Introduction
Although notable advances have been made in the
prevention, diagnosis and treatment of cancer, esophageal squamous
cell carcinoma (OSCC) remains a significant global health burden,
particularly in China, where ~70% of esophageal cancer is OSCC
(1). OSCC is an aggressive cancer and
the fourth most common cause of cancer-associated mortalities
(2,3).
The progression of esophageal cancer is rapid, and there is a poor
associated prognosis, with a 5-year overall survival rate of 10–42%
(4–6).
The association between genetic changes and clinical
characteristics can reflect the biological events that promote OSCC
invasion and metastasis, and the former may act as molecular tumor
markers for diagnosis and prognosis. The principal driver of
metastasis and invasion is epithelial-mesenchymal transition (EMT).
Therefore, EMT-associated proteins are potentially useful
diagnostic markers and therapeutic targets (7). The activation of EMT promotes tumor
epithelial cells to dedifferentiate to a mesenchymal phenotype
(8), during which cells gain the
ability to penetrate the basement membrane and move to regional
lymph nodes or distant organs (8).
EMT-associated proteins have an important role in tumor
progression, and a number of proteins are significantly associated
with clinicopathological indexes (9).
However, the EMT signalling network is extremely complex, and an
improved understanding of the role that EMT-associated proteins
have in this process may yield clinically useful information.
EMT, as demonstrated by the expression of
EMT-associated proteins, has been reported in a number of different
cancer types, including OSCC (10–12). Loss
of epithelial (E-)cadherin expression and overexpression of zinc
finger protein SNAI1, twist-related protein 1, testican-1, and
receptor of activated protein kinase C1 has been reported to occur
at the invasive front of OSCC, particularly in single or cords of
tumor cells detaching from the main tumor mass (13–18).
Although the expression of these EMT drivers have been
well-researched in other cancer types, relatively little is known
of their expression in OSCC (19).
Zinc finger E-box-binding homeobox 2 (ZEB2) is a
transcription factor that can bind Smad proteins and contains
multiple functional domains that interact with a variety of
transcriptional co-effectors (20).
ZEB2 directly binds adjacent E-boxes within the E-cadherin gene
promoter and regulates transcriptional repression by recruiting
corepressor complexes (21).
In the present study, immunohistochemistry (IHC) was
performed to investigate the expression of the EMT-associated
transcription factor ZEB2 and the cell adhesion protein E-cadherin
in OSCC. It was observed that there were significant differences in
the expression of ZEB2 and E-cadherin between OSCC and normal
esophageal mucosa epithelial membrane, and multivariate analysis
indicated that ZEB2 expression was an independent prognostic marker
in OSCC.
Materials and methods
Patients and tumor samples
All tissues were obtained from patients with OSCC
(183 males and 35 females) who underwent esophagostomy without any
preoperative therapy at the Department of Esophageal Oncology,
Cancer Hospital of Tianjin Medical University (Tianjin, China),
between June 2006 and June 2009. Histopathological diagnosis was
determined according to the National Comprehensive Cancer Network
(NCCN) guidelines (22). Informed
consent was obtained from all patients, and ethical approval was
obtained from the Institutional Review Board of the Cancer Hospital
of Tianjin Medical University.
Antibodies
A mouse anti-human monoclonal antibody against
E-cadherin was purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA), and a rabbit anti-human polyclonal antibody
against ZEB2 was obtained from Abcam (Cambridge, UK). The secondary
antibodies were obtained from Shanghai Outdo Biotech Co., Ltd.
(Shanghai, China).
Construction of tissue microarray
(TMA)
The thickness of the tissue section was 2 µm. The
slides were evaluated by a senior pathologist to identify
representative tumor areas. In brief, formalin-fixed,
paraffin-embedded tissue blocks and the corresponding hematoxylin
and eosin-stained slides were covered for TMA sampling. Multiple
0.6-mm diameter cylinders were punched from the representative
tumor areas and from the adjacent peritumoral esophageal tissue
using a tissue arrayer.
IHC
The TMA slides used an isotype control. The TMA
slides were dried overnight at 37°C, deparaffinized in xylene,
rehydrated using a graded alcohol series and immersed in 3%
hydrogen peroxide for 15 min to block endogenous peroxidase
activity at 37°C. Antigen-retrieval was performed by heating to
100°C in a pressure cooker for 3 min in EDTA buffer (pH 8.0).
Subsequently, the slides were incubated with rabbit polyclonal
anti-ZEB2 (dilution, 1:100; catalog no. ab138222) that recognized
only a single band corresponding to ZEB2, or mouse anti-E-cadherin
(dilution, 1:50; catalog no. sc-59778) for 16 h at 4°C. The slides
were subsequently incubated with a goat anti-rabbit immunoglobulin
G conjugated to horseradish peroxidase (HRP) (dilution, 1:500;
catalog no. abs20002A) for 1 h at 37°C and stained with
3,3-diaminobenzidine. Finally, the sections were counterstained
with Mayer's hematoxylin, dehydrated and mounted. As a negative
control, the primary antibody was replaced with KIT-9901 Elivision™
plus Polyer HRP (mouse/rabbit) IHC kit (Mai Xin Biotechnology Co.,
Ltd.). Patients with gastric cancer, from the Tianjin Medical
University Cancer Institute and Hospital, which express ZEB2 were
selected and used as a positive control.
Evaluation of IHC staining
All tissues were observed using a CX41 light
microscope (Olympus Corporation, Tokyo, Japan). All tissue sections
were simultaneously assessed by two independent investigators, who
were blinded to the patient clinicopathological details. The
criteria for scoring ZEB2 and E-cadherin staining were as follows:
Intensity was graded as 0 (negative), 1 (weak), 2 (moderate) or 3
(strong). The proportion of positive tumor cells was graded as 0
(<5%), 1 (5–25%), 2 (26–50%), 3 (51–75%) or 4 (>75%). A final
score was derived by multiplying the two primary scores. Final
scores of 0–4 were defined as negative expression (−), scores of
5–8 as weak positive expression (+) and scores of 9–12 as strong
positive expression (++) (23).
