Introduction
Laryngeal cancer, which is a common type of head and
neck malignancy, is the 11th most common neoplasm in males
(1). Laryngeal squamous cell
carcinomas (LSCC) account for >95% of laryngeal cancers, and
LSCC accounts for ~25% of all types of head and neck cancers
(2). In the last decade, the
incidence rate of LSCC has increased, with invasion and metastasis
being the primary factors that affect the prognosis of patients,
with a 5-year survival rate of ~60% (3,4). At
present, laryngectomy is the major effective treatment strategy
used for LSCC (5). In addition,
larynx-preservation protocols using chemotherapy or radiotherapy
are also developing (6). However, the
aforementioned treatments often do not achieve a satisfactory
clinical outcome and the overall survival of LSCC has not improved
for years (7). There is a lack of
sensitive and specific biomarkers to identify LSCC characteristics
and to predict LSCC outcomes.
Growth-related gene product β (GROβ) is an
angiogenic chemokine belonging to the CXC chemokine family, and
growing evidence has indicated that chemokines are associated with
tumor development and progression (8,9). GROβ was
initially identified in melanoma cell lines, and high expression of
GROβ was observed in human melanomas (10). Several previous studies have reported
the involvement of GROβ in tumorigenesis (11,12). GROβ
also attracted tumor cells and contributed to esophageal cancer
cell transformation and growth (13).
Blocking GROβ expression resulted in reduced proliferation and
colonization capacity of esophageal cancer cells (14). Upregulation of GROβ-chemokine receptor
2 (CXCR2) signaling significantly increased the proliferation of
cancer cells by modulating epithelial growth factor-1 (EGR-1) via
extracellular signal-regulated kinase 1/2 (ERK1/2) (15). Previous studies have detected
differentiated expression of GROβ, and high GROβ expression was
associated with several malignant features of colorectal cancer,
hepatocellular carcinoma and esophageal cancer (16–18).
However, although GROβ exhibits oncogenic functions, the
association between GROβ expression and LSCC characteristics
remains to be fully determined. Additional studies are required to
determine whether GROβ may serve as a biomarker for LSCC.
In the present study, the expression of GROβ in LSCC
tissue was detected via one-step quantitative reverse-transcription
polymerase chain reaction (RT-qPCR) and immunohistochemistry (IHC).
The associations between GROβ expression and clinicopathological
attributes of LSCC, in particular the prognostic status, were
investigated.
Materials and methods
Patient specimens
A total of 20 samples of fresh LSCC tissues and
corresponding non-cancerous tissues were collected from the
Department of Pathology, the Affiliated Hospital of Nantong
University (Nantong, China) between January 2013 and December 2014.
All of the 20 samples were obtained from males (range, 48–70 years;
average age, 60.30±5.42 years). Simultaneously, a total of 126
paraffin-embedded LSCC tissue samples (124 males and 2 females;
range, 42–87 years; average age, 64.10±8.59 years) and 28 matched
adjacent paracancerous tissue (<1 cm) samples obtained from
males (range, 52–76 years; average age, 62.93±5.80 years), were
collected from the archives of the Department of Pathology, the
Affiliated Hospital of Nantong University, between January 2002 and
December 2012. Diagnosis of LSCC was confirmed according to the
latest World Health Organization criteria and tumor-node-metastasis
(TNM) stage classification (19,20). The
original clinical data were obtained from hospital medical records,
and include details pertaining to patient sex and age, tobacco use,
alcohol consumption, TNM stage, lymph node metastasis status and
histopathological grade. None of the patients received radiotherapy
or chemotherapy prior to surgery. Written informed consent was
acquired from each patient enrolled in the present study. Ethical
approval to perform the present study was granted by the Human
Research Ethics Committee of the Affiliated Hospital of Nantong
University.
One-step RT-qPCR
A total of 20 fresh LSCC tissue samples and
corresponding non-cancerous tissue samples were used to perform
one-step qPCR. Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Expression levels of GROβ and glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) were determined by RT-qPCR using the iQ5
detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
and the SensiMix One-Step SYBR-Green kit (Quantace Ltd., London,
UK). The primers used were as follows: GROβ forward,
5′-CACCAACCACCAGGCTAC-3′ and reverse, 5′-CTTCAGGGTCAAGGCAAA-3′; and
GAPDH forward, 5′-TATTACCTGGACGAGATTCCCC-3′ and reverse,
5′-TATTACCTGGACGAGATTCCCC-3′. Amplification conditions were as
described in previous studies (21–23). GAPDH
was used as the reference gene to normalize the Cq values of cancer
and control tissue samples. The calculation formula of all sample
is 2−ΔΔCq (24). Each
experiment was repeated 3 times.
