong WG: Synergistic effects of puerarin combined with
5-fluorouracil on esophageal cancer. Mol Med Rep. 10:2535–2541.
2014.PubMed/NCBI
|
|
9
|
Tang W, Zhu J, Su S, Wu W, Liu Q, Su F and
Yu F: MiR-27 as a prognostic marker for breast cancer progression
and patient survival. PLoS One. 7:e517022012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakagawa H, Umemura A, Taniguchi K,
Font-Burgada J, Dhar D, Ogata H, Zhong Z, Valasek MA, Seki E,
Hidalgo J, et al: ER stress cooperates with hypernutrition to
trigger TNF-dependent spontaneous HCC development. Cancer Cell.
26:331–343. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Le Sage C and Agami R: Immense promises
for tiny molecules: Uncovering miRNA functions. Cell Cycle.
5:1415–1421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Croker BA, O'Donnell JA, Nowell CJ,
Metcalf D, Dewson G, Campbell KJ, Rogers KL, Hu Y, Smyth GK, Zhang
JG, et al: Fas-mediated neutrophil apoptosis is accelerated by Bid,
Bak, and Bax and inhibited by Bcl-2 and Mcl-1. Proc Natl Acad Sci
USA. 108:pp. 13135–13140. 2011; View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhai D, Jin C, Huang Z, Satterthwait AC
and Reed JC: Differential regulation of Bax and Bak by
anti-apoptotic Bcl-2 family proteins Bcl-B and Mcl-1. J Biol Chem.
283:9580–9586. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krishna S, Low IC and Pervaiz S:
Regulation of mitochondrial metabolism: Yet another facet in the
biology of the oncoprotein Bcl-2. Biochem J. 435:545–551. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death, and tumorigenesis. Br J Cancer. 96
Suppl:R40–R44. 2007.PubMed/NCBI
|
|
16
|
Zhang J, Du Y, Wu C, Ren X, Ti X, Shi J,
Zhao F and Yin H: Curcumin promotes apoptosis in human lung
adenocarcinoma cells through miR-186* signaling pathway. Oncol Rep.
24:1217–1223. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xi S, Dyer KF, Kimak M, Zhang Q, Gooding
WE, Chaillet JR, Chai RL, Ferrell RE, Zamboni B, Hunt J, et al:
Decreased STAT1 expression by promoter methylation in squamous cell
carcinogenesis. J Natl Cancer Inst. 98:181–189. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Neri M, Betta P, Marroni P, Filiberti R,
Cafferata M, Mereu C, Ivaldi G, Montanaro F, Puntoni R and
Paganuzzi M: Serum anti-p53 autoantibodies in pleural malignant
mesothelioma, lung cancer and non-neoplastic lung diseases. Lung
Cancer. 39:165–172. 2003. View Article : Google Scholar : PubMed/NCBI
|