Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most
common subtype of non-Hodgkin lymphoma (NHL), accounting for 30–40%
of all NHL patients (1–3), which is considered to be a heterogeneous
entity based on its biological characteristics and clinical
outcomes (3–5). The survivals of DLBCL patients have
notably improved since addition of rituximab to CHOP (rituximab,
cyclophosphamide, doxorubicin, vincristine, and prednisone)
chemotherapy (6,7). However, some DLBCL patients continue to
present an inferior prognosis under standard R-CHOP therapy
(1).
Ki-67, a nuclear nonhistone protein, is synthesized
at the beginning of cell proliferation (8). Ki-67 expression has been widely used in
clinical practice as an index to evaluate the proliferative
activity of lymphoma. High Ki-67 expression was highly associated
with worse OS for NHL (9). However,
the relationship between Ki-67 expression and outcome with DLBCL
are still contradictory and inconclusive in various studies
(10–12).
BCL2 protein functions as an antiapoptotic protein
inhibiting cells from programmed cell death (13). Both gene amplification and
translocation are common mechanisms causing BCL2 protein
overexpression in DLBCL. The clinical significance of BCL2 protein
expression in DLBCL is still controversial. The impact of BCL2
overexpression on survival in DLBCL is still debatable in previous
studies. Additionally, the prognostic value of BCL2 protein
overexpression is also different between GCB and ABC subtypes
(14,15).
Moreover, the predictive significance of some
prognostic factors changed following the introduction of a CD20
monoclonal antibody, rituximab, underscores the necessity for
revaluating the prognostic value of predictive factors after the
introduction of rituximab (7,16).
In the present study, we intended to investigate the
optimal prognosis cut off value of Ki-67 index in DLBCL patients,
and to confirm the specific prognostic value of BCL2 and its
association with cell of origin classification (COOC). Furthermore,
we investigated whether the BCL2/Ki-67 index has a more significant
importance on the outcome of DLBCL patients.
Materials and methods
Patient selection
Between August 2003 and January 2016, 274 patients
with de novo DLBCL were enrolled in the present study.
Patients of special types of DLBCL were excluded from this study.
All patients enrolled informed consent in accordance with
requirements of the Declaration of Helsinki, and the research
project was approved by the University and Institutional Review
Boards. Formalin-fixed, paraffin embedded (FFPE) tissue biopsy
specimens were available for all patients. All atients were treated
with R-CHOP like therapies.
Immunohistochemistry (IHC)
IHC was performed on FFPE sections. The antibodies
used were CD10 (clone 56C6; Invitrogen Life Technologies, Carlsbad,
CA, USA), BCL6 (clone PG-B6P; Dako, Carpinteria, CA, USA), MUM1
(clone MUM1p, Dako), KI-67 (clone SP6, Abcam, Cambridge, UK) and
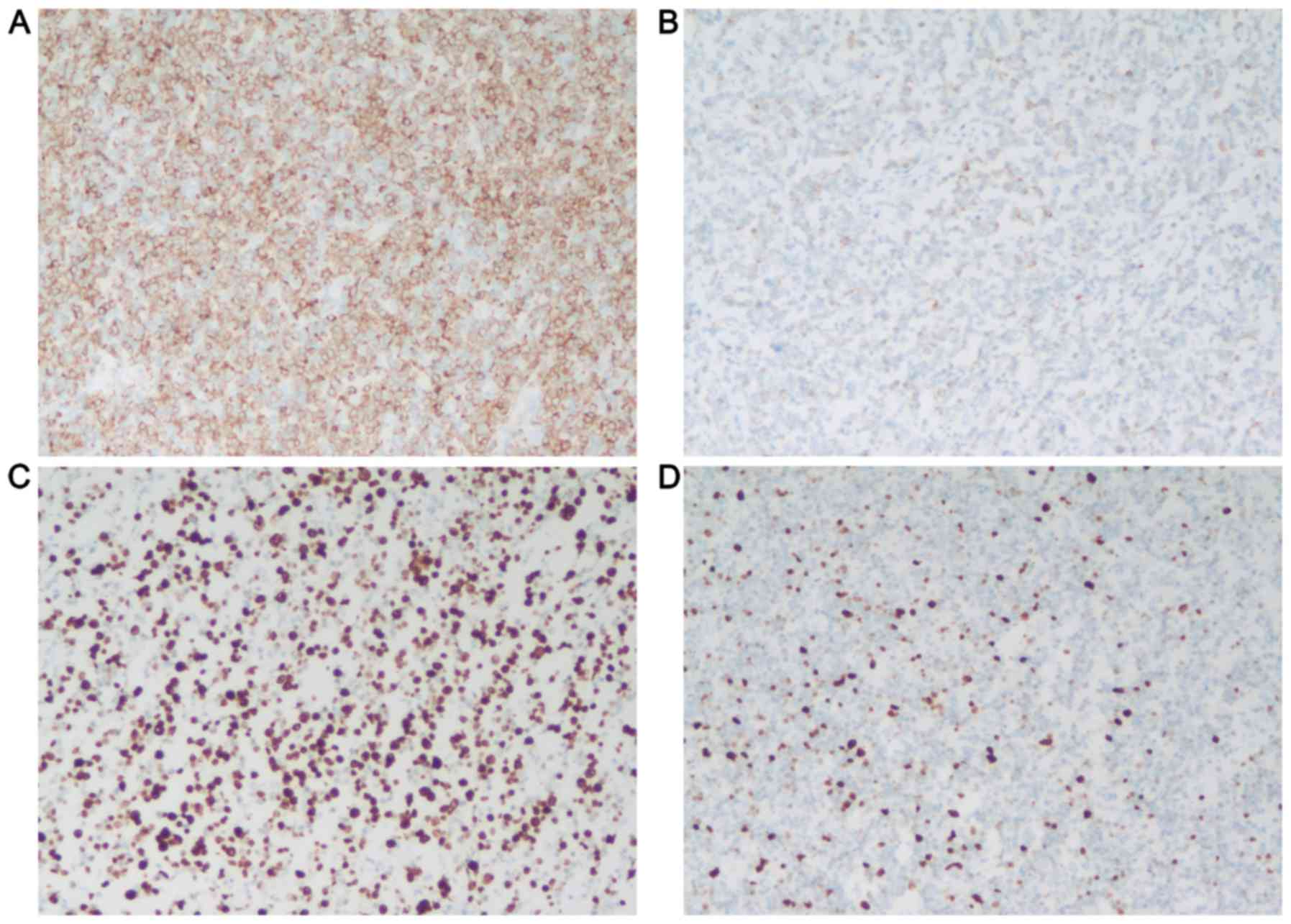
BCL2 (clone 124; Dako). The staining results were showed in
Fig. 1. COOC was performed by
immunohistochemical stains using the Hans criteria (17). Immunostains for CD10, BCL6, and MUM1
were used to classify cases as having germinal center B-cell
(GCB)-like or non-germinal center B-cell (NGC)-like
immunophenotype. The cut off scores for each antibody were
described previously (17,18). BK+ was defined as BCL2 positive and
high Ki-67 proliferation (≥90%). BK+ was defined as BCL2 negative
and low Ki-67 proliferation (<90%).