Tissue microarray (TMA) construction
and IHC
A total of 126 LSCC tissues were prepared and TMAs
were produced by Xinchao Biotech Co. Ltd. (Shanghai, China) to
proceed IHC analysis. Core tissue biopsies (diameter, 2 mm) were
taken from individual paraffin-embedded sections and arranged in
the new paraffin blocks. The tissue microarray was cut into 4 µm
sections and placed on microscope slides. IHC was performed as
described previously (25,26). Briefly, TMA sections were incubated
with a primary polyclonal anti-GROβ antibody (1:200; cat. no.
ab10366; Abcam, Cambridge, UK) diluted in PBS at 4°C for 8 h.
Following washing with PBS at 37°C for 30 min, sections were
incubated with horseradish peroxidase-conjugated secondary antibody
(1:2,000; cat. no. P0160; Dako; Agilent Technologies, Inc., Santa
Clara, CA, USA). Negative control reactions used PBS instead of the
primary antibody. The results of IHC were evaluated by a
double-blind method whereby the staining results were determined
under a light optical microscope at magnifications ×40 and ×400, by
two independent pathologists.
Expression levels of GROβ protein were evaluated by
observing the staining density and intensity of positive cells as
described previously (27,28). Staining density of positive cells was
scored as follows: 0, negative; 1, 1–10% positive cells; 2, 10–50%
positive cells, and 3, >50% positive cells. Similarly, staining
intensity was scored as: 0, no color; 1, yellow for weak positive;
2, light brown for medium positive and 3, brown for strong
positive. The two components were produced to obtain an overall
