Introduction
In patients with diffuse large B-cell lymphoma
(DLBCL), rituximab-containing chemotherapy regimens can achieve
superior long-term progression-free survival (PFS) and overall
survival (OS) rates relative to regimens that do not contain
rituximab. However, even in the rituximab era, the survival rates
of patients classified as high-intermediate risk or high risk
according to the International Prognostic Index (IPI) (1) remain unsatisfactory (2,3).
Consequently, several randomized control trials (RCTs) (4–8) have
prospectively evaluated the role of upfront autologous stem cell
transplantation (ASCT) following therapy with rituximab plus
cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP)
for high-risk DLBCL. Upfront ASCT was performed as a consolidation
treatment, and part of first-line treatment with induction
chemotherapy. However, although certain studies have reported good
outcomes following treatment with upfront high-dose chemotherapy
(HDT)/ASCT, its usefulness has not been confirmed in RCTs (4–7). Thus, its
significance in the upfront setting remains to be elucidated, and
the majority of guidelines recommend HDT/ASCT as salvage therapy.
However, in actual clinical practice, there is a dilemma regarding
the timing of ASCT in high-risk DLBCL patients who have achieved a
complete response (CR) or partial response (PR) following induction
therapy: It is unclear whether these patients should undergo
upfront HDT/ASCT, or wait until they relapse and subsequently
undergo salvage HDT/ASCT.
The aims of the present retrospective study were to
evaluate the treatment and outcomes of high-risk DLBCL patients who
had received HDT/ASCT, and to identify the clinical factors that
define the patients who achieve improved outcomes following upfront
HDT/ASCT.
Materials and methods
Patients
DLBCL patients diagnosed between January 2006 and
December 2013 at Kansai Medical University Hospital (Hirakata,
Japan) were selected from the hospital database. Overall, the
clinical data of 278 patients were collected. Among them, 66
patients were excluded as they were aged ≥75 years, and were
ineligible for HDT/ASCT. From the beginning, primary central
nervous system lymphoma was not included as its regimen differed
from the standard R-CHOP regimen. Overall, 212 patients aged <75
years were analyzed. Risk category was identified according to the
IPI at initial diagnosis. The age-adjusted (aa) IPI was used for
patients aged <60 years. High-risk patients included those with
high-intermediate-risk or high-risk tumors, while low-risk patients
include those with low-risk and low-intermediate-risk tumors
according to the aaIPI/IPI. Patients who underwent HDT/ASCT were
those who were aged <75 years in the high-risk group, with good
performance status and no severe organ dysfunction. Eligible
patients were almost all recommended to undergo HDT/ASCT at the
beginning of therapy; however, certain patients refused treatment
due to family or economic issues and other factors. Ineligible
patients were those with an Eastern Cooperative Oncology Group
performance status of 3 or 4, concomitant disease, previous
malignancy or major organ dysfunction. Upfront HDT/ASCT was
performed for patients who achieved a first CR or PR following
