Introduction
Rhabdomyosarcoma (RMS) is a highly malignant tumor
in children and is characterized by a high recurrence rate and
presence of metastases (1,2). It originates from immature striated
muscles and is mainly found in the head, neck, extremities and
genitourinary system (3). Two major
types of RMS exist: Embryonal RMS (ERMS) and alveolar RMS (ARMS).
This classification is based on the histopathology, and ERMS
accounts for approximately 60% of all RMS cases (4,5). Although
multimodal therapy including wide excision, radiotherapy, and
dose-intensive chemotherapy is used to treat RMS, these treatments
are not available for RMS patients with relapse and/or distant
metastases (6). Therefore, more
effective therapeutic approaches are needed to treat this
tumor.
Molecular targeted therapy is a promising approach
for the treatment of RMS. Recently, it has been reported that the
mitogen-activated protein kinase (MEK)/extracellular
signal-regulated kinase (ERK) pathway plays an important role in
controlling cell proliferation, differentiation, and apoptosis of
RMS cells. Blocked MEK/ERK signaling promotes apoptosis, inhibits
cell proliferation, and increases radiosensitivity of RMS cells. In
addition, the ERK signaling may be a therapeutic target for RMS
(7–9).
Based on the aforementioned, the ERK signaling may be a novel
therapeutic target for RMS.
Matrine (C15H24N2O)
is extracted from the Chinese traditional medicine Sophorae
flavescentis, and exerts anti-inflammatory effects in
vivo and in vitro with low toxicity (10,11).
Recent studies showed that it has antitumor activity in various
cancers such as, hepatocellular carcinoma (12), breast cancer (13), osteosarcoma (14), and RMS (15). Intensive studies confirmed the
anti-tumor mechanisms of matrine, including regulation of
phosphorylation of proliferation- and apoptosis-related cell
signaling molecules such as β-catenin, serine/threonine kinase
(AKT), mammalian target of rapamycin (mTOR), and ERK (16). Given that the ERK signaling is a
potential therapeutic target for RMS, we believe that matrine may
inhibit RMS cell proliferation and induce apoptosis via
inactivation of the ERK signaling. In this study, we aim to explore
the anti-ERMS effects of matrine and investigate whether the
antitumor activity of matrine is due to inhibition of the ERK
signaling in ERMS RD cells.
Materials and methods
Materials and cell lines
Matrine (C15H24N2O)
was purchased from Aladdin Ltd. (Shanghai, China) and dissolved in
Dulbecco's modified Eagle's medium (DMEM) (HyClone, Logan, UT,
USA). Fetal bovine serum (FBS), streptomycin, and penicillin were
all purchased from Gibco (Grand Island, NY, USA). Anti-MEK1,
anti-ERK 1/2, anti-phosphorylated MEK 1/2 (Thr386),
anti-phosphorylated ERK 1/2 (Thr202/Tyr204), anti-BCL2, anti-BAX,
and anti-β-actin were obtained from Cell Signaling Technology,
Inc., (Danvers, MA, USA). The ERK pathway inhibitor U0126 was
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). MEK expression plasmid pcDNA3.1(+)-MEK1 was purchased from
GeneChem Co., Ltd. (Shanghai, China). The RMS cell line RD was
purchased from the Type Culture Collection of the Chinese Academy
of Sciences (Shanghai, China).
Cell culture and transfection
RD cells, were cultured in DMEM supplemented with
10% FBS, 100 µg/ml streptomycin and 100 units/ml penicillin in a
humidified atmosphere of 5% CO2 at 37°C. For transient
transfection, cells were plated in a 6-well plate at a density of
2×105 cells per well and cultured for 24 h.
Lipofectamine® 2000 liposome transfection kit
(Invitrogen Life Technologies, Carlsbad, CA, USA) was used to
transfect pcDNA3.1(+)-MEK1 or the empty pcDNA3.1(+) into the cells
according to the manufacturer's instructions.
Cell viability assay
RD cells were plated in 96-well microtiter plates at
a density of 5×103 cells per well and treated with
matrine in various doses (0, 0.5, 1.0, 1.5, 2.0, 3.0, and 5.0 g/l)
for 24 h. Cell viabilities were assessed using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay (Sigma-Aldrich, St. Louis, MO, USA). The absorbance (A) was
detected at 490 nm using an ELISA reader. Cell viability rate was
calculated as followes: (%)=A490,
matrine/A490, control × 100%. RD cells were treated with
or without U0126 for 1 h before treatment with matrine for 24 h.
Then, cell viabilities were assessed as described above. For
transfection experiments, the cells were treated with or without
matrine after transfection for 24 h, and the cell viabilities were
assessed as described above.
Apoptosis assay
RD cells in exponential growth phase were plated in
12-well plates at a density of 2×105 cells per well. The
cells were treated with matrine (0, 0.5, 1.0, and 1.5 g/l) for 24 h
or 1.5 g/l matrine for 24 or 48 h. Apoptosis was measured using
Annexin V-FITC/PI double staining (MultiSciences Biotech, Shanghai,
China) according to the manufacturer's instructions. The apoptotic
cells were detected with flow cytometry (BD Biosciences, Franklin
Lakes, NJ, USA). Data acquisition and analysis were performed using
CellQuest software (BD Biosciences). In addition, RD cells were
treated with or without U0126 for 1 h before treatment with matrine
for 24 h, and apoptosis was measured as described above. For
transfection experiments, the cells were treated with or without
matrine after transfection for 24 h, and apoptosis was measured as
described above.
