Introduction
Pulmonary benign metastasizing leiomyoma (PBML) is a
rare disease entity that usually occurs in females of reproductive
age with a prior history of uterine myoma. PBML was first described
by Steiner in 1939 (1). PBML is
typically characterized by multiple pulmonary tumors containing
benign leiomyoma cells (2). Patients
are usually asymptomatic and the tumors grow gradually (3). In the present study, two cases of PBML
are presented, each of which include the results of
2-deoxy-2-(fluorine-18)-fluoro-D-glucose positron emission
tomography/computed tomography (18-FDG-PET/CT) scans. The first
patient demonstrated an absence of 18-FDG uptake and a quiescent
clinical course. However, the second patient exhibited a markedly
high uptake of 18-FDG and the aggressive proliferation of tumor
cells was detected. The two tumors revealed significant differences
in metabolic behavior and clinical course, yet were alike in regard
to cellular appearance. A literature review on the findings of
18-FDG-PET/CT scans in previous published cases of PBML was also
conducted and is discussed here.
Case
Case 1
A 38-year-old female was diagnosed with papillary
adenocarcinoma of the thyroid gland following a fine-needle
aspiration biopsy in Kansai Medical University Takii Hospital in
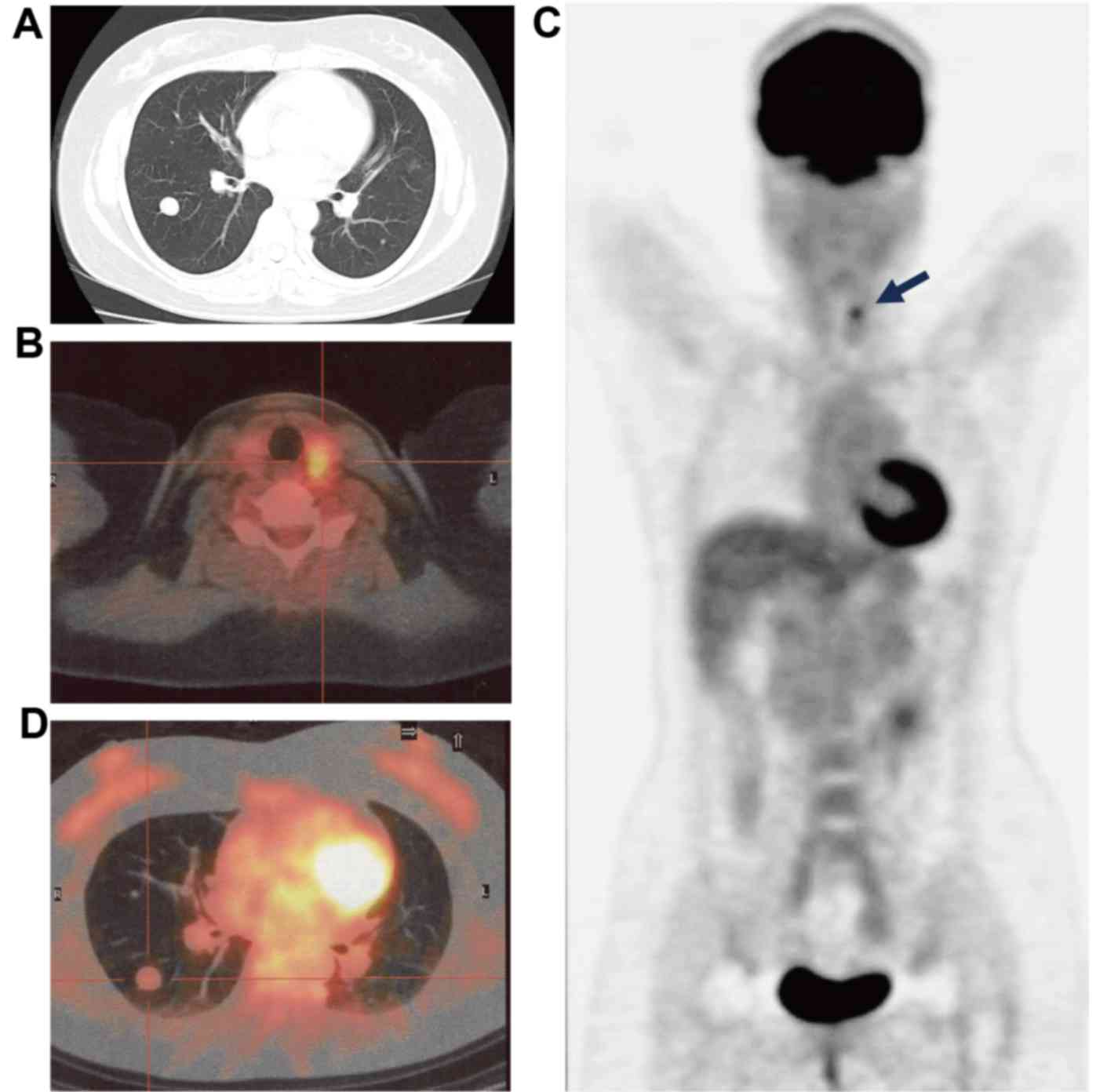
July 2009. However, a CT scan of the chest revealed the presence of
multiple nodules of varying sizes in each of the lungs (Fig. 1A). Consequently, an 18-FDG-PET/CT scan
was performed. A lesion with high 18-FDG uptake [maximum standard
uptake value (SUVmax), >4.9] was observed in the left
lobe of the thyroid gland (Fig. 1B and
C). However, the results revealed that none of the pulmonary
nodules demonstrated 18-FDG uptake (SUVmax, <1.6;
Fig. 1C and D). To elucidate whether
the pulmonary nodules were metastatic, a CT-guided needle biopsy of
the lungs was performed.