Statistical analysis
Overall survival (OS) and progression-free survival
(PFS) were the primary end points of this study. OS was calculated
from the date of diagnosis to the date of death due to any cause or
to the date of the last follow up. PFS was calculated from the date
of first progression, relapse, death, or the last follow-up.
Patients who were alive and progression-free at last follow-up were
censored for this analysis. Statistical analysis was carried out
with SPSS 16.0 software. Survival curves were plotted by using
Kaplan-Meier method and were compared by using log-rank test.
Differences were determined using a two-tailed log-rank test, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics patient
Clinic-pathologic characteristics were presented in
Table I. We examined BCL2 and Ki-67
protein expression by IHC in the R-CHOP-like cohort including
GCB-DLBCL (52/126, 41.3%), NGC-DLBCL (86/148, 58.1%). We detected
Ki-67 proliferation index in GCB-DLBCL and NGC-DLBCL (Table I).
 | Table I.Clinical and immunohistochemical
characteristics of DLBCL patients. |
Table I.
Clinical and immunohistochemical
characteristics of DLBCL patients.
| Variables | Number of cases
(%) |
|---|
| Age ≥ 60 y | 118 (43.1) |
| Male | 180 (65.7) |
| Stage III–IV | 146 (52.3) |
| Abnormal LDH
level | 114 (41.6) |
| Performance state
2–4 | 54 (19.7) |
| Extranodal
involvement ≥2 | 64 (23.4) |
| B symptom | 102 (37.2) |
| IPI ≥4 | 26 (9.5) |
| GCB | 126 (46.0) |
| Non-GCB | 148 (54.0) |
| BCL2 positive | 138 (50.4) |
| Ki-67 ≥60% | 210 (76.6) |
| Ki-67 ≥70% | 180 (65.7) |
| Ki-67 ≥80% | 122 (44.5) |
| Ki-67 ≥90% | 58 (21.2) |
Prognosis of BCL2 protein
Based on the data published previously, we selected
a cut off of ≥70% protein expression for BCL2 positivity. In the
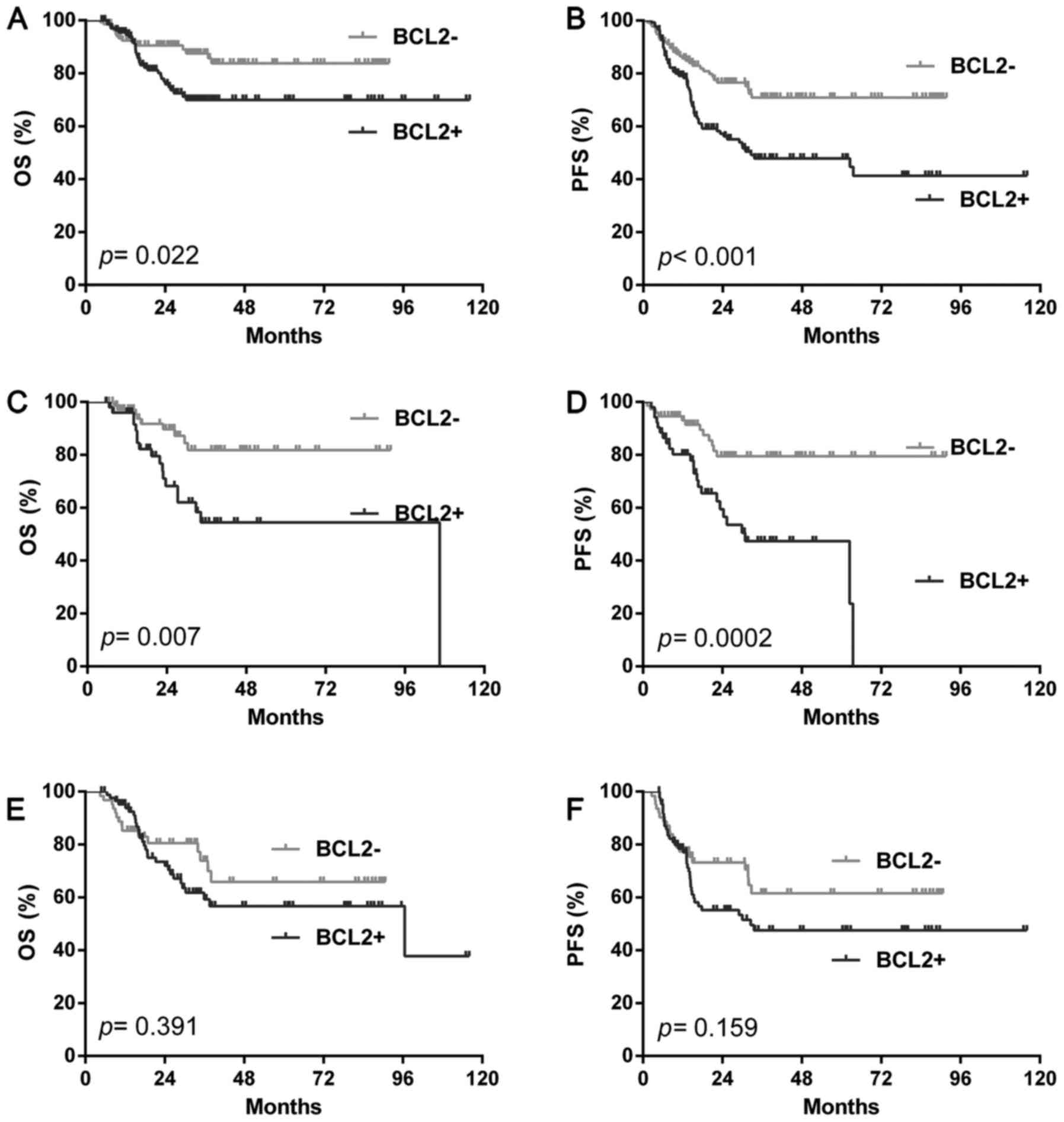
total cohort, the BCL2 positive rate was 50.4% (138/274). The BCL2
positive patients show a significantly shorter OS (P=0.022) and PFS
(P<0.001) compared with the BCL2 negative cases (Fig. 2A and B). We further analyzed BCL2
prognostic value according to different COOC. In GCB group, BCL2
positivity predict poorer outcome than negative ones (OS: P=0.007;
PFS: P=0.0002) (Fig. 2C and D).