R-CHOP. High-risk patients who did not undergo HDT/ASCT received
4–8 cycles of R-CHOP. Low-risk patients received R-CHOP or R-CHOP
in combination with radiotherapy, in accordance with the
recommended guidelines. Patients with stable disease or progressive
disease following R-CHOP, or who exhibited relapse, underwent
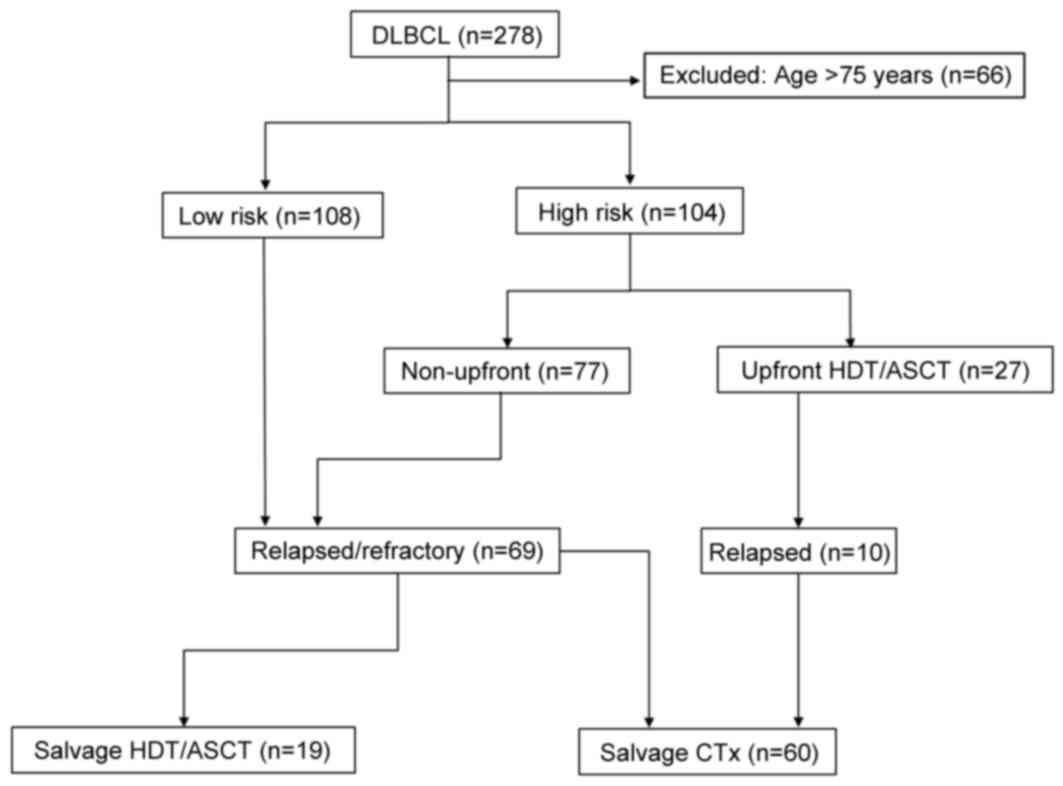
salvage therapy, including salvage HDT/ASCT (Fig. 1).
The present study was approved by the Institutional
Review Board of Kansai Medical University (Hirakata, Japan).
According to the Ethical Guidelines for Medical and Health Research
Involving Human Subjects by the Ministry of Health, Labor and
Welfare (9), the present study was a
retrospective study and did not require informed consent from
individual patients. However, the data of the present study was
made available through the website (10) and opportunities were established for
the patients to refuse.
Staging and response criteria
Clinical staging was performed using positron
emission tomography or computed tomography scanning of the neck,
thorax, abdomen and pelvis; bone marrow biopsy; cerebrospinal fluid
examination; and other tools, such as magnetic resonance imaging,
if indicated. The criteria for evaluating the efficacy of treatment
have been described by Cheson et al (11).
Induction therapy
The induction therapy regimen consisted of R-CHOP
(375 mg rituximab/m2 intravenously (i.v), plus 750 mg
cyclophosphamide/m2 i.v, 50 mg doxorubicin/m2
i.v and 2 mg vincristine/m2 i.v on day 1, and 100 mg
prednisone/m2 orally on days 1 through 5) or R-CHOP-like
regimens (for patients with poor cardiac function, doxorubicin may
be removed or replaced with pinorubicin). In the upfront setting,
the treatment response was evaluated after 4–5 cycles of R-CHOP,
and peripheral blood stem cell collection was performed using an
additional cycle of R-CHOP plus etoposide as a harvest regimen (an
established regimen for mobilizing hematopoietic stem cells into
the peripheral blood). In the non-upfront setting, patients
underwent 4–8 cycles of R-CHOP. Patients with refractory or
relapsed disease were treated with salvage regimens consisting of
rituximab, etoposide, ifosfamide and dexamethasone (R-DeVIC), or
rituximab, etoposide, methylprednisolone, cytarabine and cisplatin
(R-ESHAP).
HDT
The day of transplantation was set at day 0, and the
conditioning regimen was initiated counting back prior to
transplantation day. Patients undergoing HDT/ASCT received the
following conditioning regimen: Ranimustine (also known as MCNU),
300 mg/m2 on day −6 (6 days before ASCT); etoposide, 200
mg/m2 on days −5 to −2; cytarabine, 200 mg/m2
on days −5 to −2; and melphalan, 140 mg/m2 on day −1.
This regimen (MEAM) is a modified BEAM regimen in which carmustine
(BCNU) is replaced with MCNU due to a lack of accessibility of the
former in Japan. MEAM is one of the most frequently used
conditioning regimens for the treatment of lymphoid malignancies in
Japan (12,13).
Supportive care
Bacterial, fungal, herpes simplex virus and
pneumocystis pneumonitis prophylaxes were administered to all of
the patients in accordance with the guidelines (14,15). In
patients undergoing HDT/ASCT, granulocyte colony-stimulating factor
was administered intravenously from day +1 until neutrophil
recovery.