Histological examination was performed as part of
routine clinical practice. Briefly, the 7-µm thick sections
obtained from formalin-fixed and paraffin-embedded tissues were
used for further examination. Resected tissue was fixed in 10%
formalin neutral buffer solution (Muto Pure Chemicals Co., Ltd.,
Tokyo, Japan) at room temperature overnight. Hematoxylin and eosin
(H&E) staining was used according to standard clinical
histological examination. Light microscopy (BM43/DP27, original
magnification, ×400; Olympus Corporation, Tokyo, Japan) was used
for observation. H&E staining was performed with Tissue-Tek DRS
Slide Stainer (Sakura Fine Tek Europe B.V., Flemingweg,
Netherlands) according to manufacturer's protocol.
Immunohistochemical staining for αSMA and Ki-67 was performed with
Histofine Histostainer 36A (Nichirei Biosciences Inc., Tokyo,
Japan) using primary antibodies against αSMA and Ki-67 according to
manufacturer's protocol.
The 4 µm thick sections obtained from formalin-fixed
and paraffin-embedded tissues were deparaffinized in xylene and
rehydrated in a graded series of alcohol to water. Antigen
retrieval was performed using 10 mM citrate buffer (pH 6.0) at
121°C for 15 min. Sections were washed in TBS. Antigen retrieval
was not performed when examining the expression of αSMA. Sections
were blocked with 3% H2O2 at room temperature
for 10 min and then incubated for 1 h at room temperature with the
antibodies against αSMA (catalog no. 712021; clone no. 1A4;
pre-diluted working solution for Histostainer) or Ki-67 (catalog
no. 718017; clone no. SP6; pre-diluted working solution for
Histostainer) (both from Nichirei Biosciences Inc., Tokyo,
Japan).
The sections were subsequently incubated with the
Histofine Simple Stain MAX PO (Nichirei Biosciences Inc.) for 30
min at room temperature according to the manufacturer's protocol.
Staining was visualized by adding 3,3′diaminobenzidine (K5007;
Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) for 10 min
at room temperature. Sections were counterstained with haematoxylin
for 1 min and then dehydrated with a series of alcohols and
xylene.