However, in the NGC group, BCL2 positive patients had a similar OS
(OS: P=0.391) and PFS (PFS: P=0.159) with negative ones (Fig. 2E and F). Multivariate analysis by Cox
proportional hazards regression, BCL2 positivity remains
independent prognostic factor on PFS (P=0.006) (Tables II and III).
 | Table II.Univariate and multivariate analysis
with OS. |
Table II.
Univariate and multivariate analysis
with OS.
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Extranodal
involvement ≥2 | 2.393 | 1.679–5.326 | 0.0002 | 0.433 | 0.173–1.084 | 0.074 |
| Elevated LDH
level | 2.317 | 1.508–3.923 | 0.0003 | 0.825 | 0.300–2.266 | 0.709 |
| Stage III–IV | 1.700 | 1.060–2.693 | 0.030 | 1.498 | 0.546–4.109 | 0.432 |
| IPI ≥4 | 3.422 | 3.192–22.14 | <0.0001 | 1.141 | 0.250–5.216 | 0.865 |
| B symptom | 1.673 | 1.066–2.819 | 0.0275 | 1.067 | 0.401–2.836 | 0.897 |
| Performance state
2–4 | 2.052 | 1.313–4.663 | 0.0052 | 0.239 | 0.099–0.580 | 0.002 |
| BCL2 ≥50% | 1.987 | 1.102–3.416 | 0.022 | 0.621 | 0.368–1.049 | 0.075 |
| Ki-67 ≥90% | 1.755 | 0.9153–4.290 | 0.0850 | 0.679 | 0.372–1.241 | 0.208 |
| BK+ | 2.895 | 1.577–10.71 | 0.0041 | 0.628 | 0.234–1.682 | 0.354 |
 | Table III.Univariate and multivariate analysis
with PFS. |
Table III.
Univariate and multivariate analysis
with PFS.
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Extranodal