Statistical analysis
Statistical analyses were performed using SPSS
software (version 17.0; SPSS Inc., Chicago, IL, USA). The
χ2 test was used to assess associations between ZEB2,
E-cadherin and various clinicopathological variables. All factors
were examined using univariate analysis and significant factors
were further examined using multivariate analysis. Survival was
analyzed using the Kaplan-Meier estimator method. The statistical
significance of the association between ZEB2 and E-cadherin
expression and overall survival was estimated using the log rank
test. Multivariate Cox's proportional hazards regression was used
to identify independent factors for overall survival. P<0.05 was
considered to indicate a statistically significant difference from
a two-tailed test.
Results
Patients and baseline
characteristics
The study group comprised 183 males and 35 females,
with a median age of 67.6 years (range, 39–99 years). All patients
had undergone macroscopically curative resection, and none had
received any preoperative chemotherapy or radiotherapy.
Pathologically, all tumors were squamous cell carcinoma. A total of
2,834 lymph nodes were resected from 218 patients, with a median of
13.0 nodes per patient.
Expression of ZEB2 and E-cadherin in
esophageal tissue
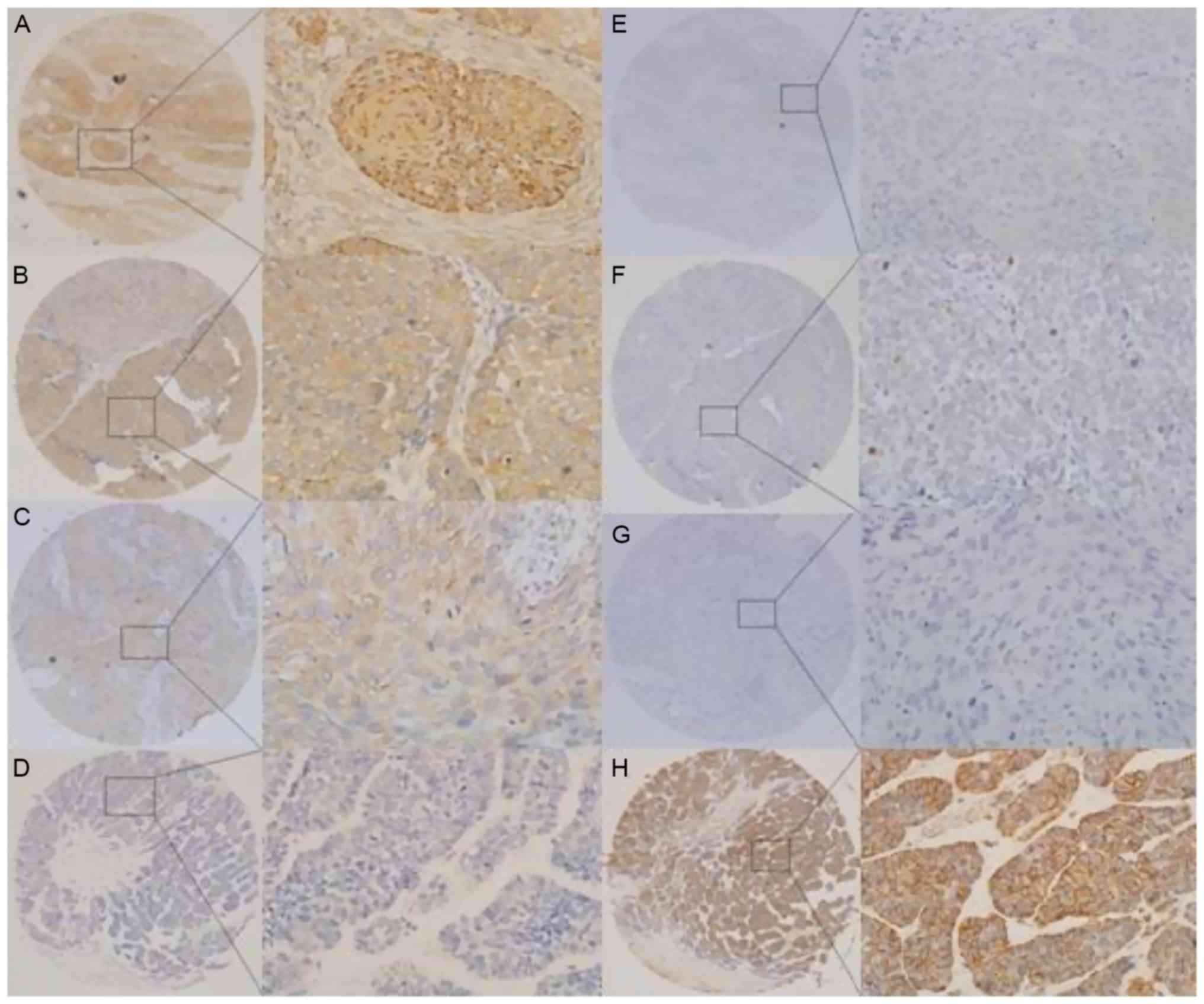
ZEB2 expression was observed in the cytoplasm of
cancer cells. By contrast, E-cadherin expression was detected at
the cell membrane (Fig. 1). IHC
analysis identified positive ZEB2 expression in 77 tumors (35.3%)
and positive E-cadherin expression in 100 tumor s (45.9%). ZEB2
expression was significantly different between OSCCs and POTs in
the patients (χ2=6.276; P<0.05). Similar results were
observed for E-cadherin expression (χ2=8.139; P<0.05)
(Table I).
 | Table I.Expression of ZEB2 and E-cadherin in
esophageal tissue. |
Table I.
