Introduction
Cell biology and genetic studies indicate that tumor
growth is not just determined by malignant cancer cells themselves,
but also by the tumor stroma (1).
Fibroblasts are non-vascular, non-inflammatory and non-epithelial
cells of the connective tissue, and are the principal cellular
component of the tumor stroma (2).
They are embedded within the fibrillar matrix of the connective
tissue and are, to a large extent, responsible for its synthesis
(2). It is becoming increasingly
evident that fibroblasts are also prominent modifiers of cancer
progression (3,4). There is evidence that a subpopulation of
fibroblasts, termed cancer-associated fibroblasts (CAFs), are
important promoters of tumor growth and progression (5). CAFs may induce epithelial-mesenchymal
transition in epithelial tumor cells, a key factor in the invasion
of squamous cell carcinoma (6).
The role of the tumor stroma on cancer progression
has also been investigated for head and neck squamous cell
carcinoma (HNSCC). HNSCCs are among the most common malignancies
worldwide (7). In the USA, it is
estimated that ~500,000 new cases of HNSCC are diagnosed per year,
equating to an incidence of 14 per 100,000 inhabitants (8). Despite the implementation of multi-modal
treatment strategies, including surgery, radiation and
chemotherapy, the survival rates have not improved significantly
over the past several decades (9).
For HNSCC, radiation is part of the majority of therapeutic
strategies, either as a primary therapy or as adjuvant radiation
following surgery (10). The effects
of radiation on patients are widely known (11–13);
short-term effects mainly comprise damage to the skin and mucosa in
the irradiated region, while long-term effects include xerostomia
and an increased risk of secondary malignancies (14).
However, the effects of a previous irradiation on
the tumor stroma are largely unknown. In vitro, it has been
demonstrated that CAFs exhibit no significant changes in
proliferation or growth when exposed to radiation (15), while another study indicated an
enhanced capability of irradiated fibroblasts to promote survival
of co-cultured cancer cells (16). In
these previous studies, however, the irradiation was delivered
in vitro, and the long-term effects on the irradiated tumor
stroma were not investigated.
Our previous study demonstrated decreased viability
of tumor cells and decreased interleukin (IL)-8 secretion when the
tumor cells were co-cultured with fibroblasts from pre-irradiated
human skin, as compared with skin-derived fibroblasts from
non-irradiated patients (17). This
raises the question of whether an irradiation of the head and neck
during cancer therapy changes the properties of fibroblasts on a
long-term basis, and what these changes consist of.
The primary objective of the present study was to
evaluate the long-term effects of irradiation during therapy for
HNSCC on skin-derived human fibroblasts compared with fibroblasts
from non-irradiated skin, in terms of viability, apoptosis,
necrosis, cell expansion and motility.
Materials and methods
Acquisition and culture of
fibroblasts
Fibroblasts were obtained from skin samples from 20
patients undergoing neck surgery at the University Hospital
Würzburg, Germany, between October 2012 and November 2013. Of the
20 patients, 10 had been treated with intensity-modulated
irradiation with 60–70 Gy for 6 weeks during head and neck cancer
therapy 6–18 months previously (Table
I). The other 10 patients underwent neck surgery for other
reasons than cancer (Table I).
Approval was obtained from the Ethics Committee of the Medical
Faculty, University of Würzburg (approval no. 12/06), and informed
consent was obtained from all patients involved. Tissue preparation
was performed as described in our previous study (17), which included a modification of the
protocol described by Vangipuram et al (18). In summary, the skin samples were
cleared of fat and cut into small pieces of 2–3 mm, which were then
seeded on 6-well plates. After 60 min of culture without medium at
37°C and 5% CO2, the tissue pieces had sufficiently
adhered to the bottom of the plates, such that Dulbecco's modified
Eagle medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with 10% fetal calf serum (Biochrom, Ltd.,
Cambridge, UK), 100 U/ml penicillin and 100 µg/ml streptomycin
[DMEM-expansion medium (DMEM-EM)] could be added without the pieces
being washed away. From these tissue pieces, the fibroblasts grew
out into the periphery. Every other day, the medium was replaced
and passaging was performed when the cells had reached 70–80%
confluence; passaging was performed by trypsinization (0.25%
trypsin; Invitrogen; Thermo Fisher Scientific, Inc.), washing in
PBS and seeding into new flasks or treatment wells.
 | Table I.Data and characteristics of the
patients. |
Table I.
Data and characteristics of the
patients.
| Patient no. | Sex | Age, years | Primary tumor
site/reason for surgery | Radiation dose,
Gy | Concurrent
chemotherapy |
|---|
| 1 | Male | 63 | Hypopharynx | 70 | Yes |
| 2 | Male | 54 | Oropharynx | 66 | No |
| 3 | Male | 59 | Larynx | 60 | No |
| 4 | Female | 72 | Hypopharynx | 69 | Yes |
| 5 | Male | 57 | Larynx | 60 | No |
| 6 | Male | 61 | Hypopharynx | 69 | Yes |
| 7 | Female | 61 | Oropharynx | 69 | Yes |
| 8 | Female | 70 | Larynx | 60 | No |
| 9 | Male | 59 | Oropharynx | 69 | Yes |
| 10 | Male | 65 | Hypopharynx | 66 | No |
| 11 | Female | 46 | Parotidectomy | n/a | n/a |
| 12 | Female | 56 | Cervical cyst | n/a | n/a |
| 13 | Male | 39 | Cervical cyst | n/a | n/a |
| 14 | Female | 65 | Parotidectomy | n/a | n/a |
| 15 | Male | 72 | Cervical cyst | n/a | n/a |
| 16 | Male | 81 | Cervical cyst | n/a | n/a |
| 17 | Male | 59 | Parotidectomy | n/a | n/a |
| 18 | Female | 66 | Cervical cyst | n/a | n/a |
| 19 | Male | 69 | Parotidectomy | n/a | n/a |
| 20 | Male | 62 | Parotidectomy | n/a | n/a |
Cell count
A total of 2×104 cells were incubated in
DMEM-EM at 37°C with 5% CO2 for 4 days, while
electronically evaluating the cell number and cell viability each
day using CASY® Technology (Innovatis AG, Reutlingen,
Germany). Only cells labeled viable by the electronic counting were
included in the analysis for the cell counting.