Statistical analysis
OS was measured from the time of ASCT or the final
chemotherapy until the time of mortality from any cause or the
final date of observation. PFS was measured from the time of ASCT
or the final chemotherapy until the time of disease relapse or
progression, mortality from any cause, or the final date of
observation. Survival estimates were calculated using the
Kaplan-Meier method, and the log-rank test was used for univariate
comparisons. To identify the clinical factors that defined the
high-risk DLBCL patients who had an improved outcome from upfront
HDT/ASCT, univariate analysis and the log-rank test were used. All
P-values were two-sided, with P<0.05 considered to indicate a
statistically significant difference.
All statistical analyses were performed using EZR
(Saitama Medical Center, Jichi Medical University, Saitama, Japan),
a graphical user interface for R (version 2.13.0; The R Foundation
for Statistical Computing, Australia); EZR is a modified version of
R commander (version 1.6–3) designed to include the statistical
functions frequently used in biostatistics (16).
Results
Patient characteristics
A total of 212 patients were divided into low-risk
(n=108) or high-risk groups (n=104) according to the aaIPI/IPI.
Among the high-risk patients, 27 underwent upfront HDT/ASCT and 77
received conventional chemotherapy. Of the 212 patients, 79
experienced relapse; there were 33 low-risk patients, 10 patients
treated in an upfront setting involving HDT/ASCT and 36 patients
treated in a non-upfront setting. Among the 79 relapsed patients,
60 patients (including 10 who had relapsed subsequent to upfront
HDT/ASCT) received salvage chemotherapy, and 19 received HDT/ASCT
as salvage therapy. These patients treated with HDT/ASCT received
3–4 cycles of the salvage regimens following relapse; 16 of these
patients achieved a second CR, 1 patient achieved a PR, and 2
patients suffered disease progression (Fig. 1).
Table I shows the
baseline characteristics of the high-risk patients: 27 of these
patients received upfront HDT/ASCT and 77 received conventional
R-CHOP without HDT/ASCT. The median age of the high-risk patients
was 62 years in the upfront setting and 67 years in the non-upfront
setting. In the upfront setting, all patients were categorized as
high-intermediate or high risk according to the IPI, and as
clinical stage III or IV. Elevated lactate dehydrogenase (LDH)
levels were observed more frequently in the upfront setting,
whereas the incidence of extranodal disease was similar to that in
the non-upfront setting.
 | Table I.Characteristics of the high-risk
patients (n=104) who received upfront HDT/ASCT and those who did
not (non-upfront). |
Table I.
Characteristics of the high-risk
patients (n=104) who received upfront HDT/ASCT and those who did
not (non-upfront).
| Characteristic | Upfront | Non-upfront |
|---|
| Total patients
(n) | 27 | 77 |
| Age (years) |
|
|
|
Median | 62 | 67 |
|
Range | 36–72 | 20–75 |
| Sex (%) |
|
|
| Male | 56 | 65 |
|
Female | 44 | 35 |
| IPI/aaIPI (%) |
|
|
|
High-intermediate | 37 | 44 |
| High | 63 | 56 |
| Stage (%) |
|
|
| I | 0 | 5 |
| II | 0 | 0 |
| III | 26 | 26 |
| IV | 74 | 69 |
| Bulky mass (%) | 7 | 4 |
| Extranodal
involvement |
|
|
| at >1 site
(%) | 56 | 58 |
| Elevated LDH level
(%) | 81 | 68 |
| HDT/ASCT as salvage
(%) | 0 | 9 |
Table II shows the
baseline characteristics of the 79 relapsed patients. A total of 19
patients received salvage HDT/ASCT and 60 received salvage
chemotherapy. Patient median age was 58 years in the salvage
HDT/ASCT setting, which was lower than that in the salvage
chemotherapy group, which is the group of patients whom relapsed
and resisted the initial treatment and received chemotherapy
without HDT/ASCT. Factors reflecting tumor burden, namely bulky
mass, extranodal DLBCL and elevated LDH, were observed more often
in the salvage chemotherapy patient group than in the salvage ASCT
patient group.
 | Table II.Characteristics of the relapsed
patients (n=79). |
Table II.