Expression of ZEB2 and E-cadherin in
esophageal tissue.
|
| ZEB2, n |
|
| E-cadherin, n |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Tumour type | – | + | χ2 | P-value | – | + | χ2 | P-value |
|---|
| OSCC | 141 | 77 | 6.276 | 0.012a | 118 | 100 | 8.139 | 0.004b |
| POT | 49 | 11 |
|
| 20 | 40 |
|
|
Association between ZEB2 and
E-cadherin expression in OSCC and POT
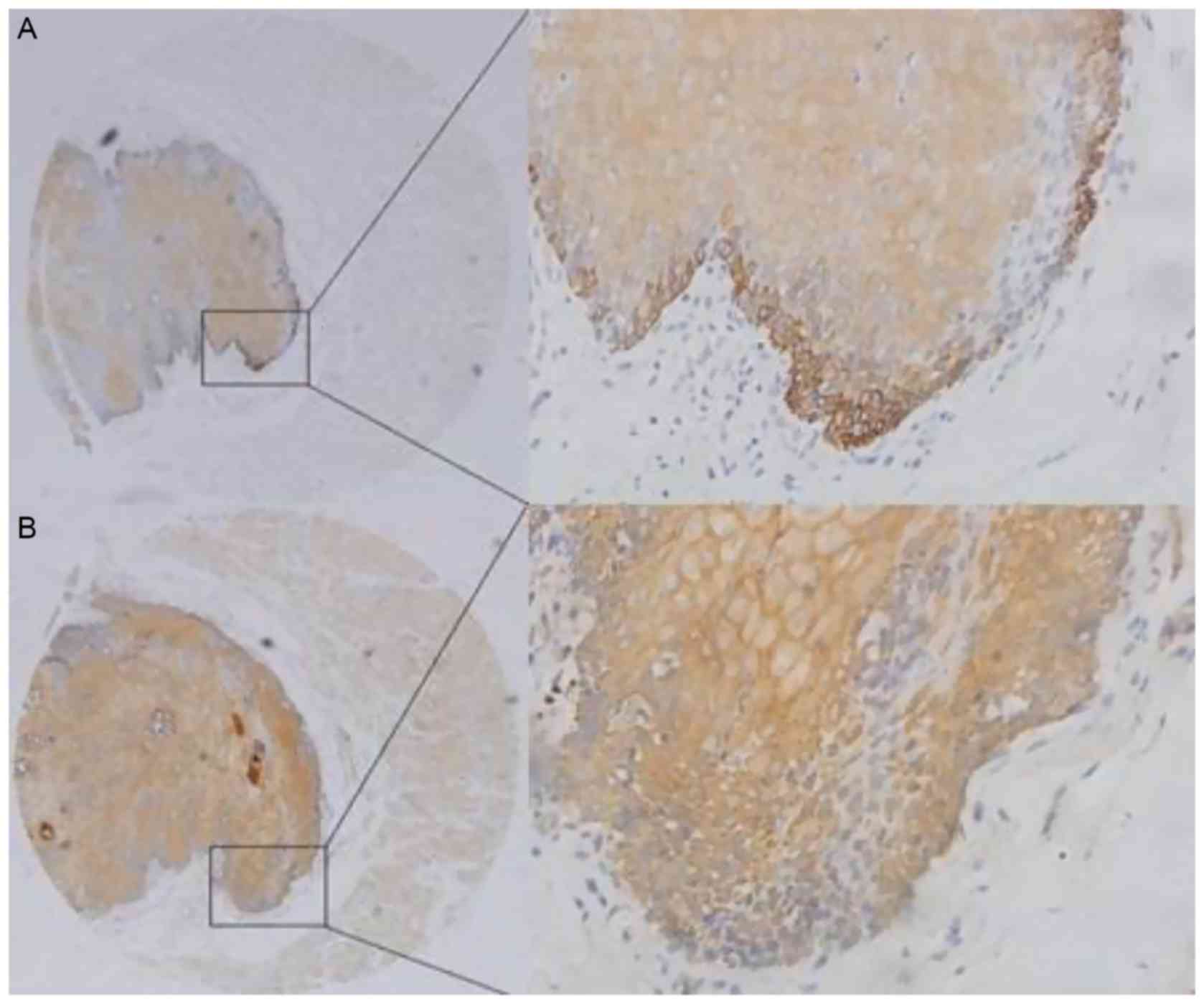
E-cadherin membrane expression and a low level of
ZEB2 expression were observed in normal esophageal epithelial
tissues (Fig. 2). IHC analysis
identified positive ZEB2 expression in 11 POT cases (18.3%), and
positive E-cadherin expression in 40 POT cases (66.7%) (Table I). Negative E-cadherin staining was
detected in 53/77 (68.8%) cases positive for ZEB2 expression.
Positive E-cadherin staining was detected in 76/141 (53.9%) cases
negative for ZEB2 expression. Therefore, the expression of ZEB2 and
E-cadherin was significantly and inversely associated in OSCC
(χ2=10.365; P<0.05) (Table
II). Similarly, the expression of ZEB2 and E-cadherin was
significantly and inversely associated in POT (χ2=4.219;
P<0.05) (Table III).
 | Table II.Association between ZEB2 and
E-cadherin immunoexpression in esophageal squamous cell
carcinoma. |
Table II.
Association between ZEB2 and
E-cadherin immunoexpression in esophageal squamous cell
carcinoma.
|
| ZEB2
expression |
|
|
|---|
|
|
|
|
|
|---|
|
Immunohistochemistry | Positive
(n=77) | Negative
(n=141) | χ2 | P-value |
|---|
| E-cadherin-positive
(n=100) | 24 | 76 | 10.365 | 0.001a |
| E-cadherin-negative
(n=118) | 53 | 65 |
|
|
 | Table III.Association between ZEB2 and
E-cadherin immunoexpression in peritumoral esophageal tissues. |
Table III.
Association between ZEB2 and
E-cadherin immunoexpression in peritumoral esophageal tissues.
|
| ZEB2 |
|
|
|---|
|
|
|
|
|
|---|
|
Immunohistochemistry | Positive
(n=11) | Negative
(n=49) | χ2 | P-value |
|---|
| E-cadherin-positive
(n=40) | 5 | 35 | 4.219 | 0.040a |
| E-cadherin-negative
(n=20) | 7 | 13 |
|
|
Association between ZEB2 and
E-cadherin expression and clinicopathological factors
Further analysis indicated that the overexpression
of ZEB2 in OSCC tissues was significantly associated with the depth
of tumor invasion, lymph node metastasis and TNM stage (P<0.05;
Table IV). By contrast, negative
expression of membrane E-cadherin was significantly associated with
lymph node metastasis (P=0.040; Table
IV). No significant associations were identified between
expression of ZEB2 and clinicopathological variables, including
sex, age, tumor position, tumor differentiation and tumor size
(P>0.05; Table IV).
 | Table IV.Clinicopathological features of
esophageal squamous cell carcinoma and associations with ZEB2 and
E-cadherin immunoexpression. |
Table IV.