MTT assay
The MTT (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) colorimetric staining method according to Mosmann
(19) was used to study the viability
of cells. All wells were incubated with 1 ml of MTT (1 mg/ml) for 5
h at 37°C with 5% CO2. MTT was then removed and 1 ml of
isopropanol was added, followed by another incubation period of 1 h
at 37°C with 5% CO2. Color changes due to the conversion
of MTT to blue formazan dye were measured using a multi-plate
reader (Titertek Multiskan PLUS MK II; Labsystems Diagnostics Oy,
Helsinski, Finland) at a wavelength of 570 nm.
Annexin V/propidium iodide
staining
A BD Pharmingen™ APC Annexin V kit (BD Biosciences,
Franklin Lakes, NJ, USA) was used to evaluate apoptosis. Cells in
suspension and adherent cells were harvested and washed twice with
PBS, followed by resuspension in 1:10 binding buffer (0.1 M HEPES,
pH 7.4, 1.4 M NaCl, 25 mM CaCl2) at a density of
1×106 cells/ml. Aliquots of this cell suspension (100
µl; 1×105 cells) were then transferred to a 5 ml culture
tube. Propidium iodide (5 µl) and Annexin V-APC (5 µl) were added
to each aliquot. Following 15 min of incubation at room temperature
in the dark, the cells were resuspended with 400 µl 1:10 binding
buffer. A FACScanto flow cytometer (BD Biosciences) was used to
analyze the samples. Propidium iodide staining indicated cells with
damaged membranes.
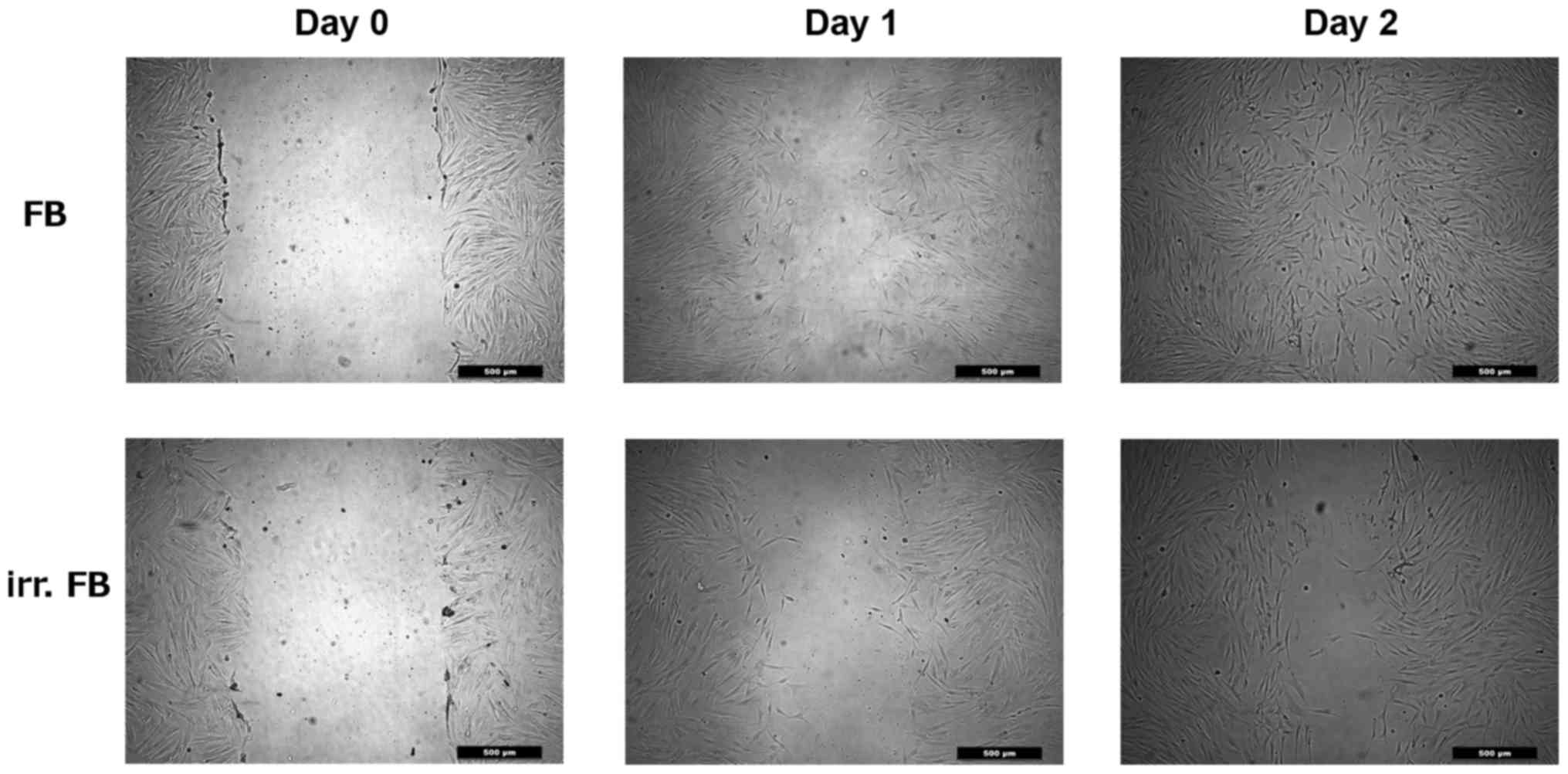
Scratch assay
A scratch assay was used to analyze cell migration
capability. Fibroblasts (1×105 cells/ml) were cultivated
in a 12-well round-bottom plate at 37°C and 5% CO2.