Wound healing assay
RD cells were seeded into a 6-well plate at a
density of 2×105 cells per well and cultured overnight
to attain 90% confluence. Cell wounds were scratched by a plastic
tip, washed twice with medium, treated with matrine (0, 0.25, 0.5,
and 0.75 mg/ml) and cultured in serum-free medium for 24 h. Images
were captured at 0 and 24 h under an inverted microscope.
Invasion assay
The RD cells invasion assay was performed using
Transwell chambers (8-µm pore size) coated with Matrigel (Corning
Inc., Acton, MA, USA). Cells (1×105) in serum-free
medium containing various concentrations of matrine (0, 0.25, 0.5,
and 0.75 mg/ml) were seeded into the upper Transwell chambers,
while 600 µl medium containing 10% FBS was added to the lower
chambers. After 24 h, cells on the upper face of the filter and the
Matrigel were removed by a cotton swab, and the cells on the bottom
were fixed, stained, and counted.
Western blot assay
RD cells were treated with matrine (0, 0.25, 0.5,
and 0.75 mg/ml) or U0126 for 48 h, and lysed in lysis buffer [50
mmol/l Tris-HCl, 1 mmol/l ethylenediaminetetraacetic acid (EDTA),
150 mmol/l NaCl, 0.1% sodium dodecyl sulfate (SDS), 1% Triton
X-100, 1 mmol/l phenylmethyl sulfonyl fluoride (PMSF)]. The protein
extracts were separated by SDS-polyacrylamide gel electrophoresis
(PAGE), and transferred to a polyvinylidene fluoride (PVDF)
membrane. After blocking in defatted milk [5% in Tris-buffered
saline/Tween-20 buffer (TBST)] at 37°C for 2 h, the membrane was
incubated overnight at 4°C with primary antibodies against MEK1,
ERK1/2, p-MEK1, p-ERK1/2, BCL2, BAX, and β-actin in TBST containing
1% bovine serum albumin and with secondary antibodies for 1 h at
room temperature. Signals were detected with ECL detection reagents
(GE Healthcare, Piscataway, NJ, USA). The optical densities of the
bands on photographic films were obtained using ImageJ software
(NIH, Bethesda, MD, USA).
Statistical analysis
Each experiment was repeated three times, outcome
variables were presented as mean ± standard deviation. The
difference between multiple groups were analyzed using the
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Matrine inhibited the proliferation of
RD cells
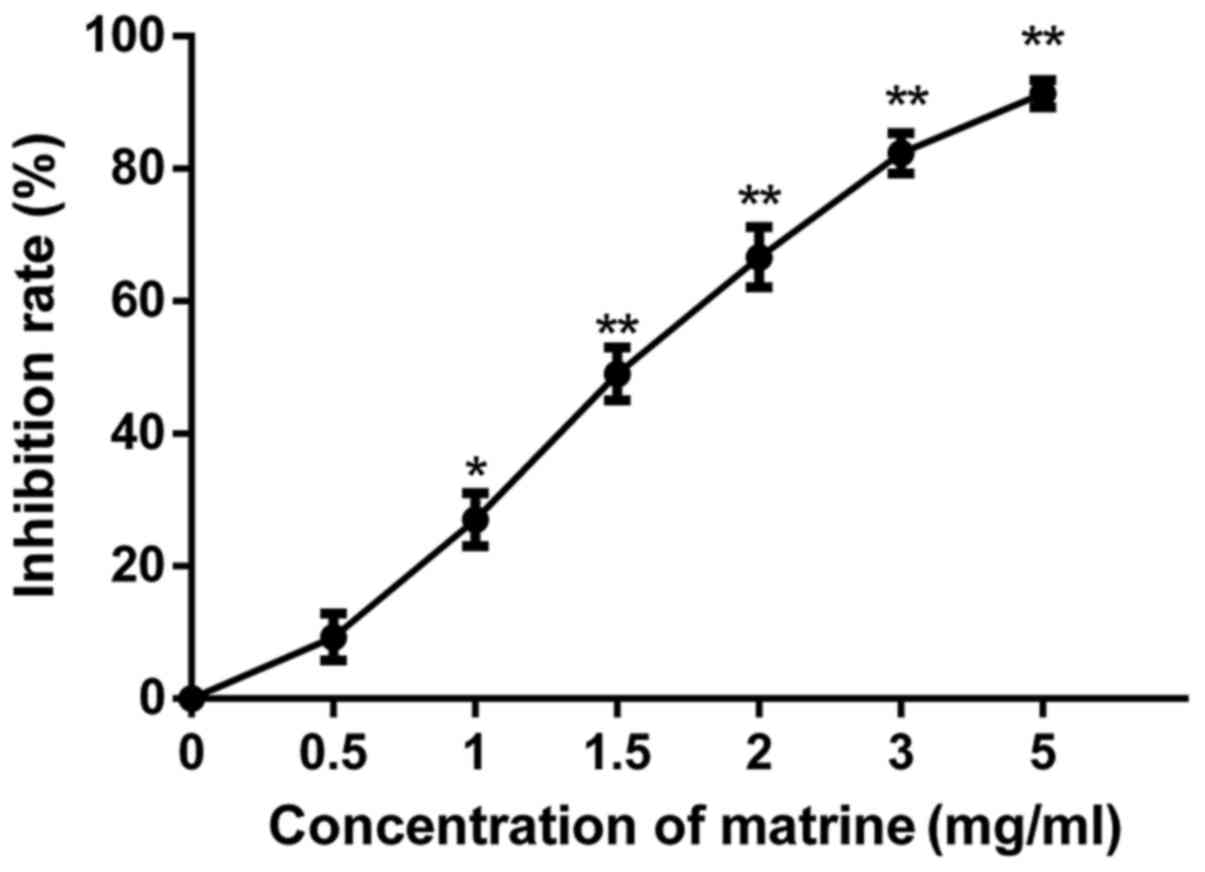
The results of the MTT assay showed that matrine
inhibited the proliferation of RD cells (Fig. 1), and increasing the concentration of
matrine leads to significant increase of inhibition rate. The
results indicated that matrine inhibited effectively the RD cell
viability. However, at concentrations below 1 mg/ml, matrine did
not significantly reduce the total viable cells. Thus, the
concentration range of matrine for the subsequent experiments was
chosen depending on these results.