involvement ≥2 | 2.094 | 1.493–4.122 | 0.0016 | 1.494 | 0.754–2.962 | 0.250 |
| Elevated LDH
level | 2.140 | 1.484–3.434 | 0.0002 | 0.461 | 0.274–0.775 | 0.003 |
| Stage III–IV | 1.641 | 1.089–2.444 | 0.018 | 0.572 | 0.337–0.971 | 0.039 |
| IPI ≥4 | 2.396 | 1.625–8.091 | 0.0018 | 0.382 | 0.150–0.973 | 0.044 |
| B symptom | 1.613 | 1.087–2.534 | 0.0192 | 2.964 | 1.730–5.079 | <0.0001 |
| Performance state
2–4 | 1.883 | 1.263–3.716 | 0.0051 | 0.555 | 0.305–1.008 | 0.053 |
| BCL2 ≥50% | 2.147 | 1.406–3.159 | 0.0003 | 0.731 | 0.585–0.912 | 0.006 |
| Ki-67 ≥90% | 1.978 | 1.363–4.083 | 0.0024 | 0.673 | 0.406–1.116 | 0.125 |
| BK+ | 3.091 | 2.033–10.27 | 0.0003 | 2.351 | 1.108–4.988 | 0.026 |
Prognosis of proliferation index
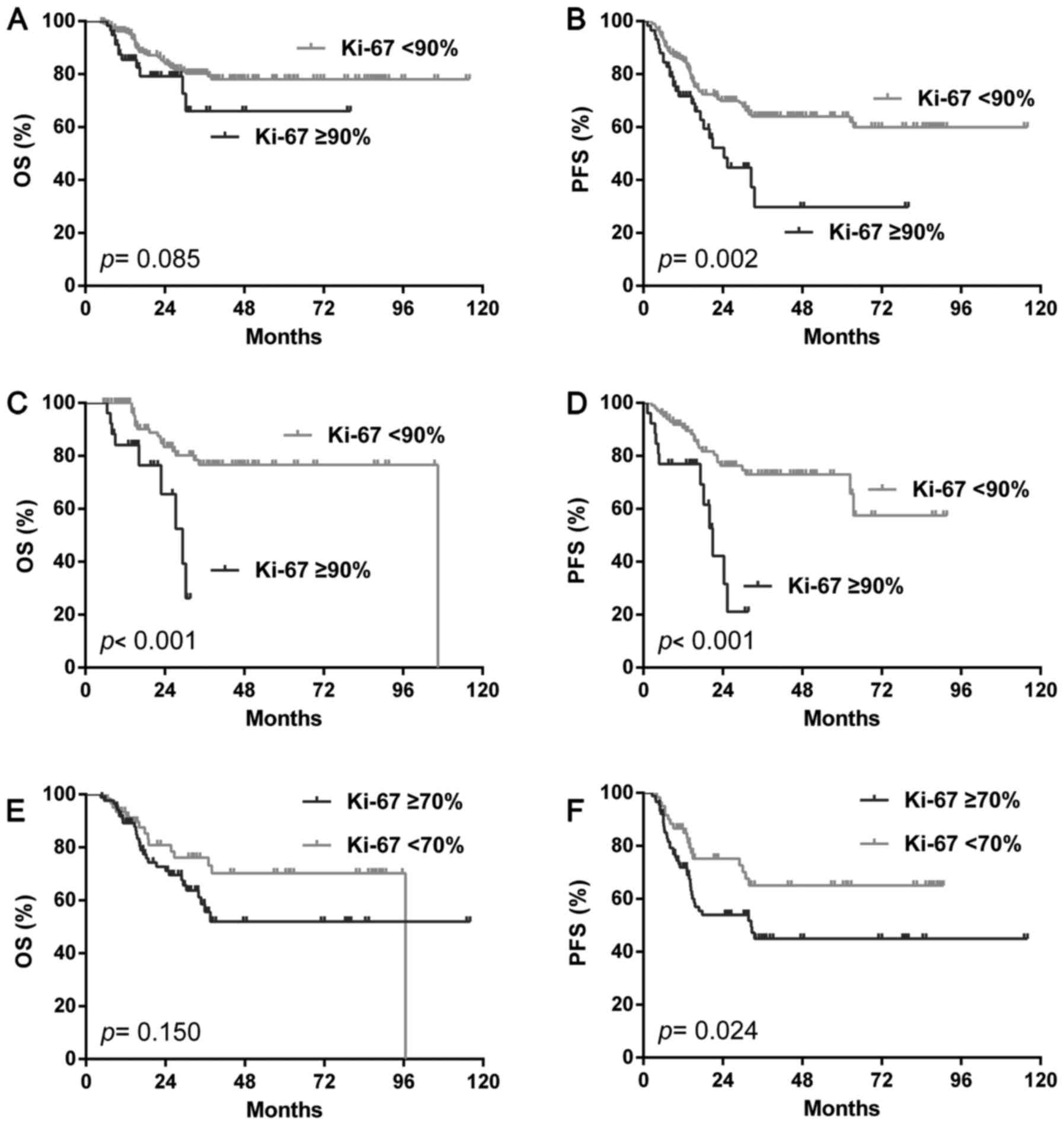
We then analyzed the prognostic value of the Ki-67
index, the incidence of Ki-67 proliferation by different cut offs
were illustrated in Table I. In the
total cohort, the Ki-67 index only showed shorter PFS (P=0.002) but
not OS (P=0.085) by the cut off of 90% (Fig. 3A, B), none of the other cut offs
showed a different outcome with both OS and PFS (data not show). In
the GCB group, the Ki-67 index predicted both poorer OS
(P<0.001) and PFS (P<0.001) by the cu toff of 90% alone
(Fig. 3C, D). In the NGC group, only
the cut off of 70% showed a shorter PFS (P=0.024) but not OS