After 24 h, a straight-line wound was induced with a sterile 1-ml
pipette tip. Subsequently, the culture plates were washed with PBS
and images were captured (day 0) with a Leica DMI 4000B Inverted
Microscope at ×40 magnification (Leica Microsystems GmbH, Wetzlar,
Germany). The cells were then incubated for a further 24 h at 37°C
with 5% CO2, before images of the plates were captured
(day 1) and the percentage of the wound closure was evaluated. This
was repeated after another 24 h of incubation (day 2). The
calculation of the area of the wound closure was investigated using
ImageJ software (version 1.43u, open source product) at day 0, day
1 and day 2.
Statistical analysis
The data collected was transferred to standard
spreadsheets and statistically analyzed using GraphPad Prism
Software (version 6.0; GraphPad Software, Inc., La Jolla, CA, USA).
The Gaussian distribution was tested via first column analysis.
Students t-test followed by Tukey's multiple comparison test was
used for statistical analysis. Data are presented as the mean ±
standard deviation, unless otherwise stated. P<0.05 was
considered to indicate a statistically significant difference.
Results
Cell count
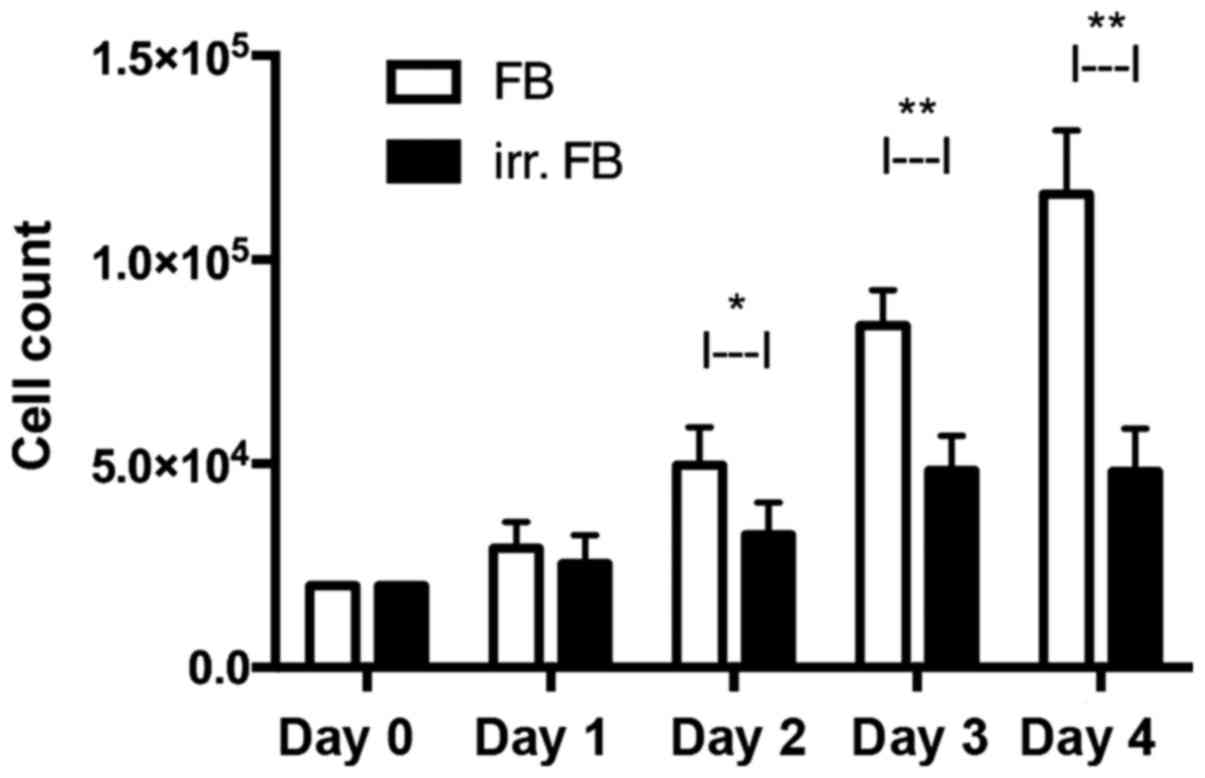
In a consecutive cell count for 4 days,
non-irradiated fibroblasts exhibited a constant increase in cell
number between days 0 and 4, reaching a median of
1.15×105 cells on day 4. Fibroblasts from pre-irradiated
tissue only had a minor increase in cell number, reaching a plateau
on day 3 with a median of 4.96×104 cells (Fig. 1). The differences between the
irradiated and non-irradiated groups were statistically significant
on days 2, 3 and 4 (P=0.0002, P=0.0001 and P=0.0001, respectively).
At day 4, non-irradiated fibroblasts had a median cell viability of
76%, whereas pre-irradiated fibroblasts had a median viability of
66%.