Clinicopathological features of
esophageal squamous cell carcinoma and associations with ZEB2 and
E-cadherin immunoexpression.
|
|
| ZEB2, n |
|
| E-cadherin, n |
|
|
|---|
| Variable | Total, n | – | + | χ2 | P-value | – | + | χ2 | P-value |
|---|
| Sex |
|
|
| 0.020 | 0.889 |
|
| 1.279 | 0.258 |
|
Male | 183 | 118 | 65 |
|
| 96 | 87 |
|
|
|
Female | 35 | 23 | 12 |
|
| 22 | 13 |
|
|
| Age, years |
|
|
| 0.304 | 0.581 |
|
| 0.266 | 0.206 |
|
<60 | 47 | 32 | 15 |
|
| 27 | 20 |
|
|
|
≥60 | 171 | 109 | 62 |
|
| 91 | 80 |
|
|
| Position |
|
|
| 0.615 | 0.735 |
|
| 0.351 | 0.839 |
|
Upper | 11 |
6 |
5 |
|
|
5 |
6 |
|
|
|
Middle | 185 | 120 | 65 |
|
| 101 | 84 |
|
|
|
Lower | 22 | 15 | 7 |
|
| 12 | 10 |
|
|
|
Differentiation |
|
|
| 2.998 | 0.223 |
|
| 4.058 | 0.131 |
|
Well | 58 | 31 | 27 |
|
| 24 | 34 |
|
|
|
Moderately | 71 | 39 | 32 |
|
| 29 | 42 |
|
|
|
Poorly | 56 | 38 | 18 |
|
| 32 | 24 |
|
|
| Diameter, cm |
|
|
| 3.792 | 0.052 |
|
| 0.914 | 0.339 |
|
<4 | 97 | 63 | 24 |
|
| 56 | 41 |
|
|
| ≥4 | 121 | 78 | 53 |
|
| 62 | 59 |
|
|
| T status |
|
|
| 4.104 | 0.043a |
|
| 0.759 | 0.384 |
|
T1-T2 | 38 | 30 | 8 |
|
| 23 | 15 |
|
|
|
T3-T4 | 180 | 111 | 69 |
|
| 95 | 85 |
|
|
| N status |
|
|
| 10.64 | 0.001a |
|
| 4.203 | 0.040a |
|
Negative | 131 | 96 | 35 |
|
| 58 | 63 |
|
|
|
Positive | 87 | 45 | 42 |
|
| 60 | 37 |
|
|
| TNM stage (22) |
|
|
| 11.08 | 0.001a | |
| 0.390 | 0.533 |
| I and
II | 104 | 79 | 25 |
|
| 54 | 50 |
|
|
|
III | 114 | 62 | 52 |
|
| 64 | 50 |
|
|
| Total | 218 | 141 | 77 |
|
| 118 | 100 |
|
|
Prognostic significance of ZEB2 and
E-cadherin expression
In OSCC tissue, univariate analyses revealed
significant associations between overall survival and ZEB2
overexpression (P=0.002), tumor size (P=0.011), depth of tumor
invasion (P=0.002), lymph node metastasis (P<0.0001) and TNM
stage (P=0.004). By contrast, a lack of E-cadherin expression, poor
differentiation, tumor site, age and sex were not significantly
associated with prognosis (Table V).
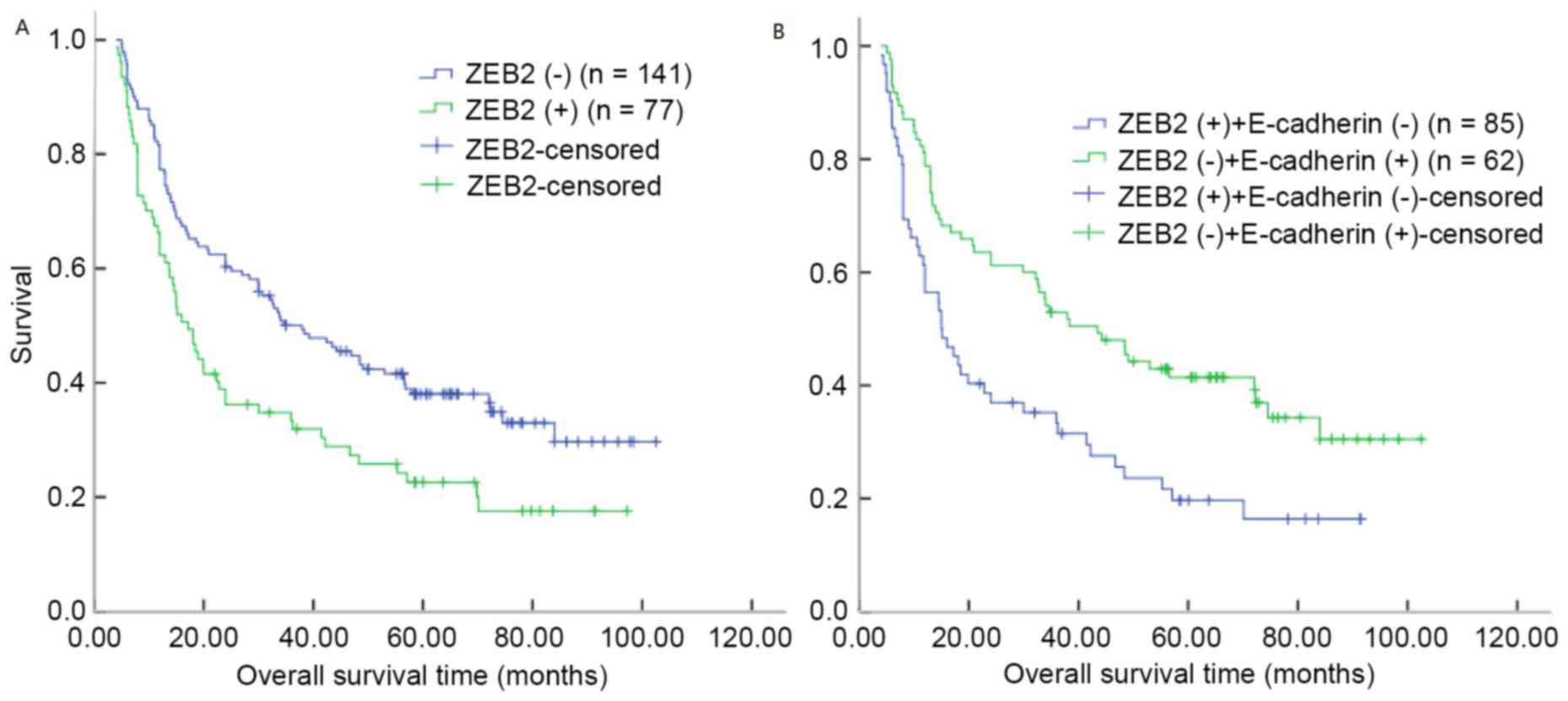
Kaplan-Meier analysis demonstrated that the median survival time
for patients with ZEB2 overexpression in OSCC was 17.20 months,
compared with 37.97 months for patients with OSCC and negative ZEB2
expression (P=0.003, log-rank test; Table VI; Fig.