Matrine induced apoptosis of RD
cells
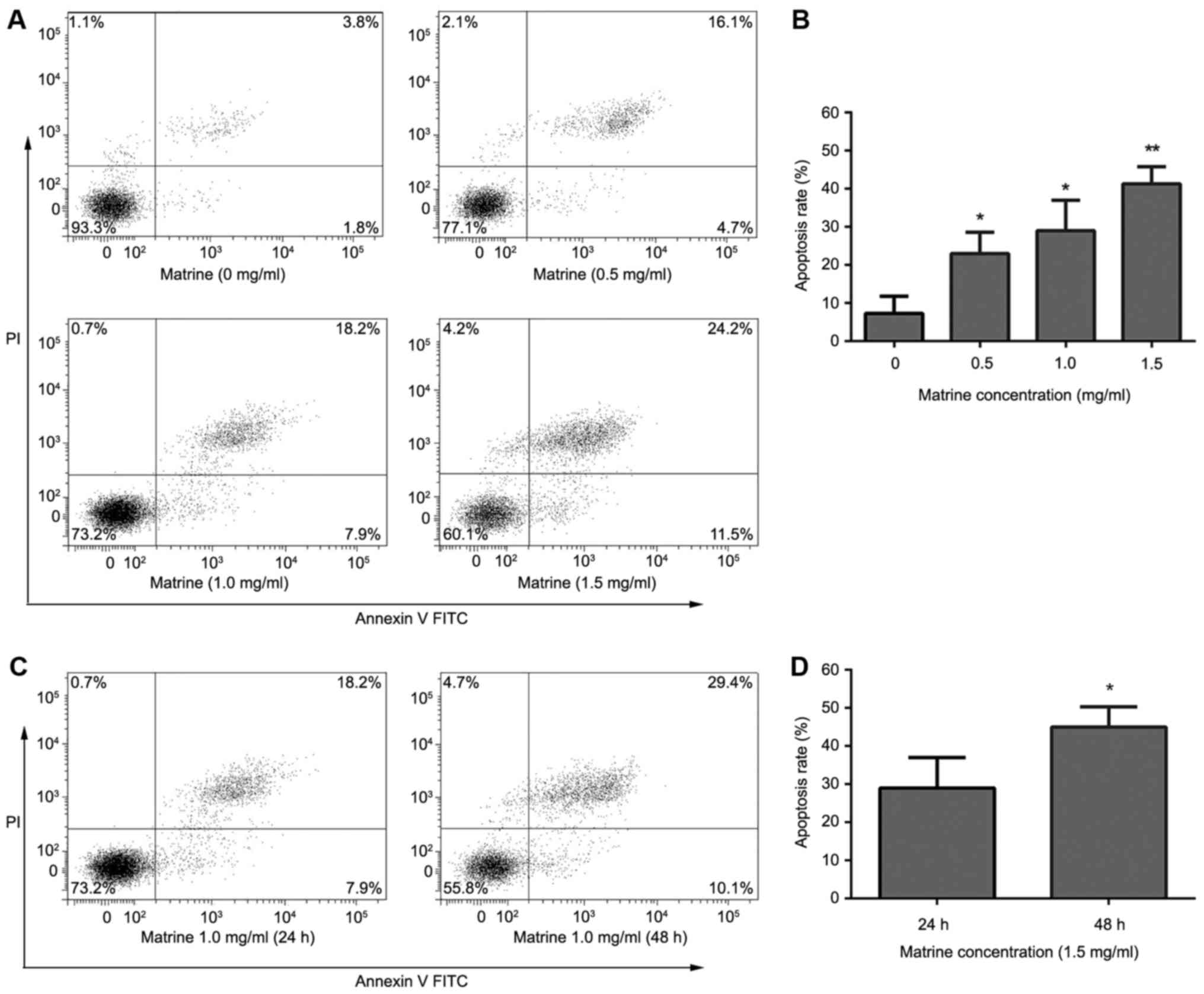
The results of the Annexin V-FITC/PI double staining
showed that matrine induced apoptosis of RD cells. After treatment
with matrine (0.5,1 and 1.5 mg/ml) for 24 h, apoptosis rates were
22.8±0.78, 26.5±1.21, 40.1±2.17%, respectively. The apoptosis rate
significantly increased with increasing drug concentrations
(Fig. 2A and B). After treatment with
matrine (1.0 mg/ml) for 24 or 48 h, apoptosis rates were 26.5±1.21,
44.7±3.03%, respectively. The apoptosis rate significantly
increased with increasing treatment times (Fig. 2C and D).