Characteristics of the relapsed
patients (n=79).
| Characteristic | Salvage HDT/ASCT | Salvage
chemotherapy |
|---|
| Total patients
(n) | 19 | 60 |
| Age (years) |
|
|
|
Median | 58 | 67 |
|
Range | 36–65 | 37–75 |
| Sex (%) |
|
|
| Male | 74 | 55 |
|
Female | 26 | 45 |
| IPI/aaIPI (%) |
|
|
| Low | 32 | 22 |
|
Low-intermediate | 37 | 17 |
|
High-intermediate | 0 | 33 |
| High | 31 | 28 |
| Stage (%) |
|
|
| I | 11 | 13 |
| II | 32 | 7 |
| III | 37 | 20 |
| IV | 20 | 60 |
| Bulky mass (%) | 0 | 3 |
| Extranodal
involvement |
|
|
| at >1 site
(%) | 21 | 63 |
| Elevated LDH level
(%) | 21 | 63 |
Treatment efficacy and prognosis
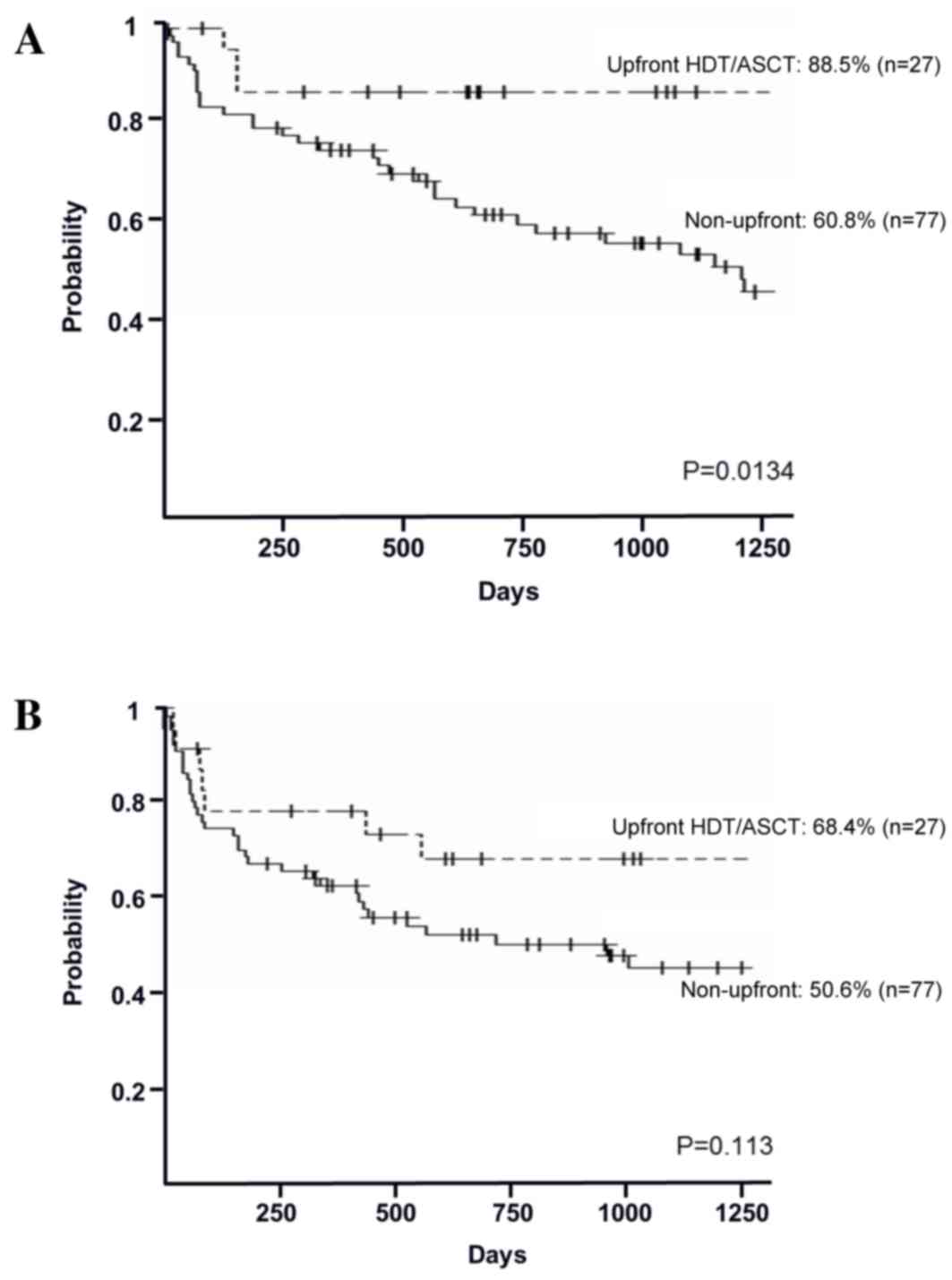
In the high-risk group, the 3-year OS rates in the