3A). However, the median survival time for patients with OSCC
and E-cadherin membrane expression was 24.00 months, compared with
34.00 months for patients with OSCC and negative E-cadherin
expression (log-rank test, P=0.055; Table VI). Notably, patients with ZEB2
overexpression and negative E-cadherin expression demonstrated
decreased overall survival times (log-rank test, P=0.002; Fig. 3B).
 | Table V.Univariate analyses of ZEB2
expression and clinicopathological variables in 218 patients with
esophageal squamous cell carcinoma (log-rank tests). |
Table V.
Univariate analyses of ZEB2
expression and clinicopathological variables in 218 patients with
esophageal squamous cell carcinoma (log-rank tests).
| Variable | Total, n | Median survival,
months | 95% confidence
interval | χ2 | P-value |
|---|
| Sex |
|
|
| 0.363 | 0.547 |
|
Male | 183 | 26.97 | 18.04–35.90 |
|
|
|
Female | 35 | 34.00 | 20.38–45.39 |
|
|
| Age, years |
|
|
| 1.963 | 0.136 |
|
<60 | 47 | 48.47 | 32.15–64.79 |
|
|
|
≥60 | 171 | 24.00 | 15.99–32.01 |
|
|
| Position |
|
|
| 3.137 | 0.208 |
|
Upper | 11 | 15.00 | 6.80–23.20 |
|
|
|
Middle | 185 | 26.97 | 18.12–35.83 |
|
|
|
Lower | 22 | 56.80 | 34.20–79.40 |
|
|
|
Differentiation |
|
|
| 4.049 | 0.129 |
|
Well | 58 | 35.46 | 23.46–48.55 |
|
|
|
Moderately | 71 | 21.78 | 12.89–30.17 |
|
|
|
Poorly | 56 | 18.95 | 9.74–25.67 |
|
|
| Diameter, cm |
|
|
| 6.447 | 0.01a |
|
<4 | 97 | 36.00 | 20.19–51.81 |
|
|
| ≥4 | 121 | 20.00 | 9.13–30.87 |
|
|
| T status (22) |
|
|
| 9.781 | 0.002a |
|
T1-T2 | 38 | 70.10 | 45.89–94.31 |
|
|
|
T3-T4 | 180 | 20.73 | 12.26–29.20 |
|
|
| N status (22) |
|
|
| 17.722 | 0.000a |
|
Negative | 131 | 43.47 | 26.98–59.96 |
|
|
|
Positive | 87 | 15.13 | 11.88–18.38 |
|
|
| bTNM stage |
|
|
| 8.210 | 0.004a |
| I and
II | 104 | 43.47 | 23.95–62.99 |
|
|
|
III | 114 | 18.10 | 13.01–23.19 |
|
|
| ZEB2
expression |
|
|
| 9.095 | 0.002a |
|
Negative | 77 | 37.97 | 25.08–50.86 |
|
|
|
Positive | 141 | 17.20 | 13.06–21.34 |
|
|
| E-cadherin
expression |
|
|
| 3.685 | 0.055 |
|
Positive | 100 | 24.00 | 14.69–33.31 |
|
|
|
Negative | 118 | 34.00 | 4.72–63.28 |
|
|
 | Table VI.Multivariate Cox's regression
analysis of overall survival in patients with esophageal squamous
cell carcinoma. |
Table VI.
Multivariate Cox's regression
analysis of overall survival in patients with esophageal squamous
cell carcinoma.
|
| Multivariate
analysis |
|---|
|
|
|
|---|
| Variables | B | Wald | Exp(B) | Hazard ratio (95%
confidence interval) | P-value |
|---|
| Diameter (<4 vs.
≥4 cm) | 0.346 |
3.836 | 1.414 | 1.000–1.999 | 0.050 |
| T statu) (positive
vs. negative) | 0.853 | 11.776 | 2.346 | 1.441–3.817 | 0.001a |
| bTNM stage (I ands (22) (T1-T2 vs. T3-T4) | 0.455 |
0.264 | 1.576 | 0.094–2.572 | 0.085 |
| N status (22 II vs.