Matrine inhibited the migration and
invasion of RD cells
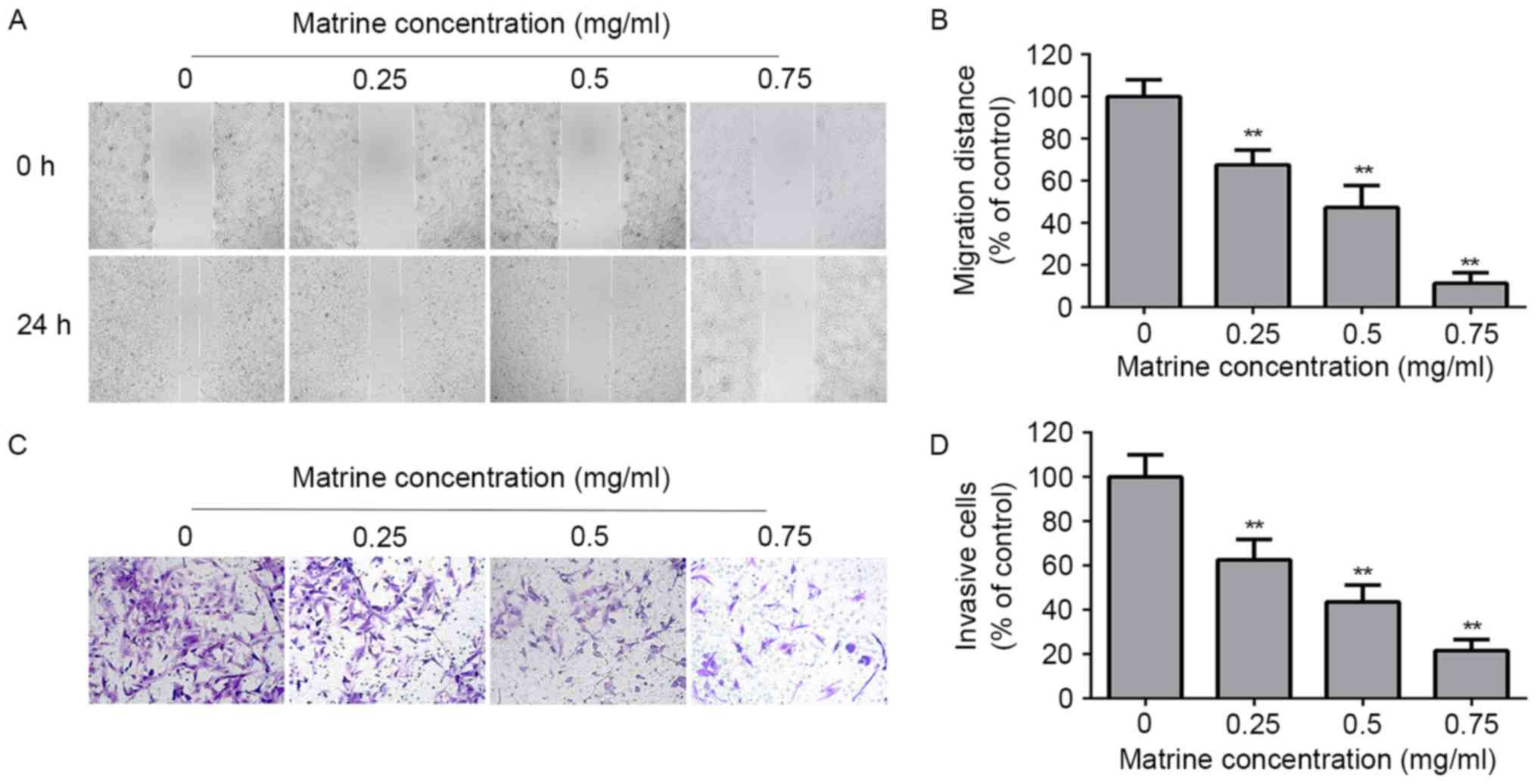
The motility of RD cells was evaluated by wound
healing assay. After treatment with matrine (0, 0.25, 0.5, and 0.75
mg/ml) for 24 h, the migration distance of RD cells were
381.2±10.3, 259.1±8.9, 177.9±14.2, 42.5±3.7 µm, respectively. The
migration distance decreased in a dose-dependent manner (Fig. 3A and B). The results suggested that
matrine inhibited the motility of RD cells.
The invasiveness of RD cells was evaluated by using
Transwell chambers coated with Matrigel. After treatment with
matrine (0, 0.25, 0.5, and 0.75 mg/ml) for 24 h, the invasion of RD
cells was reduced in a dose-dependent manner (Fig. 3C and D). The results suggested that
matrine inhibited the invasive ability of RD cells.