expression score, as follows: 0, (−); 1–3, (+); 4–6, (++); and 7–9,
(+++). The degree of GROβ staining was quantified using a two-level
grading system, and staining scores were defined as follows: ≤3,
low expression and 4–9, high expression.
Statistical analysis
The GROβ mRNA expression in fresh LSCC tissues
compared with corresponding non-cancerous tissues was analyzed with
the Wilcoxon signed-rank nonparametric test. The association of
GROβ expression on clinicopathological items of LSCC was calculated
by the χ2 test. Univariate and multivariate analyses were performed
using Cox proportional hazards regression models to explore the
prognostic factors. The Kaplan-Meier method was utilized to
evaluate the association between GROβ expression and LSCC outcomes.
For all tests, P<0.05 was considered to indicate a statistically
significant difference. All the statistical analyses were conducted
using uSTATA (version 12.0; StataCorp LLC, College Station, TX,
USA) and SPSS 18.0 statistical software (SPSS, Inc., Chicago, IL,
USA).
Results
Detection of GROβ mRNA expression in
LSCC by RT-qPCR
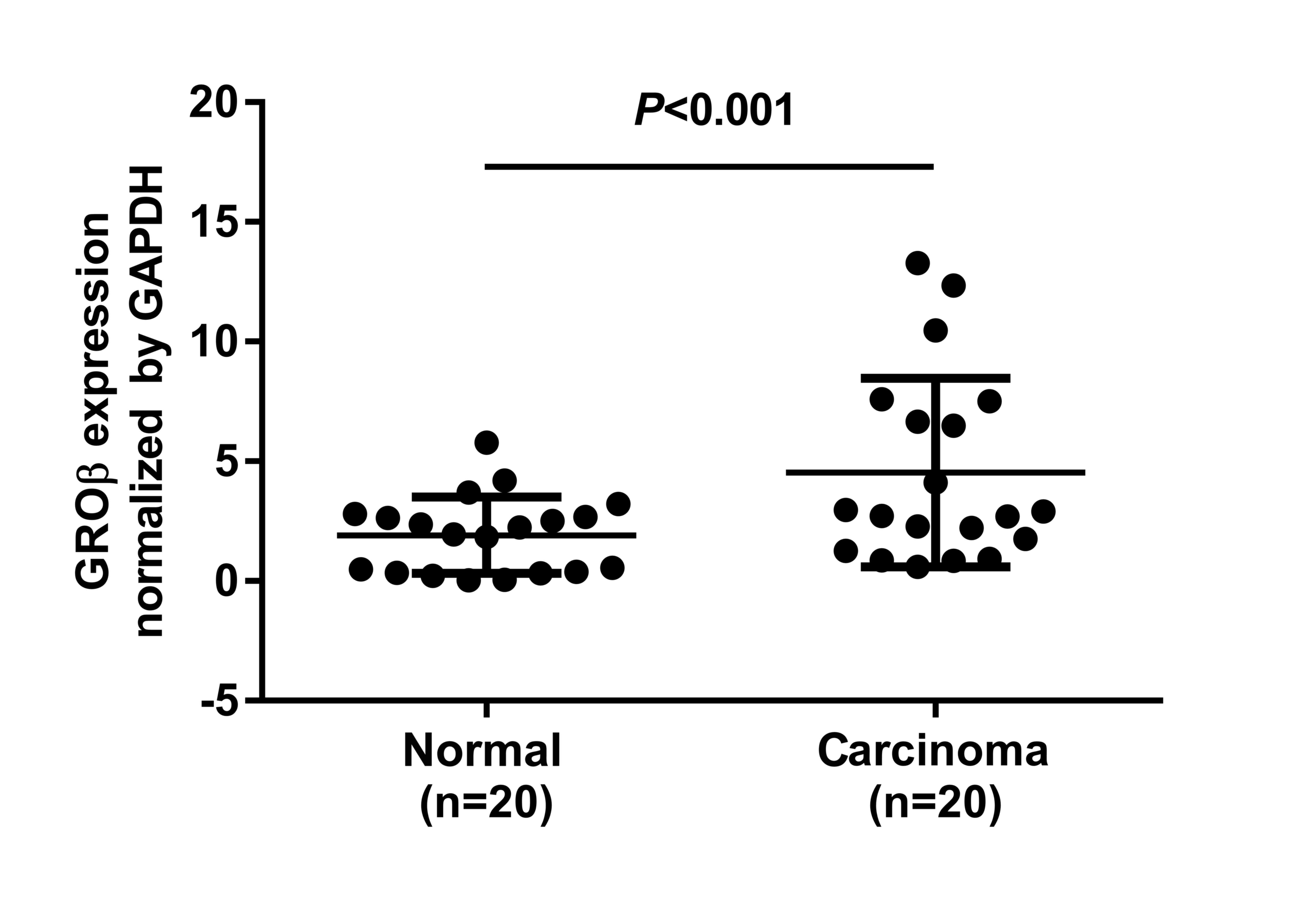
When normalized to GAPDH, the means of GROβ mRNA in
LSCC and corresponding non-cancerous tissues were 4.53±0.882 and
1.91±0.358, respectively (t=2.746; P=0.009; Fig. 1). GROβ expression averaged 2.41-fold
higher in the LSCC samples compared with the non-cancerous tissues
(Fig. 1).
Detection of GROβ protein expression
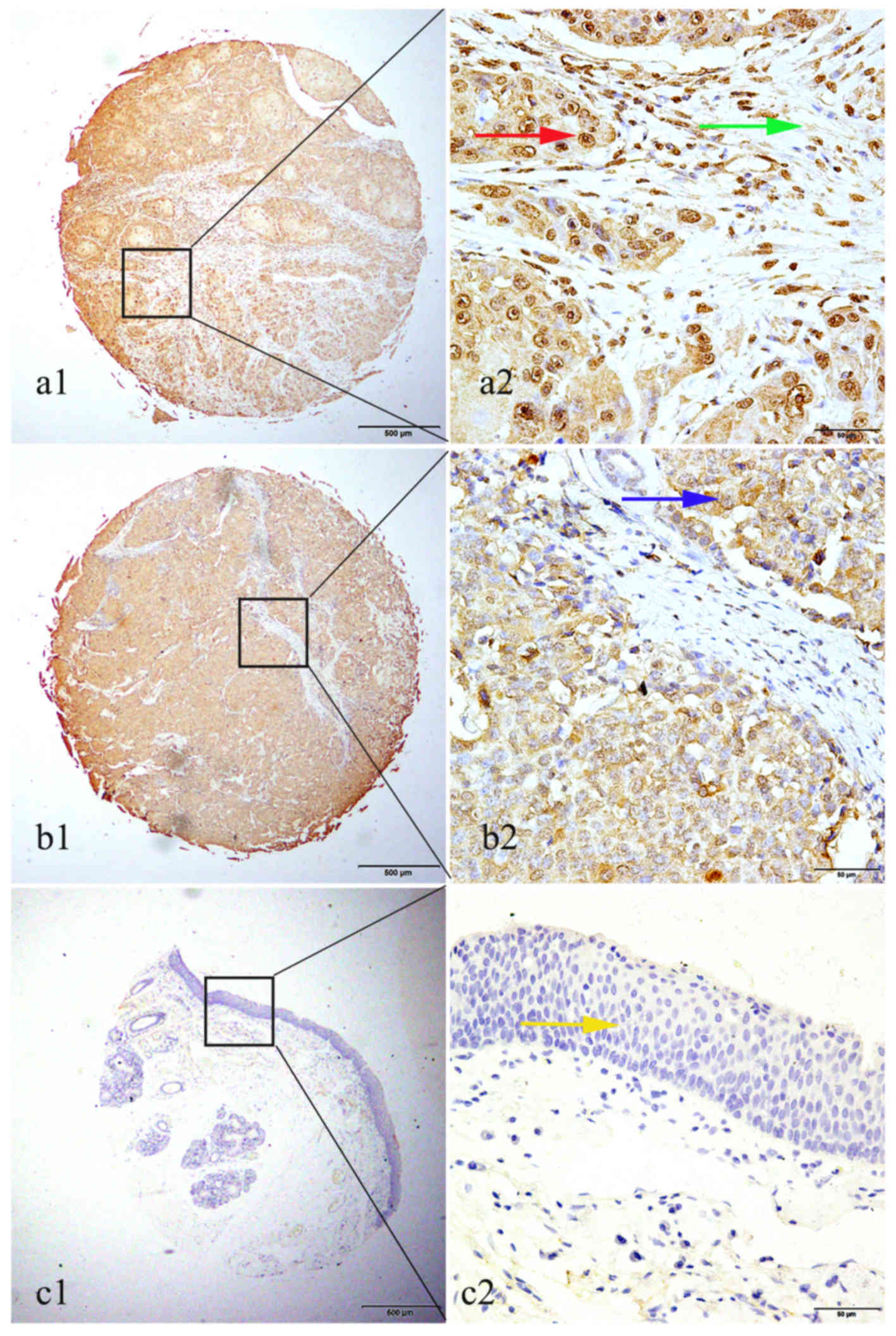
in LSCC by IHC
High GROβ expression was detected in 72 of 126
(57.1%) LSCC tissues, while only 2 cases of 28 non-cancerous