III) | 0.338 |
3.839 | 1.402 | 1.000–1.965 | 0.050 |
| ZEB2 expression
(positive vs. negative) | 0.450 |
5.707 | 1.568 | 1.084–2.269 | 0.017a |
Independent prognostic factors of
OSCC
A multivariate analysis was performed to evaluate
the variables that had been identified to be significant in the
univariate analysis. The analysis indicated that overexpression of
ZEB2 was an independent prognostic factor for favorable overall
survival among patients with OSCC (hazard ratio, 1.568; 95%
confidence interval, 1.084–2.269, P=0.017; Table VI). Additionally, lymph node
metastasis (P=0.001) was also identified to be an independent
predictive factor for overall survival.
Discussion
Highly invasive and metastatic behavior underlies
the aggressive nature of OSCC, which in turn depends on EMT
(24). Although a number of proteins,
including ZEB2, have been reported to serve key roles in EMT, not
all the proteins have been demonstrated to have prognostic
significance in OSCC (13–18,25–29). In
the present study, ZEB2 expression was examined in a large number
of samples taken from the invasive front of OSCC in order to assess
its prognostic significance. The results indicate that ZEB2 is
differentially expressed in OSCC and normal esophageal mucosal
epithelium, and that ZEB2 expression is associated with shorter
overall survival time (30).
Esophageal epithelial cells that have undergone EMT
acquire functional characteristics of activated myofibroblasts
in vitro (31). E-cadherin is
a key component of adherence junctions that anchor esophageal
epithelial cells (32). Loss of
E-cadherin expression has been frequently reported in OSCC,
particularly at the invasive tumor front (10) and is a recognized hallmark of EMT. The
EMT phenotype can be controlled by the ZEB family of transcription
factors, which is able to influence cell shape and adhesion,
leading to an increased invasive potential (33). In the present study, ZEB2 was observed
to be significantly overexpressed in >35.3% of OSCC specimens.
Consistent with the observations in the present study, ZEB2 has
previously been reported to be overexpressed in several types of
cancer, including OSCC (29,34–36).
Yoshida et al (30) reported
increased ZEB2 expression in OSCC tissues compared with normal
esophageal epithelium. ZEB2 controls the expression of matrix
metalloproteinases (37) and other
polarity proteins [protein crumbs homolog 3and lethal (2) giant larvae protein homolog 2] (38) and therefore may be regarded as a
master regulator of the EMT process (38).
In the present study, it was demonstrated that there
was an association between the ZEB2 expression and a number of
clinicopathological parameters, including depth of tumor invasion,
lymph node metastasis and TNM stage. By contrast, negative
expression of membrane E-cadherin was significantly associated with
lymph node metastasis. Notably, there was no association between
the ZEB2 or E-cadherin expression and the degree of tumor
differentiation, although ZEB2 expression has been previously
reported to be associated with histological differentiation in
gastric cancer (39). Similar to the
present study, ZEB2 overexpression in various tumors (pancreatic
cancer, eyelid sebaceous gland, pharyngeal squamous cell and oral
squamous cell carcinoma) has been significantly associated with
node metastasis (34,36,40,41),
although another previous study reported no such association
(42). The association between
decreased E-cadherin expression and lymph node metastasis remains
unclear, and whether these inconsistent findings can be accounted
for by the different type of pathogenesis remains to be
investigated (43,44).
The survival rate of patients with positive ZEB2
expression was significantly decreased compared with patients with
negative expression in univariate and multivariate analysis. ZEB2
expression was associated with invasion and metastatic
characteristics, and the overall survival rate would be expected to
be lower in patients with these characteristics. The findings of
the present study are similar to those of previous studies, which
reported that that ZEB2 is associated with poor prognosis in
patients with ovarian, mammary gland, renal cell, head and neck,
and gastric carcinoma (35,45–48). In
the present study, ZEB2 was an independent prognostic factor for
shorter survival time in postoperative patients with OSCC, which is
also in agreement with previous findings (40,49,50).
It must be noted that, even though IHC techniques
have become the focus of increasing attention, there remain a
number of limitations. However, as the application of
immunohistochemistry has increased, the estimated specificities to
the antigens of the organ or tissue have remained comparable with
some of the original expectations (51).
In summary, in the present study, it was
demonstrated that ZEB2 and E-cadherin are frequently differentially
expressed between OSCC and POT, and that ZEB2 and E-cadherin
expression is associated with certain clinicopathological
characteristics consistent with known biological function
phenotypes. High ZEB2 expression in tumors is significantly and
independently associated with poorer overall survival, particularly
in patients with lymph node metastasis. Further studies are
necessary to clarify the function of ZEB2 in EMT and to evaluate
the prognostic significance of ZEB2.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of Tianjin (Tianjin, China) (grant no.
15JCYBJC28400) and the Science Foundation of Tianjin Medical
University (Tianjin, China) (grant no. 2014KYQ27).
References
|
1
|
Xu Y, Yu X, Chen Q and Mao W: Neoadjuvant
versus adjuvant treatment: Which one is better for resectable
esophageal squamous cell carcinoma? World J Surg Oncol. 10:1732012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao P, Dai M, Chen W and Li N: Cancer
trends in China. Jpn J Clin Oncol. 40:281–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Song Y, Li L, Ou Y, Gao Z, Li E, Li X,
Zhang W, Wang J, Xu L, Zhou Y, et al: Identification of genomic
alterations in esophageal squamous cell cancer. Nature. 509:91–95.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rice TW, Rusch VW, Apperson-Hansen C,
Allen MS, Chen LQ, Hunter JG, Kesler KA, Law S, Lerut TE, Reed CE,
et al: Worldwide esophageal cancer collaboration. Dis Esophagus.
22:1–8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shapiro IM, Cheng AW, Flytzanis NC,
Balsamo M, Condeelis JS, Oktay MH, Burge CB and Gertler FB: An
EMT-driven alternative splicing program occurs in human Breast
Cancer and Modulates Cellular Phenotype. PLoS Genet.
7:e10022182011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumor progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Niwa Y, Yamada S, Koike M, Kanda M, Fujii
T, Nakayama G, Sugimoto H, Nomoto S, Fujiwara M and Kodera Y:
Epithelial to mesenchymal transition correlates with tumor budding
and predicts prognosis in esophagealsquamous cell carcinoma. J Surg
Oncol. 110:764–769. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jin H, Morohashi S, Sato F, Kudo Y,
Akasaka H, Tsutsumi S, Ogasawara H, Miyamoto K, Wajima N, Kawasaki
H, et al: Vimentin expression of esophageal squamous cell carcinoma
and its aggressive potential for lymph node metastasis. Biomed Res.