MTT assay
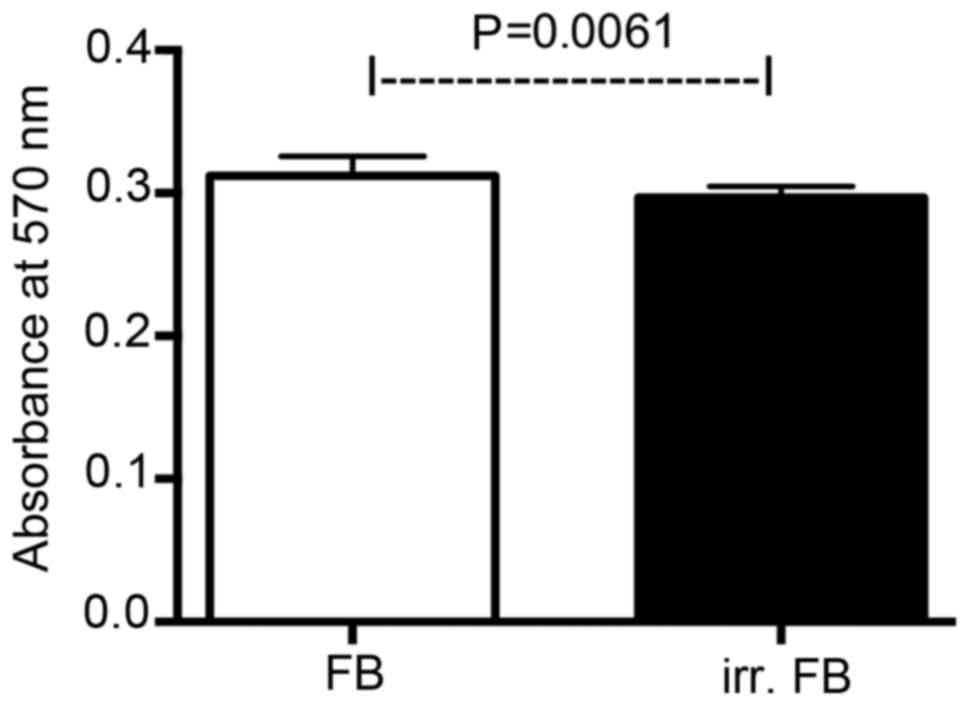
Viability of non-irradiated and pre-irradiated
fibroblasts was analyzed by MTT assay (Fig. 2). The assay revealed significantly
lower cell viability for fibroblasts cultured from pre-irradiated
skin tissue compared with non-irradiated fibroblasts
(P=0.0061).
Annexin V/propidium iodide
analysis
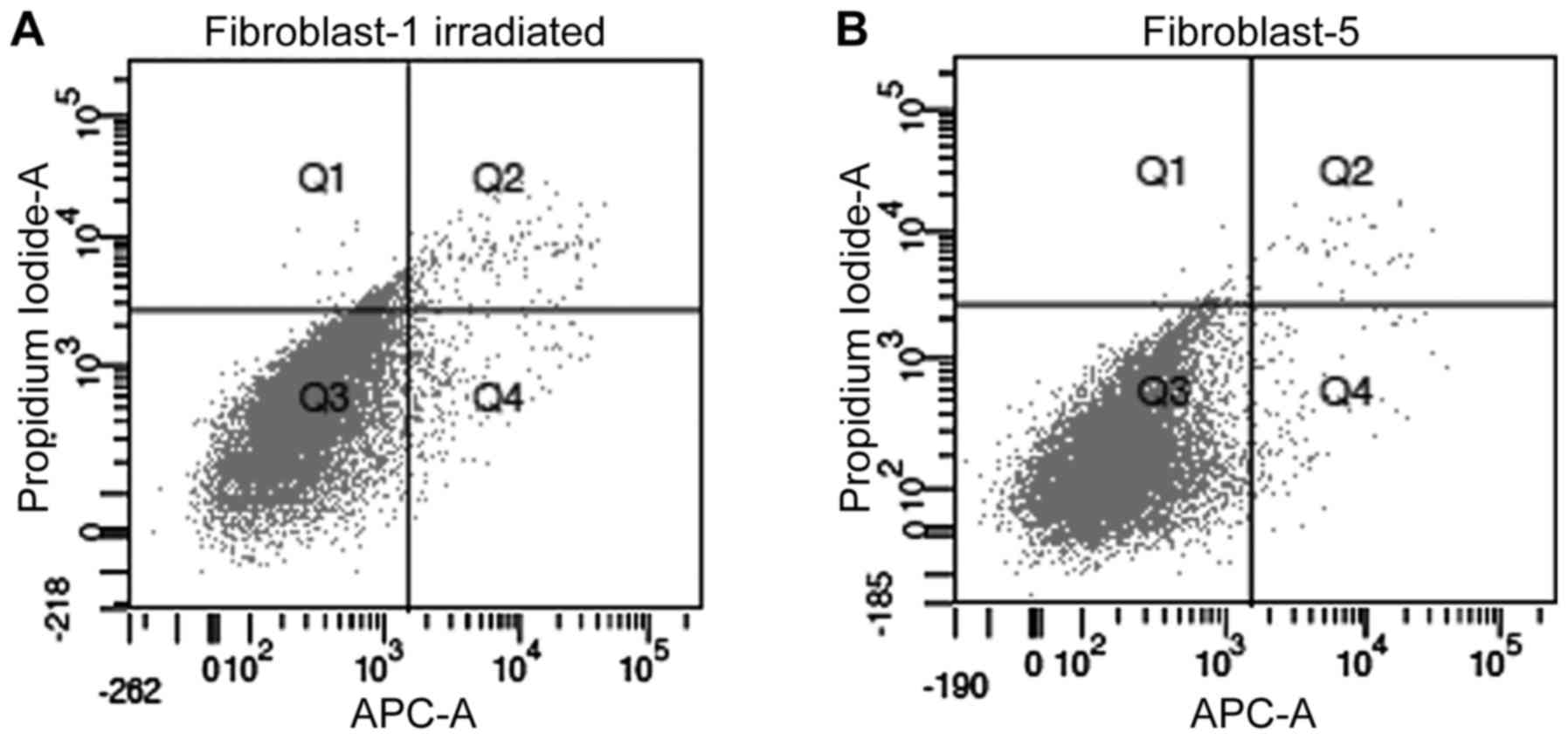
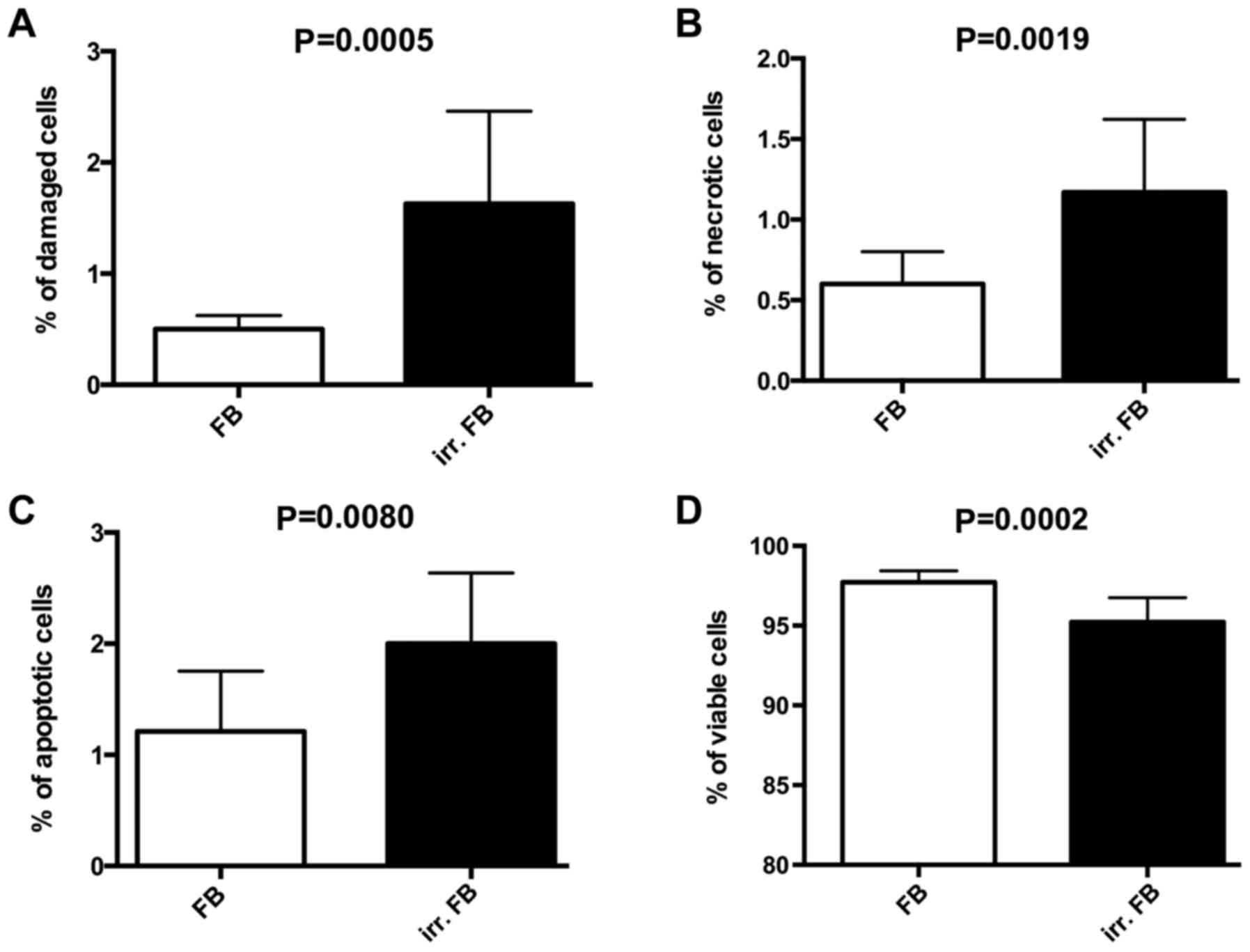
The Annexin V/propidium iodide analysis was used to
determine differences in the rates of apoptosis, necrosis and
viability between pre-irradiated fibroblasts and non-irradiated
fibroblasts (example shown in Fig.
3). Significantly higher rates of apoptosis (P=0.0080) and
necrosis (P=0.0019) were observed in pre-irradiated fibroblasts
compared with non-irradiated fibroblasts (Fig. 4). A lower percentage of viable cells
in pre-irradiated fibroblasts (P=0.0002; Fig. 4) was also observed compared with
non-irradiated fibroblasts, thus confirming the results of the
MTT-assay.