tissues (7.1%) exhibited high GROβ expression. There was a
significant difference in high expression rate of GROβ between LSCC
tissues and non-cancerous tissues (P<0.001). Positive staining
of GROβ was mainly localized in the nucleus of cancer cells
(Fig. 2). Although positive
cytoplasmic and stromal staining of GROβ was observed in certain
cases, the case number was too small to perform statistics
(Fig. 2).
Association between GROβ expression
and clinical attributes
The associations between GROβ protein expression and
the clinical characteristics of patients with LSCC are listed in
Table I. Elevated GROβ expression was
significantly associated with TNM stage (P=0.036), lymph node
metastasis (P=0.017) and histopathological grade (P=0.007). By
contrast, no association was detected between GROβ expression and
other clinical characteristics, including age, tobacco or alcohol
consumption (Table I).
 | Table I.Association of GROβ expression with
clinical attributes of laryngeal squamous cell carcinoma. |
Table I.
Association of GROβ expression with
clinical attributes of laryngeal squamous cell carcinoma.
|
|
| GROβ expression,
n |
|
|
|---|
|
|
|
|
|
|
|---|
| Groups | n | High expression | Low expression | χ2 | P-value |
|---|
| Total | 126 | 72 | 54 |
|
|
| Age |
|
|
|
|
|
| ≤60
years | 41 | 23 | 18 | 0.027 | 0.869 |
| >60
years | 85 | 49 | 36 |
|
|
| Tobacco
consumption |
|
|
|
|
|
| Yes | 68 | 41 | 27 | 0.261 | 0.609 |
| No | 31 | 17 | 14 |
|
|
|
Unknown | 27 | 14 | 13 |
|
|
| Alcohol
consumption |
|
|
|
|
|
|
Yes | 48 | 31 | 17 | 1.381 | 0.240 |
| No | 51 | 27 | 24 |
|
|
|
Unknown | 27 | 14 | 13 |
|
|