Matrine inhibited the proliferation
and induced apoptosis of RD cells via the ERK pathway
The ERK signaling pathway plays an important role in
cell growth, survival and apoptosis (17). Studies showed that the inhibition of
the ERK pathway positively reduced RMS cell growth, survival, and
epithelial-mesenchymal transition (EMT) (7–9). Thus, we
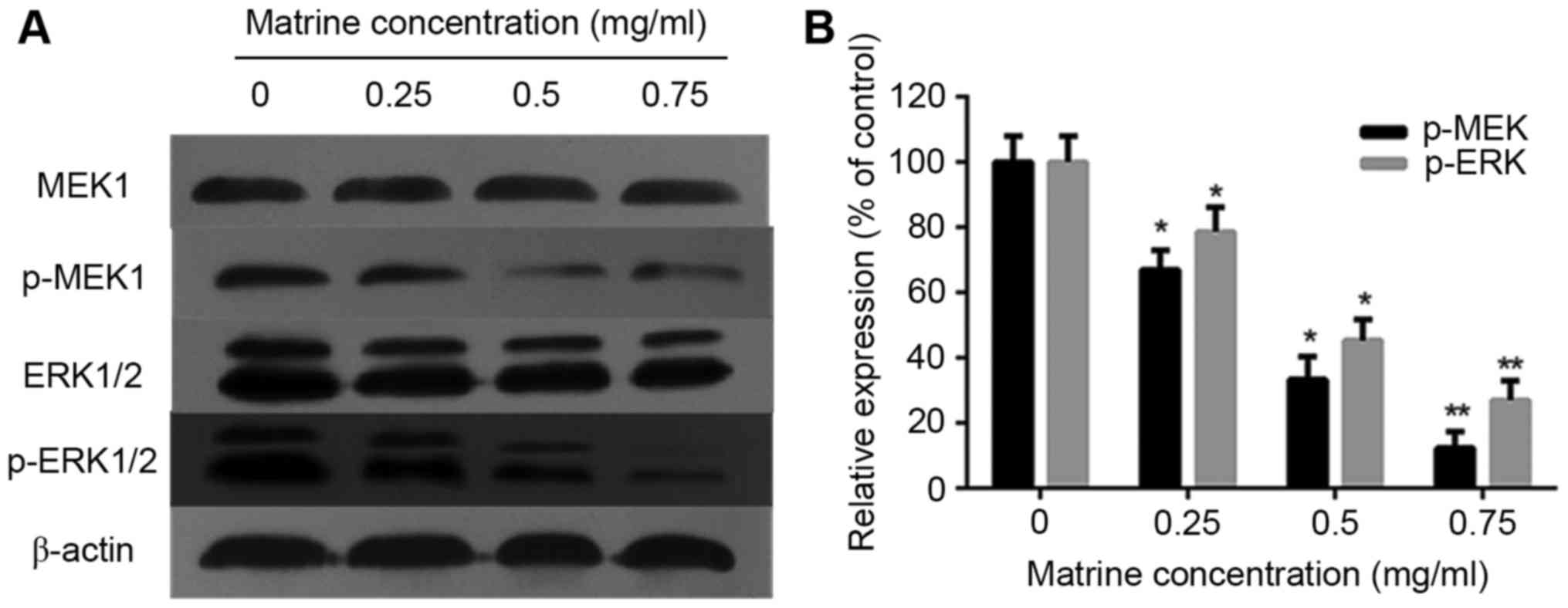
investigated the effect of matrine on ERK phosphorylation in RD
cells. The results of the western blot analysis showed that the
phosphorylation of MEK1 and ERK1/2 was reduced in a dose-dependent
manner, whereas the total MEKand ERK levels did not change
(Fig. 4A and B).
In order to further investigate the effect of
matrine on the ERK pathway, we treated cells with pcDNA3.1(+)-MEK1
to promote ERK phosphorylation. The results showed that increased
phosphorylation of ERK1/2 reversed the effect of matrine on
cellproliferation and apoptosis (Fig.
5A-C).
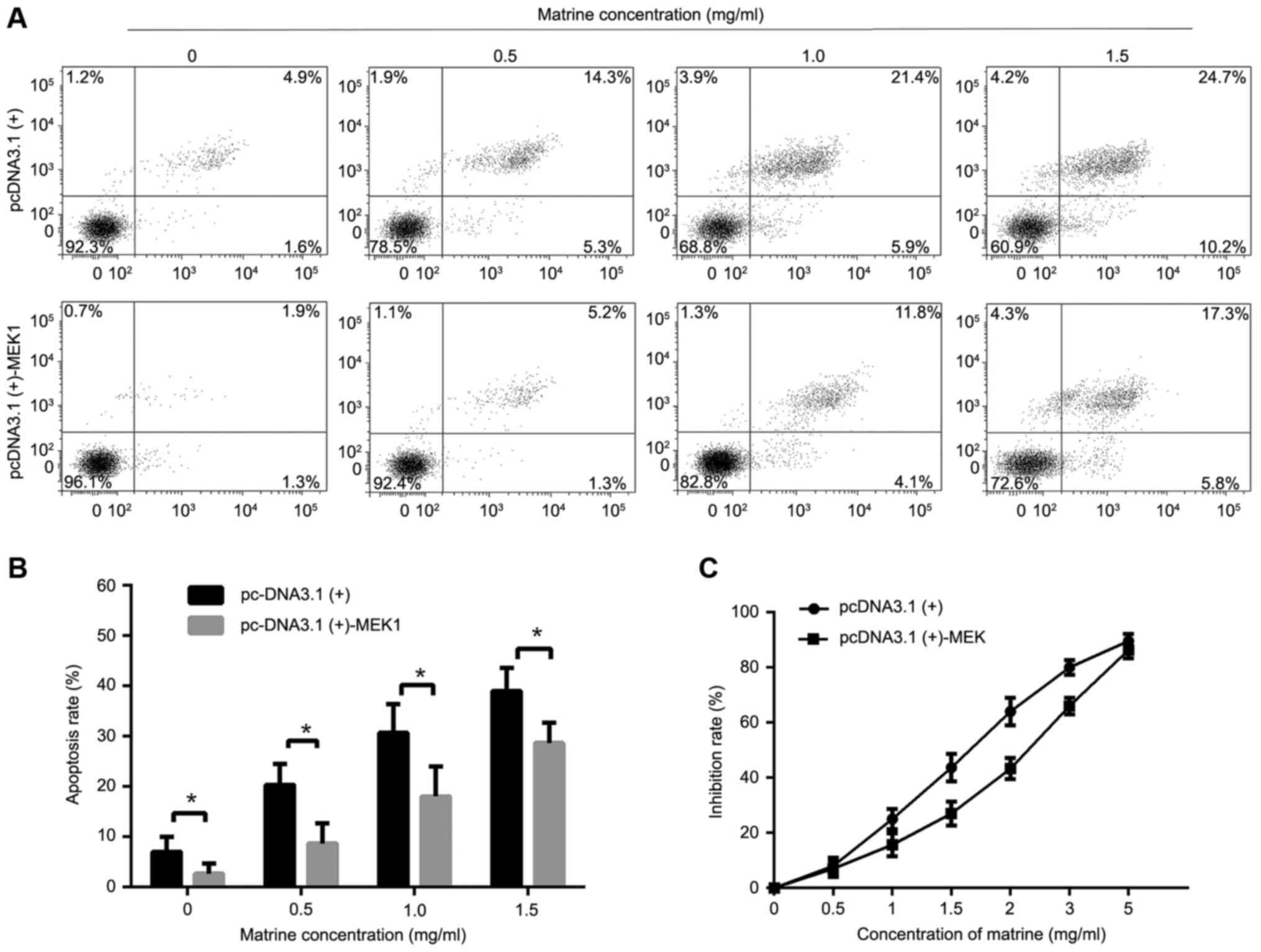 | Figure 5.Effect of matrine in the RMS cells