(P=0.150) (Fig. 3E, F).
The prognostic value of the BCL2/Ki-67
index
Since the Ki-67 index showed a better prognosis
value with the cut off of 90%, we then analyzed the prognosis of
group with BCL2 positivity and high Ki-67 proliferation (≥90%)
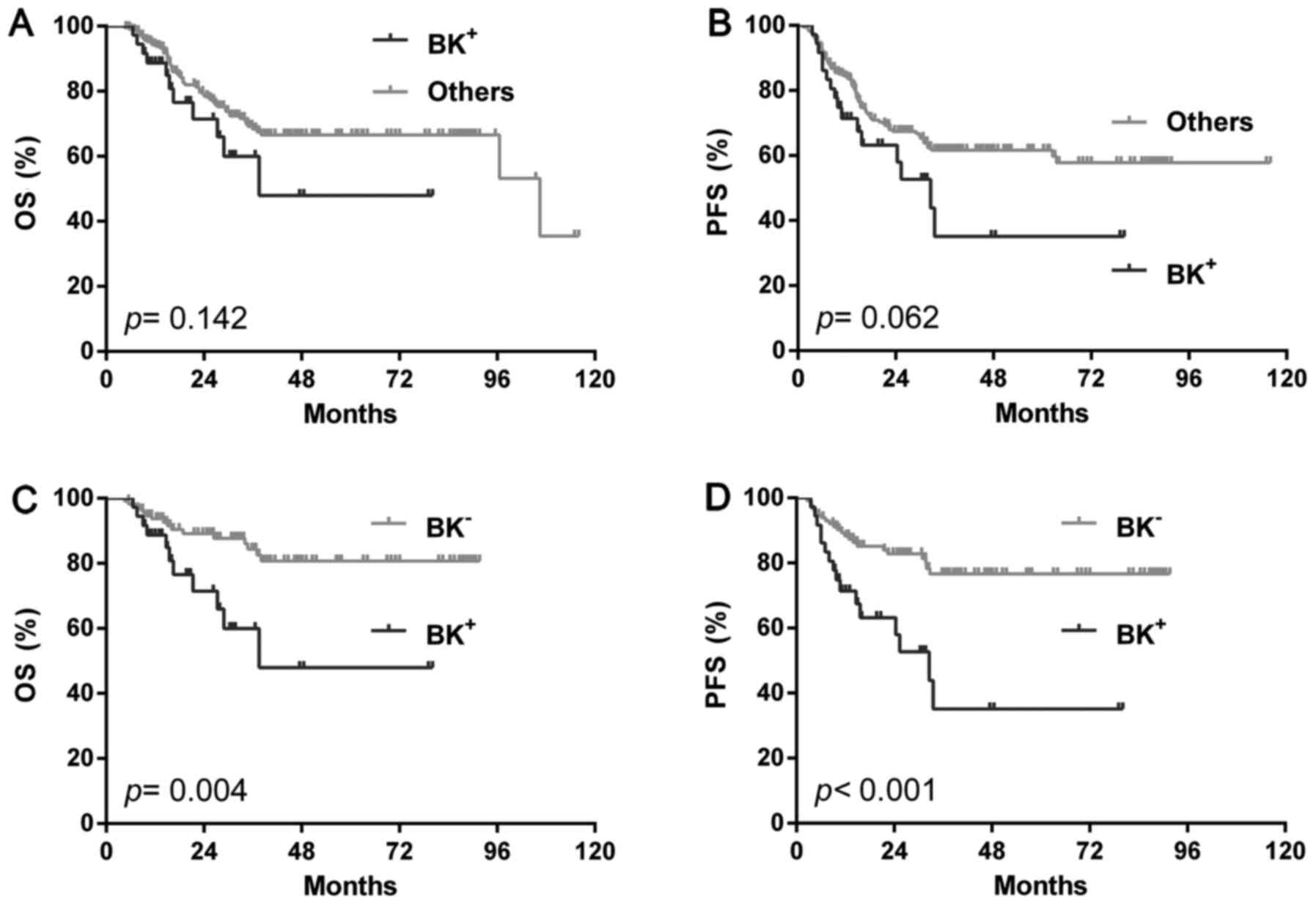
(BK+). In the total cohort, BK+ patients showed a similar OS
(P=0.142) and PFS (P=0.062) with the rest cases (single positive or
double negative) (Fig. 4A and B) or
single BCL2 positivity (OS: P=0.541, PFS: P=0.606) or high Ki-67
(OS: P=0.128, PFS: P=0.299). However, BK+ showed significantly
shorter OS (P=0.004) and PFS (P<0.001) than double negative ones
(BK-) (Fig. 4C and D). In the GCB
group, just like the total group, BK+ patients had similar OS
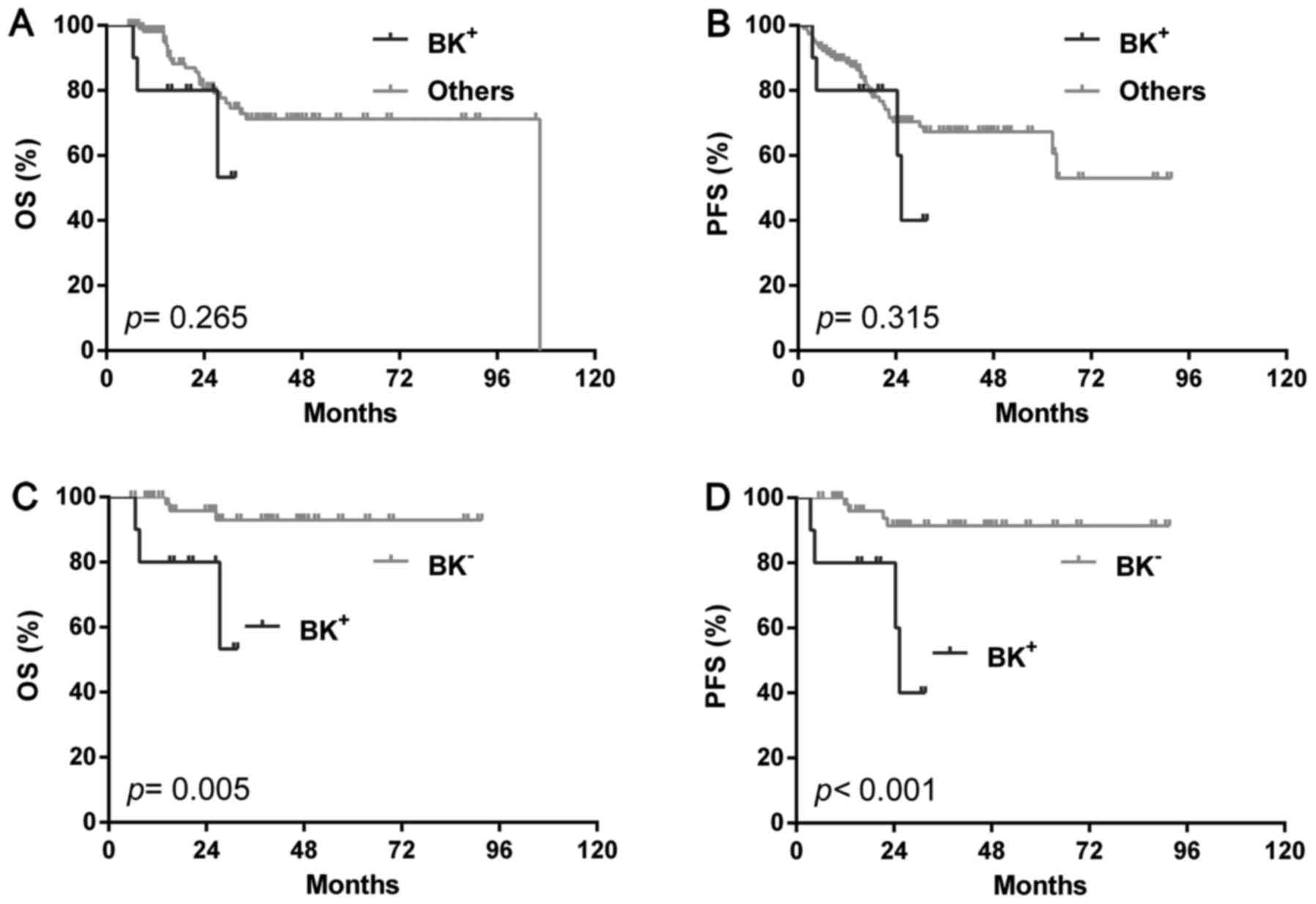
(P=0.265) and PFS (P=0.315) with the rest cases (Fig. 5A and B) or single BCL2 positivity (OS:
P=0.810, PFS: P=0.943) or high Ki-67 (OS: P=0.353, PFS: P=0.135),
but showed significantly poorer OS (P=0.005) and PFS (P<0.001)
than BK-ones (Fig. 5C and D). In the
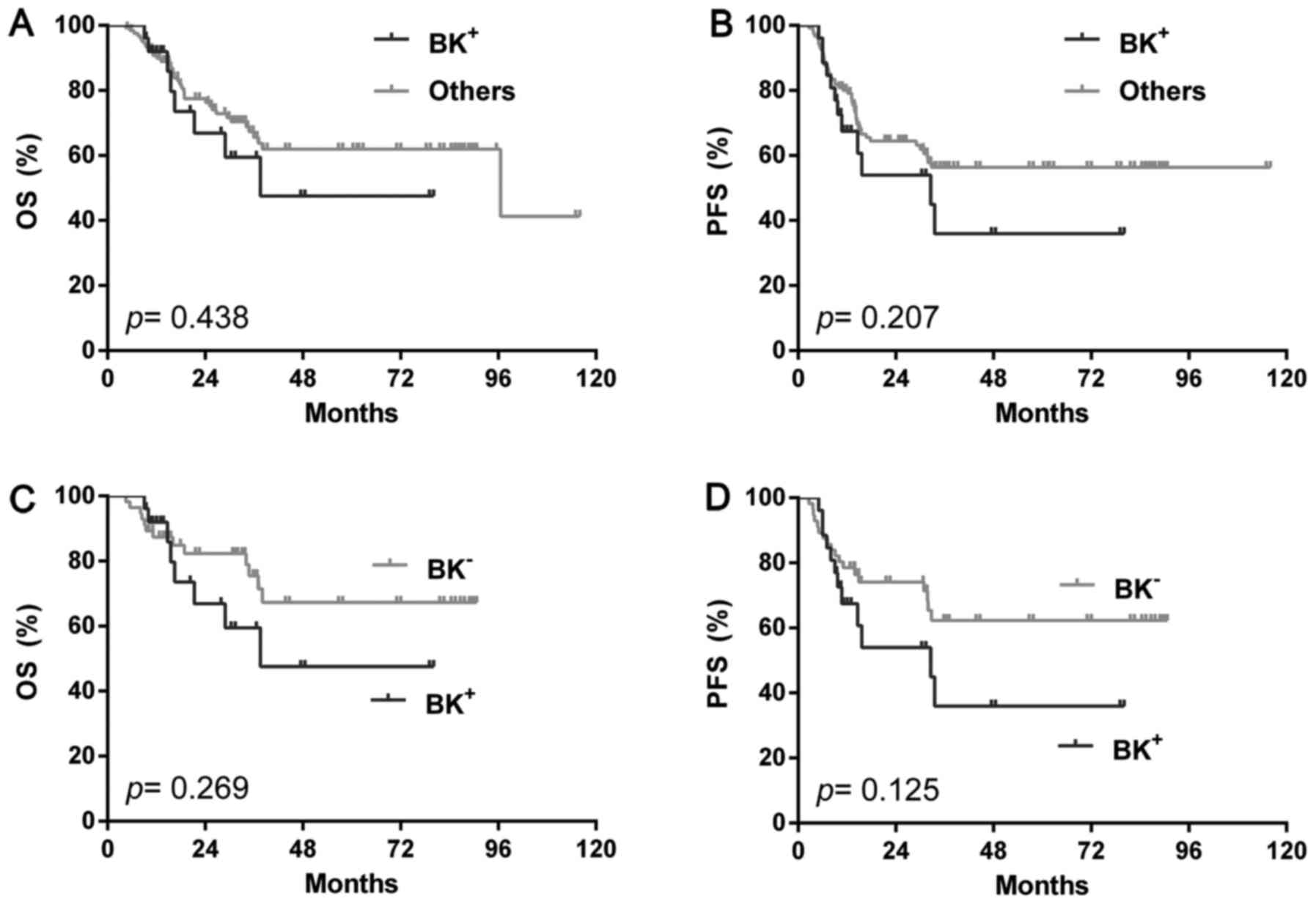
NGC group, however, BK+ showed similar outcome with either BK- (OS:
P=0.269; PFS: P=0.125) (Fig. 6A and
B) or the rest (OS: P=0.438; PFS: P=0.207) (Fig. 6C and D) or single BCL2 positivity (OS:
P=0.549, PFS: P=0.394) or high Ki-67 (OS: P=0.502, PFS: P=0.823).