upfront and non-upfront settings were 88.5% [95% confidence
interval (CI), 68.4–96.1%] and 60.8% (95% CI, 48.3–71.2%),
respectively (P=0.0134; Fig. 2A). The
3-year PFS rates in the upfront and non-upfront settings were 68.4%
(95% CI, 46.4–82.9%) and 50.6% (95% CI, 38.3–61.6%), respectively
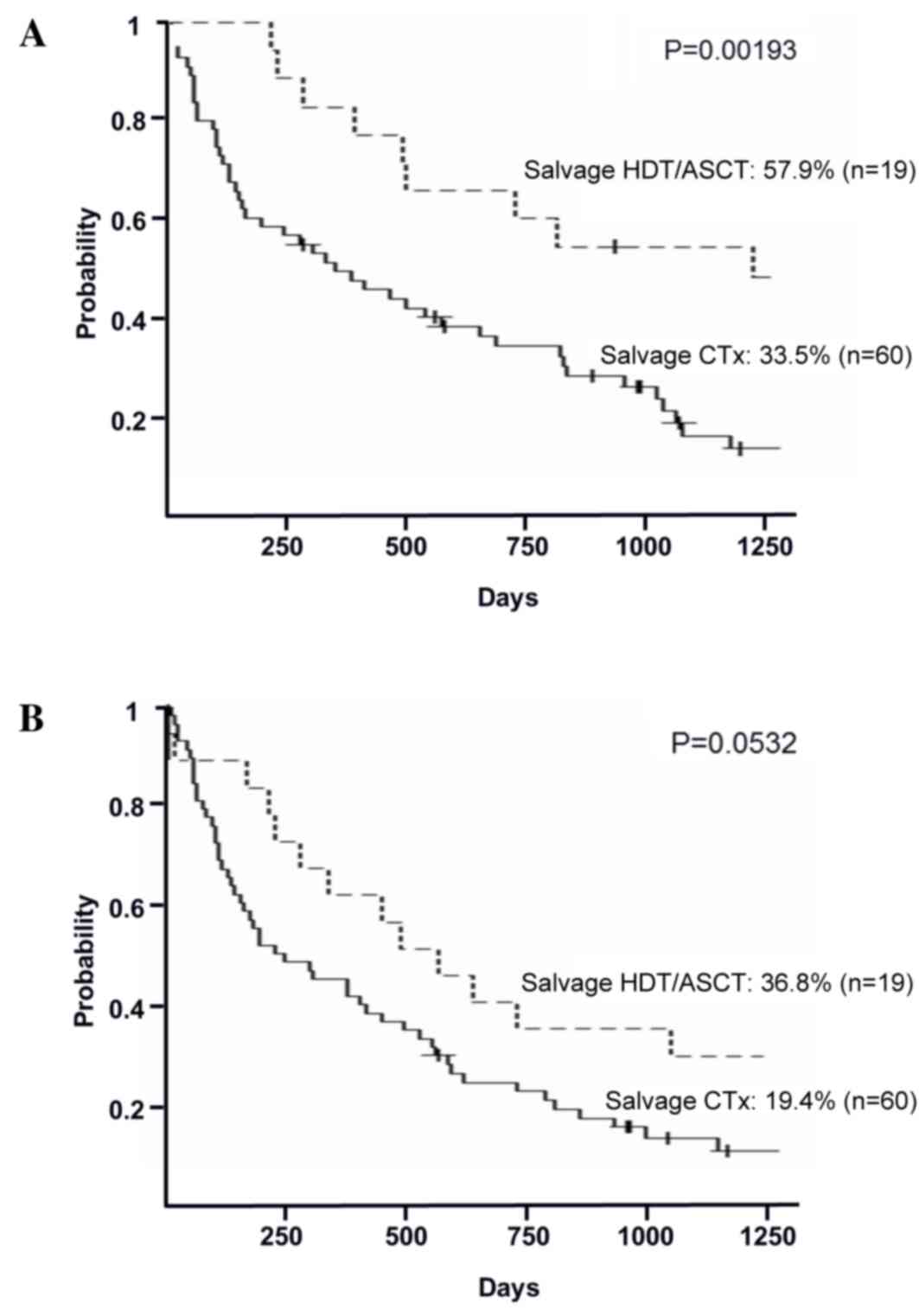
(P=0.113; Fig. 2B). In the relapsed
group, the 3-year OS rates in the salvage HDT/ASCT and the salvage
chemotherapy groups were 57.9% (95% CI, 33.2–76.3%) and 33.5% (95%
CI, 21.7–45.7%), respectively (P=0.00193; Fig. 3A). The 3-year PFS rates in the salvage
HDT/ASCT and the salvage chemotherapy groups were 36.8% (95% CI,
16.5–57.5%) and 19.4% (95% CI, 10.4–30.3%), respectively (P=0.0532;
Fig. 3B).