31:105–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang F, Li XK, Xu HY, Shan ZZ, Wang T,
Yang ZC, He W, Wang LX and Fan QX: N-cadherin participated in
invasion and metastasis of human esophageal squamous cell carcinoma
via taking part in the formation of vasculogenic mimicry. Med
Oncol. 32:4802015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong H, Xie L, Tang C, Chen S, Liu Q,
Zhang Q, Zheng W, Zheng Z and Zhang H: Snail1 correlates with
patient outcomes in E-cadherin-preserved gastroesophageal junction
adenocarcinoma. Clin Transl Oncol. 16:783–791. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Forghanifard MM, Moaven O, Farshchian M,
Montazer M, Raeisossadati R, Abdollahi A, Moghbeli M, Nejadsattari
T, Parivar K and Abbaszadegan MR: Expression analysis elucidates
the roles of MAML1 and Twist1 in esophageal squamous cell carcinoma
aggressiveness and metastasis. Ann Surg Oncol. 19:743–749. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gong T, Xue Z, Tang S, Zheng X, Xu G, Gao
L, Zhao G, Hong L, Tang G, Zhang H, et al: Nuclear expression of
Twist promotes lymphatic metastasis in esophageal squamous cell
carcinoma. Cancer Biol Ther. 13:606–613. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee KW, Kim JH, Han S, Sung CO, Do IG, Ko
YH, Um SH and Kim SH: Twist1 is an independent prognostic factor of
esophageal squamous cell carcinoma and associated with its
epithelial-mesenchymal transition. Ann Surg Oncol. 19:326–335.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song X, Han P, Liu J, Wang Y, Li D, He J,
Gong J, Li M, Tu W, Yan W, et al: Up-regulation of SPOCK1 induces
epithelial-mesenchymal transition and promotes migration and
invasion inesophageal squamous cell carcinoma. J Mol Histol.
46:347–356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang N, Liu F, Cao F, Jia Y, Wang J, Ma W,
Tan B, Wang K, Song Q and Cheng Y: RACK1 predicts poor prognosis
and regulates progression of esophageal squamous cell carcinoma
through its epithelial-mesenchymal transition. Cancer Biol Ther.
16:528–540. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Beck TN, Chikwem AJ, Solanki NR and
Golemis EA: Bioinformatic approaches to augment study of
epithelial-to-mesenchymal transition in lung cancer. Physiol
Genomics. 46:699–724. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Postigo AA, Depp JL, Taylor JJ and Kroll
KL: Regulation of Smad signaling through a differential recruitment
of coactivators and corepressors by ZEB proteins. EMBO J.
22:2453–2462. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hegarty SV, Sullivan AM and O'Keeffe GW:
Zeb2: A multifunctional regulator of nervous system development.
Prog Neurobiol. 132:81–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
National Comprehensive Cancer Network.
(NCCN) Clinical Practice Guidelines in Oncology. Esophageal and
Esophagogastric Junction Cancers. version 1. 2015.https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#esophageal
|
|
23
|
Hao XP, Pretlow TG, Rao JS and Pretlow TP:
Beta-catenin expression is altered in human colonic aberrant crypt
foci. Cancer Res. 61:8085–8088. 2001.PubMed/NCBI
|
|
24
|
Jolly MK, Boareto M, Huang B, Jia D, Lu M,
Ben-Jacob E, Onuchic JN and Levine H: Implications of the Hybrid
Epithelial/Mesenchymal Phenotype in Metastasis. Front Oncol.
5:1552015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Du HF, Ou LP, Yang X, Song XD, Fan YR, Tan
B, Luo CL and Wu XH: A new PKCα/β/TBX3/E-cadherin pathway is
involved in PLCε-regulated invasion and migration in human bladder
cancer cells. Cell Signal. 26:580–593. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu W, Tian Y, Wan H, Ma J, Song Y, Wang Y
and Zhang L: Expression of β-catenin and E- and N-cadherin in human
brainstem gliomas and clinicopathological correlations. Int J
Neurosci. 123:318–323. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koay MH, Crook M and Stewart CJ: Cyclin
D1, E-cadherin and beta-catenin expression in FIGO Stage IA
cervical squamous carcinoma: Diagnostic value and evidence for
epithelial-mesenchymal transition. Histopathology. 61:1125–1133.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Carneiro P, Figueiredo J, Bordeira-Carriço
R, Fernandes MS, Carvalho J, Oliveira C and Seruca R: Therapeutic
targets associated to E-cadherin dysfunction in gastric cancer.
Expert Opin Ther Targets. 17:1187–1201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu TA, Jan YJ, Ko BS, Liang SM, Chen SC,
Wang J, Hsu C, Wu YM and Liou JY: 14-3-3ε overexpression
contributes to epithelial-mesenchymal transition of hepatocellular
carcinoma. PLoS One. 8:e579682013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yoshida R, Morita M, Shoji F, Nakashima Y,
Miura N, Yoshinaga K, Koga T, Tokunaga E, Saeki H, Oki E, et al:
Clinical Significance of SIP1 and E-cadherin in Patients with
Esophageal Squamous Cell Carcinoma. Ann Surg Oncol. 22:2608–2614.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Muir AB, Dods K, Noah Y, Toltzis S,
Chandramouleeswaran PM, Lee A, Benitez A, Bedenbaugh A, Falk GW,
Wells RG, et al: Esophageal epithelial cells acquire functional
characteristics of activated myofibroblasts after undergoing an
epithelial to mesenchymal transition. Exp Cell Res. 330:102–110.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fang WK, Liao LD, Zeng FM, Zhang PX, Wu
JY, Shen J, Xu LY and Li EM: Desmocollin-2 affects the adhesive
strength and cytoskeletal arrangement in esophageal squamous cell
carcinoma cells. Mol Med Rep. 10:2358–2364. 2014.PubMed/NCBI
|
|
33
|
Wong TS, Gao W and Chan JY: Transcription
regulation of E-cadherin by zinc finger E-box binding homeobox
proteins in solid tumors. Biomed Res Int. 2014:9215642014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bhardwaj M, Sen S, Sharma A, Kashyap S,
Chosdol K, Pushker N, Bajaj MS and Bakhshi S: ZEB2/SIP1 as novel
prognostic indicator in eyelid sebaceous gland carcinoma. Hum
Pathol. 46:1437–1442. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Prislei S, Martinelli E, Zannoni GF,
Petrillo M, Filippetti F, Mariani M, Mozzetti S, Raspaglio G,
Scambia G and Ferlini C: Role and prognostic significance of the
epithelial-mesenchymal transition factor ZEB2 in ovarian cancer.