transfected with the plasmid pcDNA3.1(+)-MEK1. (A) RMS cells
transfected with pcDNA3.1(+)-MEK1 or the empty pcDNA3.1(+) in
12-well plates (2×105 cells/well) were treated with matrine at
concentrations of 0, 0.5, 1 and 1.5 mg/ml for 24 h. The Annexin
V-FITC/PI double staining was used to assess apoptosis, followed by
flow cytometry. (B) The apoptosis rate was calculated. (C) The
inhibition rates of matrine at concentrations of 0, 0.5, 1, 1.5, 2,
3, and 5 mg/ml for 24 h in RMS cells transfected with
pcDNA3.1(+)-MEK1 or empty pcDNA3.1(+) were detected by MTT assay.
Data represent the mean ± standard deviation of three independent
experiments, *P<0.05 vs. the control. |
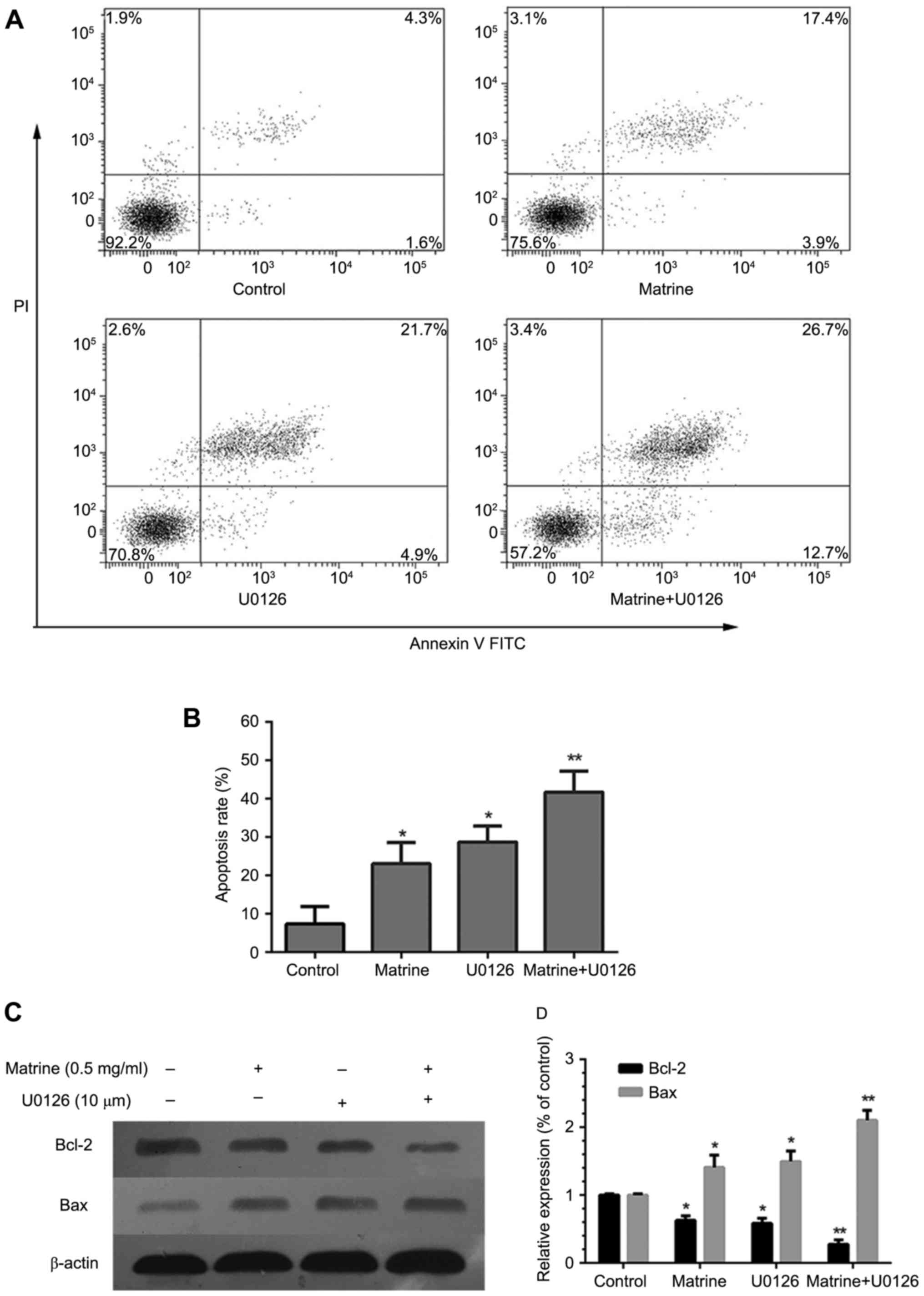
Furthermore, in order to verify whether the effects
of matrine were ERK-dependent. U0126 was used to prevent ERK
phosphorylation. The results showed that the combined use of
matrine and U0126 significantly increased apoptosis (Fig. 6A and B), reduced BCL-2 levels, and
increased BAX protein expression (Fig. 6C
and D).