Multivariate analysis by Cox proportional hazards regression,
accounting for BCL2 and Ki-67 index, demonstrated that the poor
prognostic effect of BK+ remained significant after adjusting for
the presence of the additional high risk features of extranodal
involvement ≥2, Elevated LDH level, Stage III and IV, high IPI
risk, B symptom and poor performance state (P=0.026) (Tables II and III).
Discussion
In the present study, we set out to evaluate the
prognostic value of combining Ki-67 and BCL2 as an index which
would be superior to the evaluation of the markers separately.
Ki-67 is an immunohistochemical marker of
proliferating cells. Recent studies suggested that MYC and BCL2
protein co-expression is an independent indicator of poor prognosis
in diffuse large B-cell lymphoma (19,20). Since
MYC positive DLBCL usually manifest a high proliferation rate, some
research suggested the proliferation fraction criterion to ≥90%
improved the specificity for detection MYC+
double/triple translocations, which means Ki-67 ≥90% might predict
poor outcome (21). Therefore,
further investigation is necessary to clearly delineate the
relationship between Ki-67 expression and prognosis in DLBCL. In
our study, we confirmed the prognostic value of high Ki-67 index
(≥90%) in GCB-DLBCL, which was in accordance with the prognosis of
MYC gene rearrangement (22).