High-dose regimen-related toxicity and
treatment-related mortality
Common hematological regimen-related toxicities
(RRTs), comprising neutropenia, anemia and thrombocytopenia at
grades 3 and 4, were observed in all patients. The most common
non-hematological RRTs were anorexia and nausea at grades 3 and 4,
which were noted in all patients. Diarrhea and stomatitis were also
commonly observed (80–90% of patients). Mucosal damage was
relatively severe in the salvage HDT/ASCT group. Grade 2 rash was
encountered in both upfront and salvage HDT/ASCT groups. Febrile
neutropenia (FN) was observed in 96% of patients in the upfront
setting and 55% in the salvage setting. In the salvage setting, 1
patient succumbed to FN that occurred prior to engraftment. Another
notable complication was interstitial pneumonia: In the salvage
group, 1 patient died as a result of rapid onset of interstitial
pneumonia. The overall rate of treatment-related mortality (TRM)
was 4.3%.
Univariate analysis
To identify the clinical factors that define
high-risk DLBCL patients who can achieve improved outcomes from
upfront HDT/ASCT, a univariate analysis for 3-year OS rate was
performed. There were no statistically significant prognostic
factors identified in the upfront setting; a hemoglobin level
<11 g/dl was associated with a lower OS rate, without
statistical significance in this setting (P=0.074). A high LDH
level revealed a significant association with survival rate in the
non-upfront setting (P=0.024). Although there were no statistically
differences observed, patients aged >65 years and those with
extranodal disease exhibited poorer OS rates in the non-upfront
setting (Table III).
 | Table III.Univariate analysis for 3-year OS rate
in high-risk patients. |
Table III.
Univariate analysis for 3-year OS rate
in high-risk patients.
|
| Upfront | Non-upfront |
|---|
|
|
|
|
|---|
| Characteristic | 3-year OS rate
(%) | P-value | 3-year OS rate
(%) | P-value |
|---|
| Age (years) |
| 0.234 |
| 0.393 |
| ≤65 | 83.3 |
| 62.1 |
|
|
>65 | 100.0 |
| 60.2 |
|
| Sex |
| 0.425 |
| 0.735 |
|
Female | 90.9 |
| 65.5 |
|
| Male | 85.7 |
| 58.2 |
|
| Alb (g/dl) |
| 0.386 |
| 0.527 |
|
>3.5 | 88.9 |
| 61.5 |
|
|
≤3.5 | 87.5 |
| 60.6 |
|
| LDH |
| 0.133 |
| 0.024 |
| No | 80.0 |
| 86.2 |
|
|
Yes | 90.5 |
| 50.9 |
|
| Hemoglobin
(g/dl) |
| 0.074 |
| 0.486 |
|
>11 | 94.7 |
| 59.0 |
|
|
≤11 | 71.4 |
| 64.9 |
|
| Extranodal
disease |
| 0.591 |
| 0.668 |
| No | 81.8 |
| 70.6 |
|
|
Yes | 93.3 |
| 57.2 |
|
Discussion
The findings of the present retrospective study
demonstrated the efficacy of upfront HDT/ASCT in patients with
high-risk DLBCL. While certain studies have reported good outcomes
regarding upfront HDT/ASCT (4,6,8), its usefulness has not been confirmed in
RCTs. In terms of the 2-year OS rate, Vitolo et al (4) found no significant differences between
the study arms in which patients were treated with R-CHOP alone or
R-CHOP followed by HDT/ASCT; however, the 2-year PFS rate was
significantly higher in the HDT/ASCT arm (4). Stiff et al (8) reported that in the subset of high-risk
patients alone, induction chemotherapy followed by early HDT/ASCT
significantly improved 2-year PFS and OS rates relative to
chemotherapy alone. Based on this report, as of 2015, the National
Comprehensive Cancer Network Guidelines include HDT/ASCT as one of
the optional therapies following R-CHOP for high-risk patients who
achieved a CR following induction therapy (17). However, at present, most guidelines
recommend HDT/ASCT as salvage therapy (15,17).
In the present study, a significant difference in
the 3-year OS rate was identified between the upfront ASCT setting
and the non-upfront setting: The 3-year OS rate in the upfront
setting was 88.5% (P=0.0134 vs. non-upfront setting), which was
higher than the 3-year OS rate reported in previous studies
(4–8).
However, there was no statistically significant difference in the
3-year PFS rate between the upfront and non-upfront groups,
indicating that the high-risk patients may eventually relapse, even
after upfront HDT/ASCT. The 3-year OS and PFS rates in the
non-upfront setting were 60.8 and 50.6%, respectively, and were
significantly lower than those in the upfront setting (P=0.113 for
PFS rate). These results confirmed that upfront HDT/ASCT for
high-risk DLBCL is a feasible and promising therapy.