Our results proved that matrine significantly
inhibited the proliferation and induced apoptosis of RMS cells via
the ERK1/2 signaling pathway.
Discussion
Matrine, has antitumor effects in many cancers. The
present study showed that matrine induced RMS cell growth
inhibition, apoptosis and cell cycle arrest (7–9). However,
the antitumor mechanism in RMS cells is not clear. In this study,
we treated RD cells with matrine in vitro. The results
showed that matrine inhibited cell proliferation and motility, and
induced apoptosis in a dose-dependent manner. We also showed that
matrine inhibited the phosphorylation of ERK in a dose-dependent
manner.
So far, it remains unclear how matrine inhibits cell
proliferation and induces apoptosis. Previous studies showed that
matrine targeted numerous cellular proteins, including β-catenin,
AKT, and mTOR (18–20). ERK is also targeted by matrine
(21). The ERK pathway plays an
important role in cell proliferation, migration and apoptosis
(17). Previous studies reported the
relationship between matrine and the ERK pathway. It has been shown
that the anticancer effects of matrine in several malignancies are
mediated by inactivating the phosphorylation of ERK (21,22).
Inhibition of the ERK signaling in RMS cells induced apoptosis and
arrest of tumor growth (23–25). Thus, we examined the p-ERK level in
RMS cells after treatment with matrine, and the results showed that
the expression of ERK1/2 phosphorylation was reduced in a
dose-dependent manner, These findings indicate the strong
relationship between matrine and de-phosphorylation of ERK in RMS
cells.
To clarify whether matrine inhibited RMS cell
proliferation and induced apoptosis depending on the reduced
expression of phosphorylation ERK1/2, we transfected
pcDNA3.1(+)-MEK1 into the cells to upregulate p-ERK1/2. We
demonstrated that the anti-tumor ability of matrine in RMS cells
was weakened, suggesting that matrine may exert its anticancer
activity by inactivating the phosphorylation of ERK1/2.
Furthermore, this evidence was supported by the addition of U0126.
We treated RMS cells with matrine and U0126. The anti-tumor ability
of matrine in RMS cells was enhanced. In addition, the expression
of BCL-2 was down-regulated, while the expression of BAX was
up-regulated. These results indicated that matrine inhibited RMS
cell proliferation and induced apoptosis partially depending on the
reduced expression of phosphorylation of ERK1/2.
In conclusion, this study reveals that matrine
inhibits RMS cell proliferation and motility, and induces apoptosis
in vitro. Furthermore, this study indicates that matrine
exerts antitumor effect via the ERK signaling pathway. Our findings
indicate that matrine may represent a new class of ERK inhibitors
and has the potential to be a novel therapeutic approach for
RMS.
References
|
1
|
Wexler LH: Metastatic rhabdomyosarcoma:
Still room for improvement. J Clin Oncol. 34:105–106. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shern JF, Chen L, Chmielecki J, Wei JS,
Patidar R, Rosenberg M, Ambrogio L, Auclair D, Wang J, Song YK, et
al: Comprehensive genomic analysis of rhabdomyosarcoma reveals a
landscape of alterations affecting a common genetic axis in
fusion-positive and fusion-negative tumors. Cancer Discov.
4:216–231. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hettmer S, Li Z, Billin AN, Barr FG,
Cornelison DD, Ehrlich AR, Guttridge DC, Hayes-Jordan A, Helman LJ,
Houghton PJ, et al: Rhabdomyosarcoma: Current challenges and their
implications for developing therapies. Cold Spring Harb Perspect
Med. 4:a0256502014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Arnold MA and Barr FG: Molecular
diagnostics in the management of rhabdomyosarcoma. Expert Rev Mol
Diagn. 17:189–194. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Belyea B, Kephart JG, Blum J, Kirsch DG
and Linardic CM: Embryonic signaling pathways and rhabdomyosarcoma:
Contributions to cancer development and opportunities for
therapeutic targeting. Sarcoma. 2012:4062392012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weigel BJ, Lyden E, Anderson JR, Meyer WH,
Parham DM, Rodeberg DA, Michalski JM, Hawkins DS and Arndt CA:
Intensive multiagent therapy, including dose-compressed cycles of
ifosfamide/etoposide and vincristine/doxorubicin/cyclophosphamide,
irinotecan and radiation, in patients with high-risk
rhabdomyosarcoma: A report from the children's oncology group. J
Clin Oncol. 34:117–122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zenitani M, Nojiri T, Uehara S, Miura K,
Hosoda H, Kimura T, Nakahata K, Miyazato M, Okuyama H and Kangawa
K: C-type natriuretic peptide in combination with sildenafil
attenuates proliferation of rhabdomyosarcoma cells. Cancer Med.