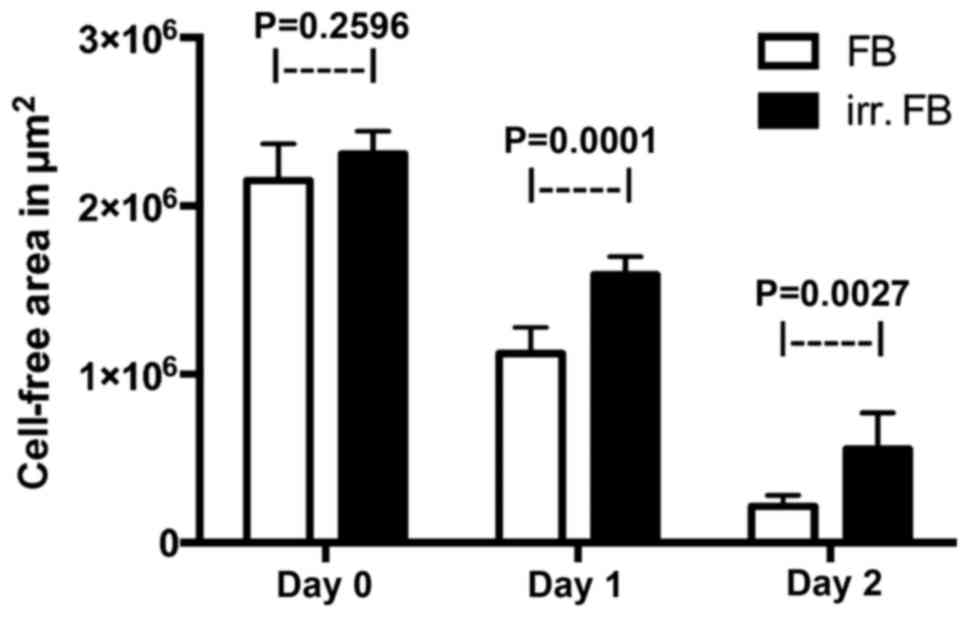
Scratch assay
The scratch assay was used to evaluate cell
migration into a wound area in monolayer conditions. When creating
the wound on day 0, no statistically significant difference was
observed between the two groups. Following periods of 24 and 48 h,
respectively, at 37°C and 5% CO2, the wound closure was
measured and compared between the two groups. The pre-irradiated
fibroblasts showed significantly slower wound closure compared with
non-irradiated fibroblasts on day 1 and day 2 (P=0.0001 and
P=0.0027, respectively), thus indicating reduced cell motility
(Figs. 5 and 6).
Discussion
The present study focused on the effects of a
previous radiation on the properties of skin-derived fibroblasts.
Tumor progression has been recognized as the product of an evolving
crosstalk between different cell types within the tumor and its
surrounding supporting tissue, or tumor stroma (20). The immune cells, capillaries, basement
membrane, activated fibroblasts and extracellular matrix
surrounding the cancer cells constitute the tumor stroma (21). Fibroblasts comprise a major component
of the tumor stroma, and numerous studies have indicated a
prominent role for these cells in cancer progression and metastasis
(2,22). CAFs have been established as key
components of tumor progression, and increasing information
indicates that they possibly contribute to a wide range of fibrotic
stromal programs of numerous different tumors (23,24). In
the context of a highly dynamic and injurious tissue
microenvironment, including damage induced by chemotherapy or
radiotherapy, CAFs may represent a resistant stromal cell type that
may be involved in tumor relapse (25).
Particularly in regard to relapsing cancer,
information about whether previous radiation changes the properties
and behavior of fibroblasts is desirable. It is already known that
cells exposed to radiation may survive, but give rise to progeny
that carry heritable damage (26).
This damage may become lethal during many generations of division
cycles of the originally irradiated progenitor cell (27,28).
Gorgojo and Little (29) previously
described the expression of lethal mutations in the progeny of
irradiated mammalian cells, thus showing an effect on surviving
cells a long time after the irradiation was administered. Chang and
Little (27,28) reported delayed reproductive deaths in
cell clones surviving irradiation, several generations following
therapy. However, these experiments were performed on established
cell lines grown and irradiated in vitro, and the relevance
of lethal mutations to irradiation of cells in vivo has been
uncertain (30). Chatterjee et
al (30) stated that the
reduction in the long-term viability of irradiated cell populations
appears to be dose-dependent and is most noticeable following large
doses of radiation. In the present study, fibroblasts derived from
pre-irradiated skin showed significantly lower viability and slower
cell growth compared with skin-derived fibroblasts from
non-irradiated patients, thereby confirming the in vitro
data available in the literature. Contrary to the aforementioned
studies, the cells used in the present study were primary human
fibroblasts from skin irradiated 6–18 months before, thus more
accurately representing the real physiological effects of
irradiation in vivo.
The levels of apoptosis and necrosis were elevated
in the pre-irradiated fibroblasts in the present study. This
indicated that there is more than one mechanism by which
irradiation damages surviving cells. O'Reilly et al
(31) reported a constant frequency
of non-lethal mutations occurring per cell division, indicating a
permanent genetic change induced by radiation. Kadhim et al
(32) also favored this hypothesis,
speculating that this mechanism may lead to cell death by an active
process such as apoptosis, rather than necrosis. O'Reilly et
al (31) also demonstrated
abnormalities in irradiated cultures a number of generations after
initial exposure, including convolution of the nuclear envelope,
increased incidence of microvilli and lysosomal accumulations,
which are characteristic of apoptosis rather than necrosis.
However, early senescence as an alternative cause of
radiation-induced changes has been discussed for mesenchymal stem
cells (MSCs) and fibroblasts (33,34).
In the present study, the scratch assay revealed
reduced motility of pre-irradiated fibroblasts. Rodriguez-Menocal
et al (35) demonstrated
decreased motility and migration capability of MSCs in an
irradiated murine delayed wound healing model. Henke et al
(36) reported decreased motility and
contractility in prostate CAFs, in their study associated with an
increase of focal adhesion kinase. By contrast, Nicolay et
al (37) found no changes in the
actin cytoskeleton or in the functional motility of irradiated MSCs
and fibroblasts. However, whether the delayed wound healing
observed in the present study is the result of reduced motility due
to radiation-induced genetic changes or a consequence of the
reduced cell division remains unclear.