BCL2 is an anti-apoptotic protein which also has an
antiproliferative effect influencing cell-cycle entry and is a
powerful prognostic marker before rituximab. Studies showed that
the addition of rituximab have eliminated the negative impact of
the BCL2 expression (23,24). However, the prognostic value of BCL2
protein in DLBCL was still controversial (14,15). In
our study, we showed BCL2 protein expression has a significant
impact on OS and PFS in GCB-DLBCL, but not in NGC-DLBCL in the
R-CHOP cohort, which was in accord with previous research (14).
Sustaining proliferative signaling and resisting
cell death were two of the ten hallmarks of cancer, which play
important role in cancer progression (25). In breast cancer, a BCL2/Ki-67 index
based on IHC was highly prognostic in ER-positive patients.
However, the prognostic value of BCL2/Ki-67 index has barely been
investigated in DLBCL. With this purpose, we combined the high
Ki-67 index (≥90%) and BCL2 positive patients together, named ‘BK’.
We showed BK+ group had significantly poor outcome than
BK− group. Stratification analysis showed GCB-DLBCL but
not NGC-DLBCL retained the prognostic value of BK+. In
multivariate analysis by Cox proportional hazards regression,
BK+ remained significantly prognostic factor of PFS in
DLBCL. Since DLBCL is a heterogeneous entity, IHC test alone may
not obtain the complete picture of this disease, hematologist need
more effective indicators to tailor therapy. Nevertheless, the
BCL2/Ki-67 index is a simple and convenient method to figure. out
the patients' outcome of DLBCL, which would be a potential
complement of current genetic heterogeneity.
Although most DLBCL patients are cured with 6–8
cycles of R-CHOP chemotherapy, about 10–15% ones have primary
refractory disease and a further 20–30% relapse. There is an urgent
need to improve outcome for these patients. In the precision
medicine era, target CD20 alone might not be enough. Studies also
suggest improvement in outcome on the use of rituximab with CHOP in
ABC-DLBCL and the BCL2 negative subset of GCB-DLBCL. However, the
BCL2 positive GCB-DLBCL has shown less improvement, and these cases
may benefit from novel agents such as inhibitors of BCL2 function
(14). Targeted inhibition of BCL2
with its highly selective inhibitor ABT-199 recently emerged as a
promising treatment strategy for some B-cell malignancies such as
CLL and MCL (26,27). ABT-199 was proved potentially
effective in BCL2 positive DLBCL (28). In GCB-DLBCL with BCL2 positive might
benefit from ABT-199 or other inhibitors of BCL2 function. Besides,
to inhibition of MYC expression via BRD4 inhibitor, such as JQ1,
might indirectly be another way to target high Ki-67 proliferation
DLBCL (29).
In conclusion, we have described a method for the
combinatorial assessment of BCL2 and Ki-67 as measured by IHC. The
BCL2/Ki-67 index was a highly effective predictor of patients'
outcome with DLBCL, especially in the GCB-DLBCL group. In
multivariate analysis, BK+ remained significantly
prognostic factor of PFS in DLBCL. In the precision medicine era,
targeting BK+ therapies might be potentially promising
ways to improve patients' outcome.
References
|
1
|
Moskowitz C: Diffuse large B cell
lymphoma: How can we cure more patients in 2012? Best Pract Res
Clin Haematol. 25:41–47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pileri SA, Agostinelli C, Sabattini E,
Bacci F, Sagramoso C, Pileri A Jr, Falini B and Piccaluga PP:
Lymphoma classification: The quiet after the storm. Semin Diagn
Pathol. 28:113–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abid MB, Nasim F, Anwar K and Pervez S:
Diffuse large B cell lymphoma (DLBCL) in Pakistan: An emerging
epidemic? Asian Pac J Cancer Prev. 6:531–534. 2005.PubMed/NCBI
|
|
4
|
Song CG, Huang JJ, Li YJ, Xia Y, Wang Y,
Bi XW, Jiang WQ, Huang HQ, Lin TY and Li ZM: Epstein-barr
virus-positive diffuse large B-cell lymphoma in the elderly: A
matched case-control analysis. PLoS One. 10:e01339732015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harris NL, Jaffe ES, Diebold J, Flandrin
G, Muller-Hermelink HK and Vardiman J: Lymphoma classification-from
controversy to consensus: The R.E.A.L. and WHO Classification of
lymphoid neoplasms. Ann Oncol. 11:(Suppl 1). S3–S10. 2000.
View Article : Google Scholar
|
|
6
|
Coiffier B: Rituximab therapy in malignant
lymphoma. Oncogene. 26:3603–3613. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sehn LH, Berry B, Chhanabhai M, Fitzgerald
C, Gill K, Hoskins P, Klasa R, Savage KJ, Shenkier T, Sutherland J,
et al: The revised International Prognostic Index (R-IPI) is a
better predictor of outcome than the standard IPI for patients with
diffuse large B-cell lymphoma treated with R-CHOP. Blood.
109:1857–1861. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He X, Chen Z, Fu T, Jin X, Yu T, Liang Y,
Zhao X and Huang L: Ki-67 is a valuable prognostic predictor of
lymphoma but its utility varies in lymphoma subtypes: Evidence from
a systematic meta-analysis. BMC Cancer. 14:1532014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song MK, Chung JS, Lee JJ, Yang DH, Kim
IS, Shin DH and Shin HJ: High Ki-67 expression in involved bone
marrow predicts worse clinical outcome in diffuse large B cell
lymphoma patients treated with R-CHOP therapy. Int J Hematol.