| TNM stage |
|
|
|
|
|
| Stage
I, II | 65 | 31 | 33 | 4.383 | 0.036 |
| Stage
III, IV | 49 | 34 | 16 |
|
|
|
Unknown | 12 | 7 | 5 |
|
|
| Lymph node
metastasis |
|
|
|
|
|
|
Yes | 18 | 15 | 3 | 5.667 | 0.017 |
| No | 105 | 56 | 49 |
|
|
|
Unknown | 3 | 1 | 2 |
|
|
| Histopathological
grade |
|
|
|
|
|
|
High | 11 | 9 | 0 | 9.861 | 0.007 |
|
Moderate | 65 | 37 | 28 |
|
|
|
Low | 47 | 26 | 21 |
|
|
|
Unknown | 3 | 0 | 5 |
|
|
Survival analysis
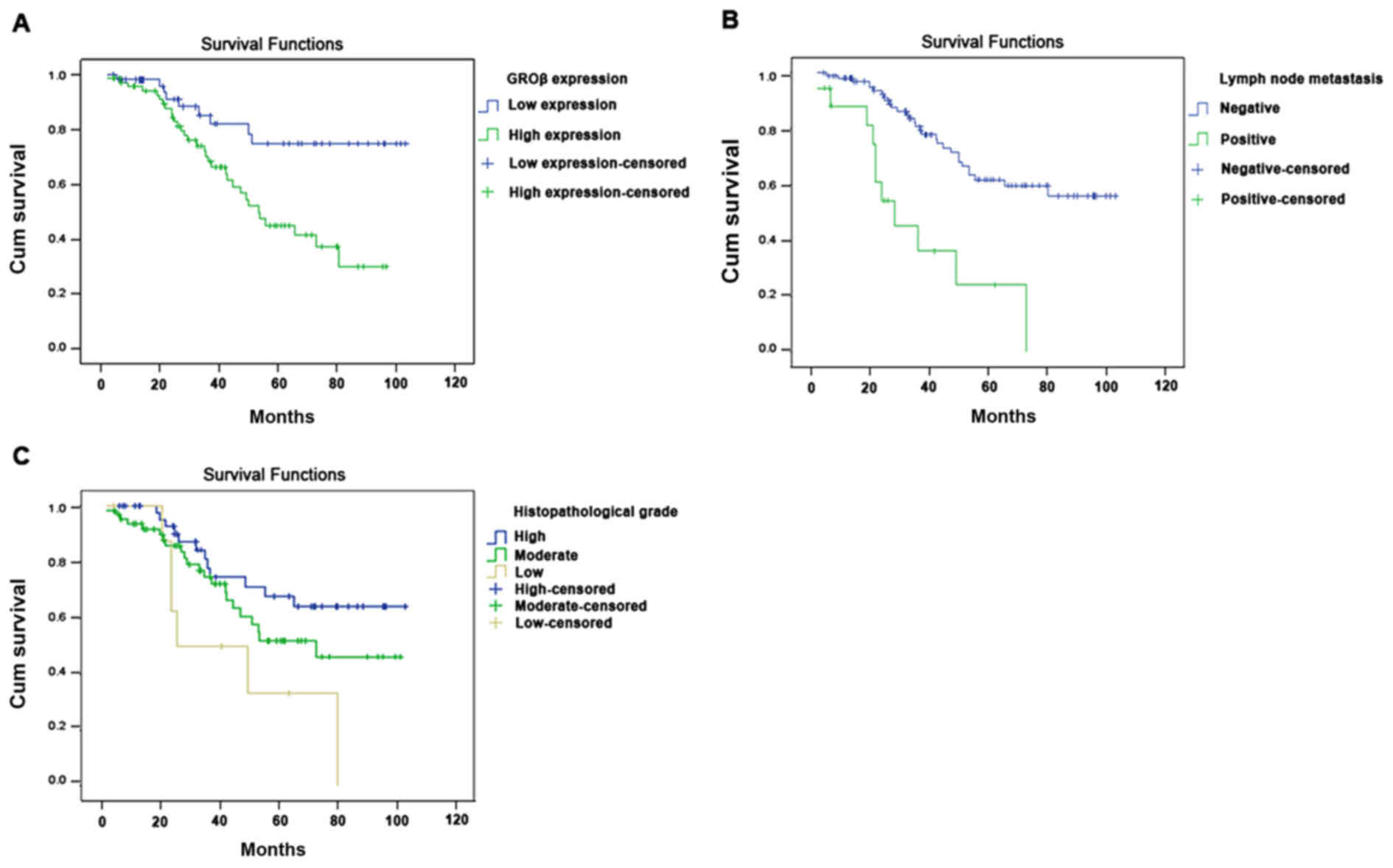
Univariate analysis revealed that the overall
survival of patients with LSCC was associated with high GROβ
expression (P=0.004), TNM stage (P=0.002), lymph node metastasis
(P=0.001) and histopathological grade (P=0.017). Multivariate
analysis identified that high GROβ expression (P=0.048), lymph node
metastasis (P=0.007) and histopathological grade (P=0.038) were
independent prognostic factors for overall survival (Table II). Furthermore, Kaplan-Meier
survival curves indicated that patients with LSCC with low GROβ
expression, negative lymph node metastasis and high
histopathological grade had a significantly longer overall survival
time (Fig. 3).
 | Table II.Univariate and multivariate analysis
of prognostic factors in laryngeal squamous cell carcinoma for
overall survival time. |
Table II.
Univariate and multivariate analysis
of prognostic factors in laryngeal squamous cell carcinoma for
overall survival time.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
| P-value | 95% CI | P-value | 95% CI |
|---|
| GROβ
expression |
|
|
|
|
| High
vs. low | 0.004 | 1.411–6.249 | 0.043 | 1.025–5.164 |
| Age |
|
|
|
|
| ≤60 vs.