As the majority of guidelines recommend HDT/ASCT as
salvage therapy, a comparison between salvage HDT/ASCT and salvage
chemotherapy was performed in the present study. The 3-year OS rate
of the salvage HDT/ASCT group was 57.9% (P=0.00193), which is
unsatisfactory in comparison with the OS rate of the upfront
HDT/ASCT group. The 3-year PFS rate in this setting was 36.8%
(P=0.0532), which revealed that relapse was frequent even after
salvage HDT/ASCT. These results indicate that DLBCL is frequently
resistant to chemotherapy following relapse. The patients in the
salvage HDT/ASCT group were younger on average than those in the
salvage chemotherapy group; this indicates that patients who are
able to undergo salvage HDT/ASCT must be limited due to age or
co-morbidity. Thus, the timing of HDT/ASCT administration is
critical. If patients are eligible, it would be beneficial for them
to undergo HDT/ASCT as an early treatment before they become
therapy-resistant or more elderly with more complications.
In the present study, the common RRTs were anorexia,
nausea, diarrhea, stomatitis and FN. In total, 2 patients died due
to interstitial pneumonia or FN; as these 2 patients were treated
in the salvage setting, potential organ damage caused by prior
chemotherapies may have caused these lethal adverse effects.
However, according to the present results, the TRM rate was low
(4.3%); thus, we suggest that there is no reason to avoid HDT
following ASCT if patients are eligible.
HDT/ASCT may be tolerable and effective; however,
serious adverse effects can occur. Therefore, it is necessary to
identify patient groups that will gain the maximum benefit from
upfront HDT/ASCT. In this regard, the most accurate prognostic
factors must be determined. Using univariate analysis, the present
study did not reveal any significant prognostic factors in the
upfront setting. Notably, patients in the upfront setting with
advanced age (>65 years), elevated LDH levels or extranodal
disease had a better prognosis than younger patients, those with
normal LDH, or those without extranodal disease, respectively; this
suggests that HDT/ASCT may overcome the unfavorable outcomes caused
by these prognostic factors.
The present study had a number of limitations.
First, a small patient cohort was evaluated and the follow-up
period was short. To determine the true efficacy of HDT/ASCT, a
longer follow-up period will be required. Furthermore, biological
features, such as genetic abnormalities or CD5 expression, were not
considered. These factors are expected to be important in the
prediction of survival. In the present study, the 3-year OS and PFS
rates were superior to those previously reported for the following
reasons. First, as our institute is a university hospital, patients
had already been selected before they attended our out-patient
department; consequently, there would have been a selection bias.
Second, it is suspected that the use of the IPI will fail in the
classification of appropriate patients for upfront HDT/ASCT; it has
been reported that the IPI cannot be used to predict the outcome of
a patient group with poor prognosis where the 5-year OS rate is
<60% (3). Thus, the population
must have included patients with a good prognosis who did not
require upfront treatment with HDT/ASCT. Therefore, it is necessary
to establish prognostic factors additional to the IPI that can be
used to identify the patients who will benefit from upfront
HDT/ASCT. In the current study, no significant prognostic factors
associated with upfront HDT/ASCT could be determined. A
sufficiently large study population will be required to provide the
statistical power to adequately assess these factors, and
prospective studies will be required to confirm the efficacy of
upfront HDT/ASCT.
In conclusion, the use of upfront HDT/ASCT in
patients with high-risk DLBCL is feasible and may improve their
outcome. HDT/ASCT should be administered as an early treatment
before patients become therapy-resistant.
References
|
1
|
A predictive model for aggressive
non-Hodgkin's lymphoma. The International Non-Hodgkin's Lymphoma
Prognostic Factors Project. N Engl J Med. 329:987–994. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ziepert M, Hasenclever D, Kuhnt E, Glass
B, Schmitz N, Pfreundschuh M and Loeffler M: Standard international
prognostic index remains a valid predictor of outcome for patients
with aggressive CD20+ B-cell lymphoma in the rituximab era. J Clin
Oncol. 28:2373–2380. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sehn LH, Berry B, Chhanabhai M, Fitzgerald
C, Gill K, Hoskins P, Klasa R, Savage KJ, Shenkier T, Sutherland J,
et al: The revised International Prognostic Index (R-IPI) is a
better predictor of outcome than the standard IPI for patients with
diffuse large B-cell lymphoma treated with R-CHOP. Blood.