101:140–147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li ZM, Huang JJ, Xia Y, Zhu YJ, Zhao W,
Wei WX, Jiang WQ, Lin TY, Huang HQ and Guan ZZ: High Ki-67
expression in diffuse large B-cell lymphoma patients with
non-germinal center subtype indicates limited survival benefit from
R-CHOP therapy. Eur J Haematol. 88:510–517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hasselblom S, Ridell B, Sigurdardottir M,
Hansson U, Nilsson-Ehle H and Andersson PO: Low rather than high
Ki-67 protein expression is an adverse prognostic factor in diffuse
large B-cell lymphoma. Leuk Lymphoma. 49:1501–1509. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jerkeman M, Anderson H, Dictor M, Kvaløy
S, Akerman M and Cavallin-Ståhl E: Nordic Lymphoma Group study:
Assessment of biological prognostic factors provides clinically
relevant information in patients with diffuse large B-cell
lymphoma-a Nordic Lymphoma Group study. Ann Hematol. 83:414–419.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ali HR, Dawson SJ, Blows FM, Provenzano E,
Leung S, Nielsen T, Pharoah PD and Caldas C: A Ki67/BCL2 index
based on immunohistochemistry is highly prognostic in ER-positive
breast cancer. J Pathol. 226:97–107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Iqbal J, Meyer PN, Smith LM, Johnson NA,
Vose JM, Greiner TC, Connors JM, Staudt LM, Rimsza L, Jaffe E, et
al: BCL2 predicts survival in germinal center B-cell-like diffuse
large B-cell lymphoma treated with CHOP-like therapy and rituximab.
Clin Cancer Res. 17:7785–7795. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iqbal J, Neppalli VT, Wright G, Dave BJ,
Horsman DE, Rosenwald A, Lynch J, Hans CP, Weisenburger DD, Greiner
TC, et al: BCL2 expression is a prognostic marker for the activated
B-cell-like type of diffuse large B-cell lymphoma. J Clin Oncol.
24:961–968. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pfreundschuh M, Ho AD, Cavallin-Stahl E,
Wolf M, Pettengell R, Vasova I, Belch A, Walewski J, Zinzani PL,
Mingrone W, et al: Prognostic significance of maximum tumour (bulk)
diameter in young patients with good-prognosis diffuse large-B-cell
lymphoma treated with CHOP-like chemotherapy with or without
rituximab: An exploratory analysis of the MabThera International
Trial Group (MInT) study. Lancet Oncol. 9:435–444. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hans CP, Weisenburger DD, Greiner TC,
Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E,
Braziel RM, Jaffe ES, et al: Confirmation of the molecular
classification of diffuse large B-cell lymphoma by
immunohistochemistry using a tissue microarray. Blood. 103:275–282.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu S, Xu-Monette ZY, Tzankov A, Green T,
Wu L, Balasubramanyam A, Liu WM, Visco C, Li Y, Miranda RN, et al:
MYC/BCL2 protein coexpression contributes to the inferior survival
of activated B-cell subtype of diffuse large B-cell lymphoma and
demonstrates high-risk gene expression signatures: A report from
The International DLBCL Rituximab-CHOP Consortium Program. Blood.
121:4021–4031. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Johnson NA, Slack GW, Savage KJ, Connors
JM, Ben-Neriah S, Rogic S, Scott DW, Tan KL, Steidl C, Sehn LH, et
al: Concurrent expression of MYC and BCL2 in diffuse large B-cell
lymphoma treated with rituximab plus cyclophosphamide, doxorubicin,
vincristine, and prednisone. J Clin Oncol. 30:3452–3459. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Green TM, Young KH, Visco C, Xu-Monette
ZY, Orazi A, Go RS, Nielsen O, Gadeberg OV, Mourits-Andersen T,
Frederiksen M, et al: Immunohistochemical double-hit score is a
strong predictor of outcome in patients with diffuse large B-cell
lymphoma treated with rituximab plus cyclophosphamide, doxorubicin,
vincristine, and prednisone. J Clin Oncol. 30:3460–3467. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mationg-Kalaw E, Tan LH, Tay K, Lim ST,
Tang T, Lee YY and Tan SY: Does the proliferation fraction help
identify mature B cell lymphomas with double- and triple-hit
translocations? Histopathology. 61:1214–1218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Akyurek N, Uner A, Benekli M and Barista
I: Prognostic significance of MYC, BCL2, and BCL6 rearrangements in
patients with diffuse large B-cell lymphoma treated with
cyclophosphamide, doxorubicin, vincristine, and prednisone plus
rituximab. Cancer. 118:4173–4183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wilson KS, Sehn LH, Berry B, Chhanabhai M,
Fitzgerald CA, Gill KK, Klasa R, Skinnider B, Sutherland J, Connors
JM and Gascoyne RD: CHOP-R therapy overcomes the adverse prognostic
influence of BCL-2 expression in diffuse large B-cell lymphoma.
Leuk Lymphoma. 48:1102–1109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mounier N, Briere J, Gisselbrecht C, Emile
JF, Lederlin P, Sebban C, Berger F, Bosly A, Morel P, Tilly H, et
al: Rituximab plus CHOP (R-CHOP) overcomes bcl-2-associated
resistance to chemotherapy in elderly patients with diffuse large
B-cell lymphoma (DLBCL). Blood. 101:4279–4284. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Montraveta A, Xargay-Torrent S, Rosich L,
López-Guerra M, Roldán J, Rodríguez V, Lee-Vergés E, de Frías M,
Campàs C, Campo E, et al: Bcl-2high mantle cell lymphoma cells are
sensitized to acadesine with ABT-199. Oncotarget. 6:21159–21172.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cervantes-Gomez F, Lamothe B, Woyach JA,
Wierda WG, Keating MJ, Balakrishnan K and Gandhi V: Pharmacological
and protein profiling suggests venetoclax (ABT-199) as optimal
partner with ibrutinib in chronic lymphocytic Leukemia. Clin Cancer
Res. 21:3705–3715. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Klanova M, Andera L, Brazina J, Svadlenka
J, Benesova S, Soukup J, Prukova D, Vejmelkova D, Jaksa R, Helman
K, et al: Targeting of BCL2 family proteins with ABT-199 and
homoharringtonine reveals BCL2- and MCL1-Dependent subgroups of
diffuse large B-cell lymphoma. Clin Cancer Res. 22:1138–1149. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shao Q, Kannan A, Lin Z, Stack BC Jr, Suen
JY and Gao L: BET protein inhibitor JQ1 attenuates Myc-amplified
MCC tumor growth in vivo. Cancer Res. 74:7090–7102. 2014.
View Article : Google Scholar : PubMed/NCBI
|