>60 years | 0.197 |
| 0.125 |
|
| Tobacco
consumption |
|
|
|
|
| Yes vs.
no | 0.210 |
| 0.398 |
|
| Alcohol
consumption |
|
|
|
|
| Yes vs.
no | 0.595 |
| 0.226 |
|
| TNM stage |
|
|
|
|
| Stage
I, II vs. Stage III, IV | 0.002 | 1.409–5.046 | 0.226 | 0.774–3.633 |
| Lymph node
metastasis |
|
|
|
|
| Yes vs.
no | 0.001 | 2.122–8.706 | 0.007 | 1.418–9.339 |
| Histopathological
grade |
|
|
|
|
| High
vs. moderate vs. low | 0.017 | 1.112–2.971 | 0.038 | 1.031–3.073 |
Discussion
Chemokines are a superfamily of small, cytokine-like
proteins that interact with cell-surface receptors during
development of the host immune response (29,30).
Previous data have revealed that chemokines are involved in human
cancer, in addition to their functions in development and
inflammatory responses (31). GRO is
a member of the CXC chemokine family, which is composed of GROα,
GROβ and GROγ (32). GROα is
expressed at high levels in a variety of tumors and is associated
with tumor proliferation, angiogenesis and metastasis (33,34). A
previous study also confirmed that high GROα expression is
associated with an aggressive malignant phenotype of LSCC, and GROα
may be a valuable prognostic biomarker for patients with LSCC
(35). Several studies have explored
the involvement of GROβ in tumor formation and development. For
example, Wang et al (15) and
Dong et al (18) reported that
GROβ is highly expressed in esophageal squamous cell carcinoma.
Doll et al (36) also reported
a significantly elevated level of GROβ expression in colon
carcinoma compared with normal tissue. GROβ may form an autocrine
loop by binding its receptor CXCR2 and activating the Ras-ERK1/2
signaling pathway, which is important for cell proliferation
(15). This pathway in turn enhances
the transcription and expression of EGR-1, a transcription factor
that regulates the expression of downstream factors associated with
cell growth and cell cycle regulation, thereby promoting tumor
progression (37). Based on this
information, although the exact function of GROβ in LSCC remains to
be investigated, it is reasonable to speculate that the GROβ/CXCR2
axis is involved in LSCC development. In the present study, the
clinicopathological significance of GROβ in LSCC was detected with
a particular focus on its prognostic characteristics.
The results of RT-qPCR demonstrated that GROβ mRNA
levels were increased in LSCC compared with non-cancerous tissues.
This data was consistent with that reported in a series of previous
studies, in which the expression of GROβ was revealed to be
significantly elevated in cancer tissues compared with normal
tissues (15,18,36). The
expression of GROβ was confirmed by conducting IHC. Consistent with
the results of RT-qPCR, the IHC results revealed increased GROβ
expression in LSCC tissues compared with non-cancerous tissues. The
IHC staining pattern revealed that GROβ protein was mainly
localized in the nucleus of LSCC cells. In addition, small LSCC
cases exhibited positive cytoplasmic and stromal staining of GROβ.
However, Ye et al (38)
reported that GROβ was principally detected in the cytoplasm in
ovarian cancer, and it was presumed that the reason for the
differential distribution of GROβ may be due to the differences in
cancer type, antibody used and experimental protocol. Additional
studies that enroll a larger number of clinical samples of LSCC in
particular cancer categories are necessary to validate the findings
of the present study.
GROβ overexpression (including in serum, plasma and
tissue) has been reported to be associated with several malignant
features of human cancers (36,37). In
the present study, high GROβ expression in LSCC was associated with
three clinical pathological characteristics, namely TNM stage,
lymph node metastasis and histopathological grade. In addition,
univariate and multivariate analysis revealed the prognostic value
of GROβ overexpression, indicating that patients with LSCC with
high GROβ expression may have poor prognoses. The Kaplan-Meier
curve also implied that high GROβ expression in patients with LSCC
indicated unfavorable overall survival. The obtained data were
consistent with the results of a previous study, which illustrated
that high GROβ expression was associated with poor prognosis and
contributed to ovarian cancer tumorigenesis and metastasis
(38).
In conclusion, to the best of our knowledge, the
present study was the first to examine GROβ mRNA expression with
RT-qPCR and protein expression with IHC in LSCC. The results
revealed that high GROβ expression may be associated with the
development and progression of LSCC. Therefore, GROβ may be a
useful biomarker for predicting the prognosis of LSCC, and
targeting GROβ may provide a novel strategy for LSCC treatment.
Acknowledgements
The present study was supported by grants from the
Science and Technique Development Fund (grant no. 20120066) of
Nantong, Jiangsu, China and Youth Medical Personnel of Scientific
Research Fund (grant no. WQ2016066) of Nantong Municipal Commission
of Health, and Family Planning and Nantong Tumor Hospital (Nantong,
China).
References
|
1
|
Shen Z, Li Q, Deng H, Lu D, Song H and Guo
J: Long non-coding RNA profiling in laryngeal squamous cell
carcinoma and its clinical significance: Potential biomarkers for
LSCC. PLoS One. 9:e1082372014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Almadori G, Bussu F, Cadoni G, Galli J,
Paludetti G and Maurizi M: Molecular markers in laryngeal squamous
cell carcinoma: Towards an integrated clinicobiological approach.