The effects of the radiation-induced changes
observed in the fibroblasts in the present study of tumor cells
differed from the data available in previous studies. Kamochi et
al (38) presented data
indicating that irradiated fibroblasts promote growth and invasion
of co-cultured HNSCC. Other studies also reported of increased
invasiveness of pancreatic and mammalian tumor cells co-cultured
with irradiated fibroblasts (39,40).
However, in all these studies the radiation was administered in
vitro to the fibroblasts, so the long-term effects could not be
examined. The use of fibroblasts from human skin, which has been
exposed to therapeutic irradiation a number of months prior,
appears to be more comparable to the physiological conditions in
vivo. Using this approach, previous studies have already
demonstrated a decrease in viability of HNSCC co-cultured with
pre-irradiated fibroblasts (17). In
addition, fibroblasts from pre-irradiated human skin decreased the
secretion of IL-8 by HNSCC cells in a co-culture of these two cell
types (17).
A notable drawback of the present study was that
functional analysis regarding cytokine secretion and protein
synthesis was not included. A quantitative evaluation of the
secretory profile of the fibroblasts with or without radiation may
elucidate the mechanisms behind the changes observed in the present
study. In particular, ILs such as IL-6 and IL-8 have been shown to
be prominent modifiers of cancer cell behavior (41–44).
Whether the amount of these ILs produced by fibroblasts changes
following irradiation, however, has not been investigated thus far.
These analyses will be part of future studies at our
institution.
In conclusion, previous irradiation is associated
with changes in the properties of fibroblasts derived from human
skin in the irradiated area. Reduced cell viability, increased
rates of apoptosis and necrosis, slower cell growth and reduced
cell motility may be demonstrated. Since the effects of these
radiation-induced changes of the fibroblasts on tumor cells have
already been demonstrated, more information regarding the genetic
and secretory alterations of the fibroblasts are warranted to fully
elucidate the long-term effects of radiation. These
radiation-induced changes in fibroblasts (and, therefore, the tumor
stroma) may be a possible novel target for therapeutic strategies
for recurring cancer, and therefore require additional
investigation.
Acknowledgements
The present study was supported in part by the
Rudolf Bartling Foundation.
References
|
1
|
Kalluri R: Basement membranes: Structure,
assembly and role in tumour angiogenesis. Nat Rev Cancer.
3:422–433. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kalluri R and Zeisberg M: Fibroblasts in
cancer. Nat Rev Cancer. 6:392–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tlsty TD and Hein PW: Know thy neighbor:
Stromal cells can contribute oncogenic signals. Curr Opin Genet
Dev. 11:54–59. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Elenbaas B and Weinberg RA: Heterotypic
signaling between epithelial tumor cells and fibroblasts in
carcinoma formation. Exp Cell Res. 264:169–184. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mueller MM and Fusenig NE: Friends or foes
- bipolar effects of the tumour stroma in cancer. Nat Rev Cancer.
4:839–849. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Higashikawa K, Yoneda S, Taki M, Shigeishi
H, Ono S, Tobiume K and Kamata N: Gene expression profiling to
identify genes associated with high-invasiveness in human squamous
cell carcinoma with epithelial-to-mesenchymal transition. Cancer
Lett. 264:256–264. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chan GG, Tai BC, Liang S, Lim DT and Soo
KC: Squamous cell carcinoma of the head and neck
(HNSCC)-multi-modality treatment and impact on survival. Asian J
Surg. 25:35–40. 2002.PubMed/NCBI
|
|
10
|
Ganzer U and Gobel U: Modern treatment
strategies in head and neck tumors in childhood and adolescence-a
review. Laryngol Rhinol Otol (Stuttg). 63:113–119. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Million RR, Parsons JT and Mendenhall WM:
Effect of radiation on normal tissues in the head and neck. Bone,
cartilage and soft tissue. Front Radiat Ther Oncol. 23:221–237,
251–254. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Poland JM: The role of Candida
infections as an adverse effect upon head and neck cancer patients
undergoing therapeutic radiation and the effect of antimycotic
treatment. Mycoses. 32:(Suppl 2). 39–41. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Parker RG: Radiation-induced cancer as a
factor in clinical decision making (the 1989 ASTRO Gold Medal
address). Int J Radiat Oncol Biol Phys. 18:993–1000. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Larson DL: Management of complications of
radiotherapy of the head and neck. Surg Clin North Am. 66:169–182.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Affolter A, Schmidtmann I, Mann WJ and
Brieger J: Cancer-associated fibroblasts do not respond to combined
irradiation and kinase inhibitor treatment. Oncol Rep. 29:785–790.
2013.PubMed/NCBI
|
|
16
|
Tsai KK, Chuang EY, Little JB and Yuan ZM:
Cellular mechanisms for low-dose ionizing radiation-induced
perturbation of the breast tissue microenvironment. Cancer Res.
65:6734–6744. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gehrke T, Scherzad A, Hackenberg S,
Schenzielorz P, Hagen R and Kleinsasser N: Differences in tumor
stroma derived from irradiated versus non-irradiated fibroblasts in
a co-culture model with head and neck squamous cell carcinoma.
Oncol Lett. 12:3549–3554. 2016.PubMed/NCBI
|
|
18
|
Vangipuram M, Ting D, Kim S, Diaz R and
Schule B: Skin punch biopsy explant culture for derivation of
primary human fibroblasts. J Vis Exp. e37792013.PubMed/NCBI
|
|
19
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liotta LA and Kohn EC: The
microenvironment of the tumour-host interface. Nature. 411:375–379.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ronnov-Jessen L, Petersen OW and Bissell
MJ: Cellular changes involved in conversion of normal to malignant
breast: Importance of the stromal reaction. Physiol Rev. 76:69–125.