Oncotarget. 6:18966–18979. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Galván JA, Zlobec I, Wartenberg M, Lugli
A, Gloor B, Perren A and Karamitopoulou E: Expression of E-cadherin
repressors SNAIL, ZEB1 and ZEB2 by tumor and stromal cells
influences tumor-budding phenotype and suggests heterogeneity of
stromal cells in pancreatic cancer. Br J Cancer. 112:1944–1950.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miyoshi A, Kitajima Y, Sumi K, Sato K,
Hagiwara A, Koga Y and Miyazaki K: Snail and SIP1 increase cancer
invasion by upregulating MMP family in hepatocellular carcinoma
cells. Br J Cancer. 90:1265–1273. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Davalos V, Moutinho C, Villanueva A, Boque
R, Silva P, Carneiro F and Esteller M: Dynamic epigenetic
regulation of the microRNA-200 family mediates epithelial and
mesenchymal transitions in human tumorigenesis. Oncogene.
31:2062–2074. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun X, Sun Z, Zhu Z, Li C, Zhang J, Xu H
and Sun M: Expression of SIP1 is strongly correlated with LDHA and
shows a significantly poor outcome in gastric cancer. Tumour Biol.
36:7521–7530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jouppila-Mättö A, Mannermaa A, Sironen R,
Kosma VM, Soini Y and Pukkila M: SIP1 predicts progression and poor
prognosis in pharyngeal squamous cell carcinoma. Histol
Histopathol. 30:569–579. 2015.PubMed/NCBI
|
|
41
|
Kong YH, Syed Zanaruddin SN, Lau SH,
Ramanathan A, Kallarakkal TG, Vincent-Chong VK, Wan Mustafa WM,
Abraham MT, Abdul Rahman ZA, Zain RB and Cheong SC: Co-Expression
of TWIST1 and ZEB2 in oral squamous cell carcinoma is associated
with poor Survival. PLoS One. 10:e01340452015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Miura N, Yano T, Shoji F, Kawano D,
Takenaka T, Ito K, Morodomi Y, Yoshino I and Maehara Y:
Clinicopathological significance of Sip1-associated epithelial
mesenchymal transition in non-small cell lung cancer progression.
Anticancer Res. 29:4099–4106. 2009.PubMed/NCBI
|
|
43
|
Mehendiratta M, Solomon MC, Boaz K,
Guddattu V and Mohindra A: Clinico-pathological correlation of
Ecadherin expression at the invasive tumor front of Indian oral
squamous cell carcinomas: An immunohistochemical study. J Oral
Maxillofac Pathol. 18:217–222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mostaan LV, Khorsandi MT, Sharifian SM,
Shandiz FH, Mirashrafi F, Sabzari H, Badiee R, Borghei H and
Yazdani N: Correlation between E-cadherin and CD44 adhesion
molecules expression and cervical lymph node metastasis in oral
tongue SCC: Predictive significance or not. Pathol Res Pract.
207:448–451. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gamba CO, Campos LC, Negreiros-Lima GL,
Maciel-Lima K, Sousa LP, Estrela-Lima A, Ferreira E and Cassali GD:
ZEB2 and ZEB1 expression in a spontaneous canine model of invasive
micropapillary carcinoma of the mammary gland. Res Vet Sci.
97:554–559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fang Y, Wei J, Cao J, Zhao H, Liao B, Qiu
S, Wang D, Luo J and Chen W: Protein expression of ZEB2 in renal
cell carcinoma and its prognostic significance in patient survival.
PLoS One. 8:e625582013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chu PY, Hu FW, Yu CC, Tsai LL, Yu CH, Wu
BC, Chen YW, Huang PI and Lo WL: Epithelial-mesenchymal transition
transcription factor ZEB1/ZEB2 co-expression predicts poor
prognosis and maintains tumor-initiating properties in head and
neck cancer. Oral Oncol. 49:34–41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Okugawa Y, Inoue Y, Tanaka K, Kawamura M,
Saigusa S, Toiyama Y, Ohi M, Uchida K, Mohri Y and Kusunoki M: Smad
interacting protein 1 (SIP1) is associated with peritoneal
carcinomatosis in intestinal type gastric cancer. Clin Exp
Metastasis. 30:417–429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sayan AE, Griffiths TR, Pal R, Browne GJ,
Ruddick A, Yagci T, Edwards R, Mayer NJ, Qazi H, Goyal S, et al:
SIP1 protein protects cells from DNA damage-induced apoptosis and
has independent prognostic value in bladder cancer. Proc Natl Acad
Sci USA. 106:14884–14889. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Maeda G, Chiba T, Okazaki M, Satoh T, Taya
Y, Aoba T, Kato K, Kawashiri S and Imai K: Expression of SIP1 in
oral squamous cell carcinomas: Implications for E-cadherin
expression and tumor progression. Int J Oncol. 27:1535–1541.
2005.PubMed/NCBI
|
|
51
|
Torlakovic EE, Nielsen S, Vyberg M and
Taylor CR: Getting controls under control: The time is now for
immunohistochemistry. J Clin Pathol. 68:879–882. 2015. View Article : Google Scholar : PubMed/NCBI
|