5:795–805. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ciccarelli C, Vulcano F, Milazzo L,
Gravina GL, Marampon F, Macioce G, Giampaolo A, Tombolini V, Di
Paolo V, Hassan HJ and Zani BM: Key role of MEK/ERK pathway in
sustaining tumorigenicity and in vitro radioresistance of embryonal
rhabdomyosarcoma stem-like cell population. Mol Cancer. 15:162016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marampon F, Gravina GL, Di Rocco A,
Bonfili P, Di Staso M, Fardella C, Polidoro L, Ciccarelli C,
Festuccia C, Popov VM, et al: MEK/ERK inhibitor U0126 increases the
radiosensitivity of rhabdomyosarcoma cells in vitro and in vivo by
downregulating growth and DNA repair signals. Mol Cancer Ther.
10:159–168. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu G, Zhou W, Zhao J, Pan X, Sun Y, Xu H,
Shi P, Geng C, Gao L and Tian X: Matrine alleviates
lipopolysaccharide-induced intestinal inflammation and oxidative
stress via CCR7 signal. Oncotarget. 8:11621–11628. 2017.PubMed/NCBI
|
|
11
|
Sun D, Wang J, Yang N and Ma H: Matrine
suppresses airway inflammation by downregulating SOCS3 expression
via inhibition of NF-κB signaling in airway epithelial cells and
asthmatic mice. Biochem Biophys Res Commun. 477:83–90. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xie SB, He XX and Yao SK: Matrine-induced
autophagy regulated by p53 through AMP-activated protein kinase in
human hepatoma cells. Int J Oncol. 47:517–526. 2015.PubMed/NCBI
|
|
13
|
Li H, Li X, Bai M, Suo Y, Zhang G and Cao
X: Matrine inhibited proliferation and increased apoptosis in human
breast cancer MCF-7 cells via upregulation of Bax and
downregulation of Bcl-2. Int J Clin Exp Pathol. 8:14793–14799.
2015.PubMed/NCBI
|
|
14
|
Ma K, Huang MY, Guo YX and Hu GQ:
Matrine-induced autophagy counteracts cell apoptosis via the ERK
signaling pathway in osteosarcoma cells. Oncol Lett. 12:1854–1860.
2016.PubMed/NCBI
|
|
15
|
Guo L, Xue TY, Xu W and Gao JZ: Matrine
promotes G0/G1 arrest and down-regulates cyclin D1 expression in
human rhabdomyosarcoma cells. Panminerva Med. 55:291–296.
2013.PubMed/NCBI
|
|
16
|
Liu Y, Xu Y, Ji W, Li X, Sun B, Gao Q and
Su C: Anti-tumor activities of matrine and oxymatrine: literature
review. Tumour Biol. 35:5111–5119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Herrero A, Pinto A, Colón-Bolea P, Casar
B, Jones M, Agudo-Ibáñez L, Vidal R, Tenbaum SP, Nuciforo P,
Valdizán EM, et al: Small molecule inhibition of ERK dimerization
prevents tumorigenesis by RAS-ERK pathway oncogenes. Cancer Cell.
28:170–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang HQ, Jin JJ and Wang J: Matrine
induces mitochondrial apoptosis in cisplatin-resistant non-small
cell lung cancer cells via suppression of β-catenin/survivin
signaling. Oncol Rep. 33:2561–2566. 2015.PubMed/NCBI
|
|
19
|
Wu J, Hu G, Dong Y, Ma R, Yu Z, Jiang S,
Han Y, Yu K and Zhang S: Matrine induces Akt/mTOR signalling
inhibition-mediated autophagy and apoptosis in acute myeloid
leukaemia cells. J Cell Mol Med. 21:1171–1181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Niu H, Zhang Y, Wu B, Zhang Y, Jiang H and
He P: Matrine induces the apoptosis of lung cancer cells through
downregulation of inhibitor of apoptosis proteins and the Akt
signaling pathway. Oncol Rep. 32:1087–1093. 2014.PubMed/NCBI
|
|
21
|
Li Y, Zhang ZN, Zhao HM, Tong ZC, Yang J,
Wang H and Liang XJ: Matrine inhibits the invasive properties of
human osteosarcoma cells by downregulating the ERK-NF-κB pathway.
Anti-Cancer Drugs. 25:1035–1043. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qian L, Liu Y, Xu Y, Ji W, Wu Q, Liu Y,
Gao Q and Su C: Matrine derivative WM130 inhibits hepatocellular
carcinoma by suppressing EGFR/ERK/MMP-2 and PTEN/AKT signaling
pathways. Cancer Lett. 368:126–134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Marampon F, Bossi G, Ciccarelli C, Di
Rocco A, Sacchi A, Pestell RG and Zani BM: MEK/ERK inhibitor U0126
affects in vitro and in vivo growth of embryonal rhabdomyosarcoma.
Mol Cancer Ther. 8:543–551. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guenther MK, Graab U and Fulda S:
Synthetic lethal interaction between PI3K/Akt/mTOR and Ras/MEK/ERK
pathway inhibition in rhabdomyosarcoma. Cancer Lett. 337:200–209.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Otabe O, Kikuchi K, Tsuchiya K, Katsumi Y,
Yagyu S, Miyachi M, Iehara T and Hosoi H: MET/ERK2 pathway
regulates the motility of human alveolar rhabdomyosarcoma cells.
Oncol Rep. 37:98–104. 2017.PubMed/NCBI
|