Eur J Cancer. 41:683–693. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu Y, Su Z, Li G, Yu C, Ren S, Huang D,
Fan S, Tian Y, Zhang X and Qiu Y: Increased expression of
metadherin protein predicts worse disease-free and overall survival
in laryngeal squamous cell carcinoma. Int J Cancer. 133:671–679.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma J, Wang J, Fan W, Pu X, Zhang D, Fan C,
Xiong L, Zhu H, Xu N, Chen R and Liu S: Upregulated TIMP-1
correlates with poor prognosis of laryngeal squamous cell
carcinoma. Int J Clin Exp Pathol. 7:246–254. 2013.PubMed/NCBI
|
|
5
|
Rodrigo JP, Coca-Pelaz A and Suárez C: The
current role of partial surgery as a strategy for functional
preservation in laryngeal carcinoma. Acta Otorrinolaringol Esp.
62:231–238. 2011.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ajithkumar T, Price S, Horan G, Burke A
and Jefferies S: Prevention of radiotherapy-induced neurocognitive
dysfunction in survivors of paediatric brain tumours: The potential
role of modern imaging and radiotherapy techniques. Lancet Oncol.
18:e91–e100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mountzios G, Kostopoulos I, Kotoula V,
Sfakianaki I, Fountzilas E, Markou K, Karasmanis I, Leva S,
Angouridakis N, Vlachtsis K, et al: Insulin-like growth factor 1
receptor (IGF1R) expression and survival in operable squamous-cell
laryngeal cancer. PLoS One. 8:e540482013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Messina JL, Fenstermacher DA, Eschrich S,
Qu X, Berglund AE, Lloyd MC, Schell MJ, Sondak VK, Weber JS and
Mulé JJ: 12-Chemokine gene signature identifies lymph node-like
structures in melanoma: Potential for patient selection for
immunotherapy? Sci Rep. 2:7652012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mitkin NA, Hook CD, Schwartz AM, Biswas S,
Kochetkov DV, Muratova AM, Afanasyeva MA, Kravchenko JE,
Bhattacharyya A and Kuprash DV: p53-dependent expression of CXCR5
chemokine receptor in MCF-7 breast cancer cells. Sci Rep.
5:93302015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Richmond A and Thomas HG: Melanoma growth
stimulatory activity: Isolation from human melanoma tumors and
characterization of tissue distribution. J Cell Biochem.
36:185–198. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao H, Zhu H, Jin Q, Zhang S, Wang W,
Wang D and Huang J: Association of high expression of Groβ with
clinical and pathological characteristics of unfavorable prognosis
in gastrointestinal stromal tumors. Dis Markers. 2015:1710352015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng Z, Zheng M, Bi J, Feng Q, Yue Z,
Zhou Y, Hu W, Zhang H and Gao H: Serum GROβ: A potential
tumor-associated biomarker for colorectal cancer. Int J Clin Exp
Med. 8:2526–2535. 2015.PubMed/NCBI
|
|
13
|
Dong QM, Zhang JQ, Li Q, Bracher JC,
Hendricks DT and Zhao XH: Clinical significance of serum expression
of GROβ in esophageal squamous cell carcinoma. World J
Gastroenterol. 17:2658–2662. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bruyère C, Lonez C, Duray A, Cludts S,
Ruysschaert JM, Saussez S, Yeaton P, Kiss R and Mijatovic T:
Considering temozolomide as a novel potential treatment for
esophageal cancer. Cancer. 117:2004–2016. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang B, Hendricks DT, Wamunyokoli F and
Parker MI: A growth-related oncogene/CXC chemokine receptor 2
autocrine loop contributes to cellular proliferation in esophageal
cancer. Cancer Res. 66:3071–3077. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng Z, Zheng M, Bi J, Feng Q, Yue Z,
Zhou Y, Hu W, Zhang H and Gao H: Serum GROβ: A potential
tumor-associated biomarker for colorectal cancer. Int J Clin Exp
Med. 8:2526–2535. 2015.PubMed/NCBI
|
|
17
|
Li Y, Wang Y and Zhang P: Clinical
significance of serum expression of GROβ in hepatocellular
carcinoma. Tumour Biol. 36:6445–6449. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dong QM, Zhang JQ, Li Q, Bracher JC,
Hendricks DT and Zhao XH: Clinical significance of serum expression
of GROβ in esophageal squamous cell carcinoma. World J
Gastroenterol. 17:2658–2662. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thompson L: World Health Organization
classification of tumours: Pathology and genetics of head and neck
tumours. Ear Nose Throat J. 85:742006.PubMed/NCBI
|
|
20
|
Webber C, Gospodarowicz M, Sobin LH,