109:1857–1861. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vitolo U, Chiappella A, Brusamolino E, et
al: A randomized multicenter phase III study for first line
treatment of young patients with high risk (AAIPI 2–3) diffuse
large B-cell lymphoma (DLBCL): Rituximab (R) plus dose-dense
chemotherapy CHOP14/MEGA CHOP14 with or without intensified
high-dose chemotherapy (HDT) and autologous stem cell
transplantation (ASCT). Results of DLCL04 trial of Italian lymphoma
foundation (FIL). Ann Oncol. 22:1062011.
|
|
5
|
Schmitz N, Nickelsen M, Ziepert M, Haenel
M, Borchmann P, Schmidt C, Viardot A, Bentez N, Peter N, Ehninger
G, et al: Conventional chemoimmunotherapy (R-CHOEP)-14 or high-dose
therapy (R-Mega-CHOEP) for young, high-risk patients with
aggressive B-cell lymphoma: Final results of the randomized
Mega-CHOEP trial of the German High-Grade Non-Hodgkin Lymphoma
Study Group (DSHNHL). J Clin Oncol. 29:80022011. View Article : Google Scholar
|
|
6
|
Stiff PJ, Unger JM, Cook J, Constine S,
Couban S, Shea TC, Winter JN, Miller TP, Tubbs RR, Marcellus DC, et
al: Randomized phase III U.S./Canadian intergroup trial (Swog
S9704) comparing CHOP (+/−) R for eight cycles to CHOP (+/−) R for
six cycles followed by autotransplantation for patients with
high-intermediate (H-int) or high IPI grade diffuse aggressive
non-Hodgkin lymphoma (NHL). J Clin Oncol. 29:80012011. View Article : Google Scholar
|
|
7
|
Le Gouill S, Milpied NJ, Lamy T, Delwail
V, Gressin R, Guyotat D, Damaj GL, Foussard C, Cartron G,
Maisonneuve H, et al: First-line rituximab (R) high-dose therapy
(R-HDT) versus R-CHOP14 for young adults with diffuse large B-Cell
lymphoma: Preliminary results of the GOELAMS 075 prospective
multicenter randomized trial. J Clin Oncol. 29:80032011. View Article : Google Scholar
|
|
8
|
Stiff PJ, Unger JM, Cook JR, Constine LS,
Couban S, Stewart DA, Shea TC, Porcu P, Winter JN, Kahl BS, et al:
Autologous transplantation as consolidation for aggressive
non-Hodgkin's lymphoma. N Engl J Med. 369:1681–1690. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
The Ministry of Health, Labor and Welfare,
. Ethical Guidelines for Medical and Health Research Involving
Human Subjects. http://www.mhlw.go.jp/file/06-Seisakujouhou-10600000-Daijinkanboukouseikagakuka/0000080278.pdf
|
|
10
|
http://www.kmu.ac.jp/hirakata/visit/treatment/medical_section/ketuekishuyounaika.html
|
|
11
|
Cheson BD, Horning SJ, Coiffier B, Shipp
MA, Fisher RI, Connors JM, Lister TA, Vose J, Grillo-López A,
Hagenbeek A, et al: Report of an international workshop to
standardize response criteria for non-Hodgkin's lymphomas. NCI
Sponsored International Working Group. J Clin Oncol. 17:12441999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kameoka Y, Takahashi N, Ishizawa K, Kato
Y, Ito J, Sasaki O, Murai K, Noji H, Hirokawa M, Tajima K, et al:
Safety and feasibility of high-dose ranimustine (MCNU),
carboplatin, etoposide and cyclophosphamide (MCVC) therapy followed
by autologous stem cell transplantation for malignant lymphoma. Int
J Hematol. 96:624–630. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kawabata K, Hagiwara S, Takenouchi A,
Tanimura A, Tanuma J, Tachikawa N, Miwa A and Oka S: Autologous
stem cell transplantation using MEAM regimen for relapsed
AIDS-related lymphoma patients who received highly active
anti-retroviral therapy: A report of three cases. Intern Med.
48:111–114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
NCCN Clinical Practice Guidelines in
Oncology: Prevention and Treatment of Cancer-Related Infections.
Version 1. 2017.https://www.nccn.org/professionals/physician_gls/f_guidelines.asp
|
|
15
|
Japanese Society of Hematology, . clinical
practice guideline. http://www.jshem.or.jp/gui-hemali/table.html
|
|
16
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
NCCN Clinical Practice Guidelines in
Oncology: Non-Hodgkin's lymphomas. Version 2. 2016.https://www.nccn.org/professionals/physician_gls/f_guidelines.asp
|