1996.PubMed/NCBI
|
|
22
|
Ohlund D, Elyada E and Tuveson D:
Fibroblast heterogeneity in the cancer wound. J Exp Med.
211:1503–1523. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ostman A and Augsten M: Cancer-associated
fibroblasts and tumor growth-bystanders turning into key players.
Curr Opin Genet Dev. 19:67–73. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marsh T, Pietras K and McAllister SS:
Fibroblasts as architects of cancer pathogenesis. Biochim Biophys
Acta. 1832:1070–1078. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kalluri R: The biology and function of
fibroblasts in cancer. Nat Rev Cancer. 16:582–598. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lyng FM, O'Reilly S, Cottell DC, Seymour
CB and Mothersill C: Persistent expression of morphological
abnormalities in the distant progeny of irradiated cells. Radiat
Environ Biophys. 35:273–283. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang WP and Little JB: Delayed
reproductive death as a dominant phenotype in cell clones surviving
X-irradiation. Carcinogenesis. 13:923–928. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chang WP and Little JB: Delayed
reproductive death in X-irradiated Chinese hamster ovary cells. Int
J Radiat Biol. 60:483–496. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gorgojo L and Little JB: Expression of
lethal mutations in progeny of irradiated mammalian cells. Int J
Radiat Biol. 55:619–630. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chatterjee A, Hodgkiss RJ and Rojas A:
Contribution of lethal mutations to excision assays for tumour cell
survival. Acta Oncol. 34:493–498. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
O'Reilly S, Mothersill C and Seymour CB:
Postirradiation expression of lethal mutations in an immortalized
human keratinocyte cell line. Int J Radiat Biol. 66:77–83. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kadhim MA, Macdonald DA, Goodhead DT,
Lorimore SA, Marsden SJ and Wright EG: Transmission of chromosomal
instability after plutonium alpha-particle irradiation. Nature.
355:738–740. 1992. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Alessio N, Bohn W, Rauchberger V, Rizzolio
F, Cipollaro M, Rosemann M, Irmler M, Beckers J, Giordano A and
Galderisi U: Silencing of RB1 but not of RB2/P130 induces cellular
senescence and impairs the differentiation potential of human
mesenchymal stem cells. Cell Mol Life Sci. 70:1637–1651. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cmielova J, Havelek R, Soukup T, Jiroutová
A, Visek B, Suchánek J, Vavrova J, Mokry J, Muthna D, Bruckova L,
et al: Gamma radiation induces senescence in human adult
mesenchymal stem cells from bone marrow and periodontal ligaments.
Int J Radiat Biol. 88:393–404. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rodriguez-Menocal L, Shareef S, Salgado M,
Shabbir A and Van Badiavas E: Role of whole bone marrow, whole bone
marrow cultured cells, and mesenchymal stem cells in chronic wound
healing. Stem Cell Res Ther. 6:242015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Henke A, Franco OE, Stewart GD, Riddick
AC, Katz E, Hayward SW and Thomson AA: Reduced contractility and
motility of prostatic cancer-associated fibroblasts after
inhibition of heat shock protein 90. Cancers (Basel). 8:E772016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nicolay NH, Sommer E, Lopez R, Wirkner U,
Trinh T, Sisombath S, Debus J, Ho AD, Saffrich R and Huber PE:
Mesenchymal stem cells retain their defining stem cell
characteristics after exposure to ionizing radiation. Int J Radiat
Oncol Biol Phys. 87:1171–1178. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kamochi N, Nakashima M, Aoki S, Uchihashi
K, Sugihara H, Toda S and Kudo S: Irradiated fibroblast-induced
bystander effects on invasive growth of squamous cell carcinoma
under cancer-stromal cell interaction. Cancer Sci. 99:2417–2427.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ohuchida K, Mizumoto K, Murakami M, Qian
LW, Sato N, Nagai E, Matsumoto K, Nakamura T and Tanaka M:
Radiation to stromal fibroblasts increases invasiveness of
pancreatic cancer cells through tumor-stromal interactions. Cancer
Res. 64:3215–3222. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Barcellos-Hoff MH and Ravani SA:
Irradiated mammary gland stroma promotes the expression of
tumorigenic potential by unirradiated epithelial cells. Cancer Res.
60:1254–1260. 2000.PubMed/NCBI
|
|
41
|
Harigai M, Hara M, Kitani A, Norioka K,
Hirose T, Hirose W, Suzuki K, Kawakami M, Masuda K, Shinmei M, et
al: Interleukin 1 and tumor necrosis factor-alpha synergistically
increase the production of interleukin 6 in human synovial
fibroblast. J Clin Lab Immunol. 34:107–113. 1991.PubMed/NCBI
|
|
42
|
Lu C, Vickers MF and Kerbel RS:
Interleukin 6: A fibroblast-derived growth inhibitor of human
melanoma cells from early but not advanced stages of tumor
progression. Proc Natl Acad Sci USA. 89:9215–9219. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Anderson IC, Mari SE, Broderick RJ, Mari
BP and Shipp MA: The angiogenic factor interleukin 8 is induced in
non-small cell lung cancer/pulmonary fibroblast cocultures. Cancer
Res. 60:269–272. 2000.PubMed/NCBI
|
|
44
|
Doldi V, Callari M, Giannoni E, D'Aiuto F,
Maffezzini M, Valdagni R, Chiarugi P, Gandellini P and Zaffaroni N:
Integrated gene and miRNA expression analysis of prostate cancer
associated fibroblasts supports a prominent role for interleukin-6
in fibroblast activation. Oncotarget. 6:31441–31460. 2015.
View Article : Google Scholar : PubMed/NCBI
|