Wittekind C, Greene FL, Mason MD, Compton C, Brierley J and Groome
PA: Improving the TNM classification: Findings from a 10-year
continuous literature review. Int J Cancer. 135:371–378. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang J, Fan X, Wang X, Lu Y, Zhu H, Wang
W, Zhang S and Wang Z: High ROR2 expression in tumor cells and
stroma is correlated with poor prognosis in pancreatic ductal
adenocarcinoma. Sci Rep. 5:129912015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu Y, Wang C, Zhang Y, Jia L and Huang J:
Overexpression of MAGE-A9 Is predictive of poor prognosis in
epithelial ovarian cancer. Sci Rep. 5:121042015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen R, Lu M, Wang J, Zhang D, Lin H, Zhu
H, Zhang W, Xiong L, Ma J, Mao Y, et al: Increased expression of
Trop2 correlates with poor survival in extranodal NK/T cell
lymphoma, nasal type. Virchows Arch. 463:713–719. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han L, Jiang B, Wu H, Wang X, Tang X,
Huang J and Zhu J: High expression of CXCR2 is associated with
tumorigenesis, progression and prognosis of laryngeal squamous cell
carcinoma. Med Oncol. 29:2466–2472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu H, Lu J, Wang X, Zhang H, Tang X, Zhu
J and Mao Y: Upregulated ZO-1 correlates with favorable survival of
gastrointestinal stromal tumor. Med Oncol. 30:6312013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Welsh JB, Sapinoso LM, Kern SG, Brown DA,
Liu T, Bauskin AR, Ward RL, Hawkins NJ, Quinn DI, Russell PJ, et
al: Large-scale delineation of secreted protein biomarkers
overexpressed in cancer tissue and serum. Proc Natl Acad Sci USA.
100:3410–3415. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Han L, Jiang B, Wu H, Zhang S and Lu X:
Expression and prognostic value of MAGE-A9 in laryngeal squamous
cell carcinoma. Int J Clin Exp Pathol. 7:6734–6742. 2014.PubMed/NCBI
|
|
29
|
Charo IF and Ransohoff RM: The many roles
of chemokines and chemokine receptors in inflammation. N Engl J
Med. 354:610–621. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ebnet K and Vestweber D: Molecular
mechanisms that control leukocyte extravasation: The selectins and
the chemokines. Histochem Cell Biol. 112:1–23. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang B, Khachigian LM, Esau L, Birrer MJ,
Zhao X, Parker MI and Hendricks DT: A key role for early growth
response-1 and nuclear factor-kappaB in mediating and maintaining
GRO/CXCR2 proliferative signaling in esophageal cancer. Mol Cancer
Res. 7:755–764. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang D, Yang W, Du J, Devalaraja MN, Liang
P, Matsumoto K, Tsubakimoto K, Endo T and Richmond A:
MGSA/GRO-mediated melanocyte transformation involves induction of
Ras expression. Oncogene. 19:4647–4659. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Belperio JA, Keane MP, Arenberg DA,
Addison CL, Ehlert JE, Burdick MD and Strieter RM: CXC chemokines
in angiogenesis. J Leukoc Biol. 68:1–8. 2000.PubMed/NCBI
|
|
34
|
Loukinova E, Dong G, Enamorado-Ayalya I,
Thomas GR, Chen Z, Schreiber H and Van Waes C: Growth regulated
oncogene-alpha expression by murine squamous cell carcinoma
promotes tumor growth, metastasis, leukocyte infiltration and
angiogenesis by a host CXC receptor-2 dependent mechanism.
Oncogene. 19:3477–3486. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Han L, Liu W, Chen Y, Wu H, Zhang Y and
Jiang B: GROα expression and its prognostic implications in
laryngeal squamous cell carcinoma. Neoplasma. 62:152–158. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Doll D, Keller L, Maak M, Boulesteix AL,
Siewert JR, Holzmann B and Janssen KP: Differential expression of
the chemokines GRO-2, GRO-3, and interleukin-8 in colon cancer and
their impact on metastatic disease and survival. Int J Colorectal
Dis. 25:573–581. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dong Q, Zhang J, Hendricks DT and Zhao X:
GROβ and its downstream effector EGR1 regulate cisplatin-induced
apoptosis in WHCO1 cells. Oncol Rep. 25:1031–1037. 2011.PubMed/NCBI
|
|
38
|
Ye Q, Zhai X, Wang W, Zhang S, Zhu H, Wang
D and Wang C: Overexpression of growth-related oncogene-β Is
associated with tumorigenesis, metastasis and poor prognosis in
ovarian cancer. Dis Markers. 2015:3873822015. View Article : Google Scholar : PubMed/NCBI
|