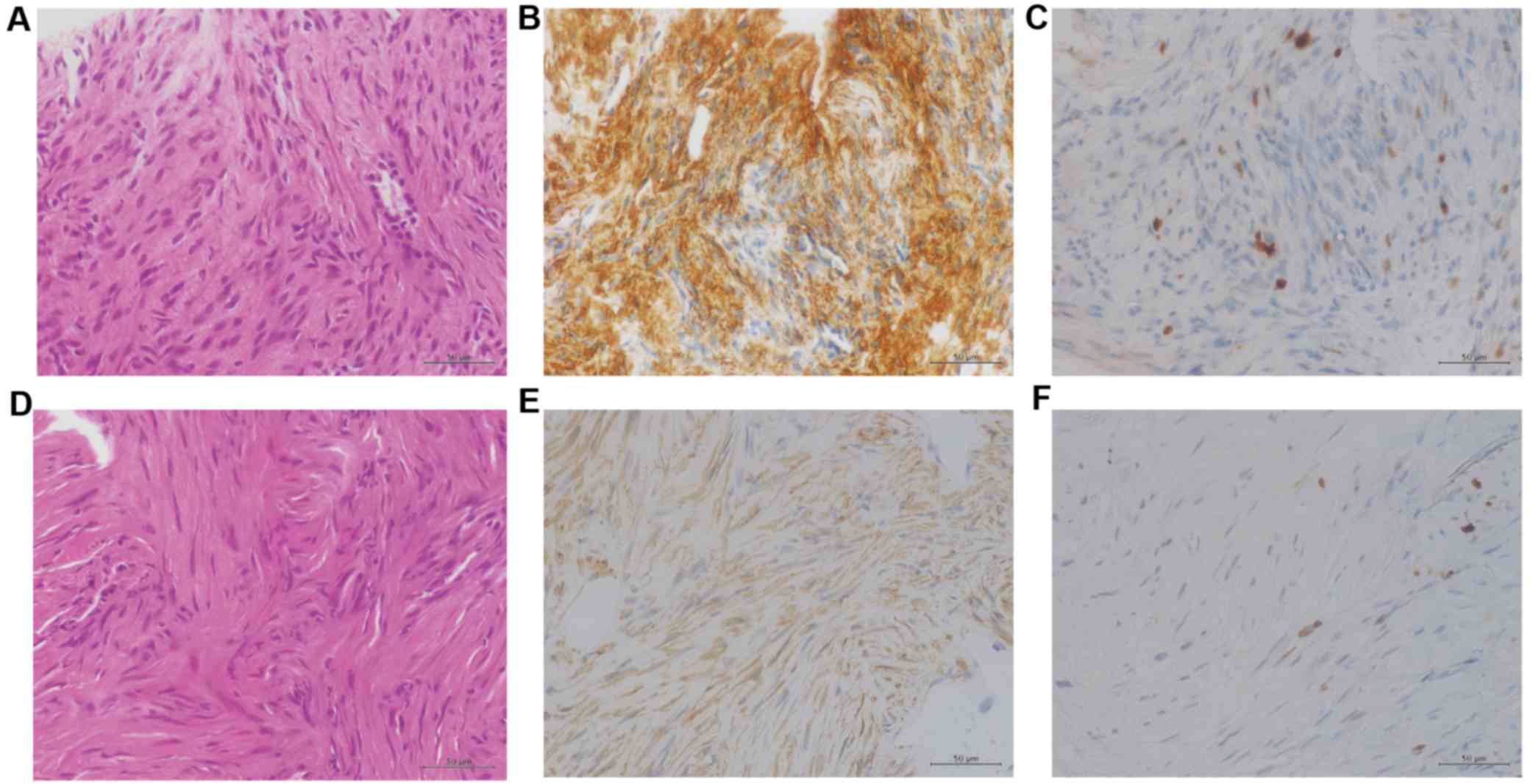
The lung biopsy tissue specimen revealed a
disordered arrangement of spindle-shaped tumor cells (Fig. 2A), and immunohistochemical examination
indicated that the cells stained positive for α-smooth muscle actin
(α-SMA). The Ki-67 ratio was <1% (Fig.
2B and C). These findings were consistent with the phenotypic
characteristics of benign leiomyoma (1). At the age of 37, the patient had
experienced extensive genital bleeding due to a uterine myoma, and
an emergency total hysterectomy with a right oophorectomy was
performed. Histological examination of the preexisting uterine
myoma tissue specimens, which were obtained during a previous
surgical resection (Japan Community Health Care Organization
Hoshigaoka Medical Center, Osaka, Japan), was performed as
aforementioned. The results of the histological examination
revealed the presence of intravascular leiomyomatosis, which is
associated with the pathogenesis of PBML (4). Thus, a definitive diagnosis of PBML was
established.
As the pulmonary lesions were not attributable to
metastases of thyroid cancer, the patient underwent a subtotal
thyroidectomy with a curative intention. The tumor-node-metastasis
classification pathological stage of the tumor was identified to be
pT1aN1aM0 (5). With regard to PBML,
observation without the use of aggressive therapy is recommended
when the tumor is clinically quiescent (3).
Case 2
A 62-year-old post-menopausal female was referred to
Kansai Medical University Takii Hospital for an investigation due
to the presence of numerous pulmonary nodules detected in a routine
chest radiography in August 2013. The patient had experienced
bilateral upper back pain for two weeks prior to the first
appointment at this hospital. The patient had no history of uterine
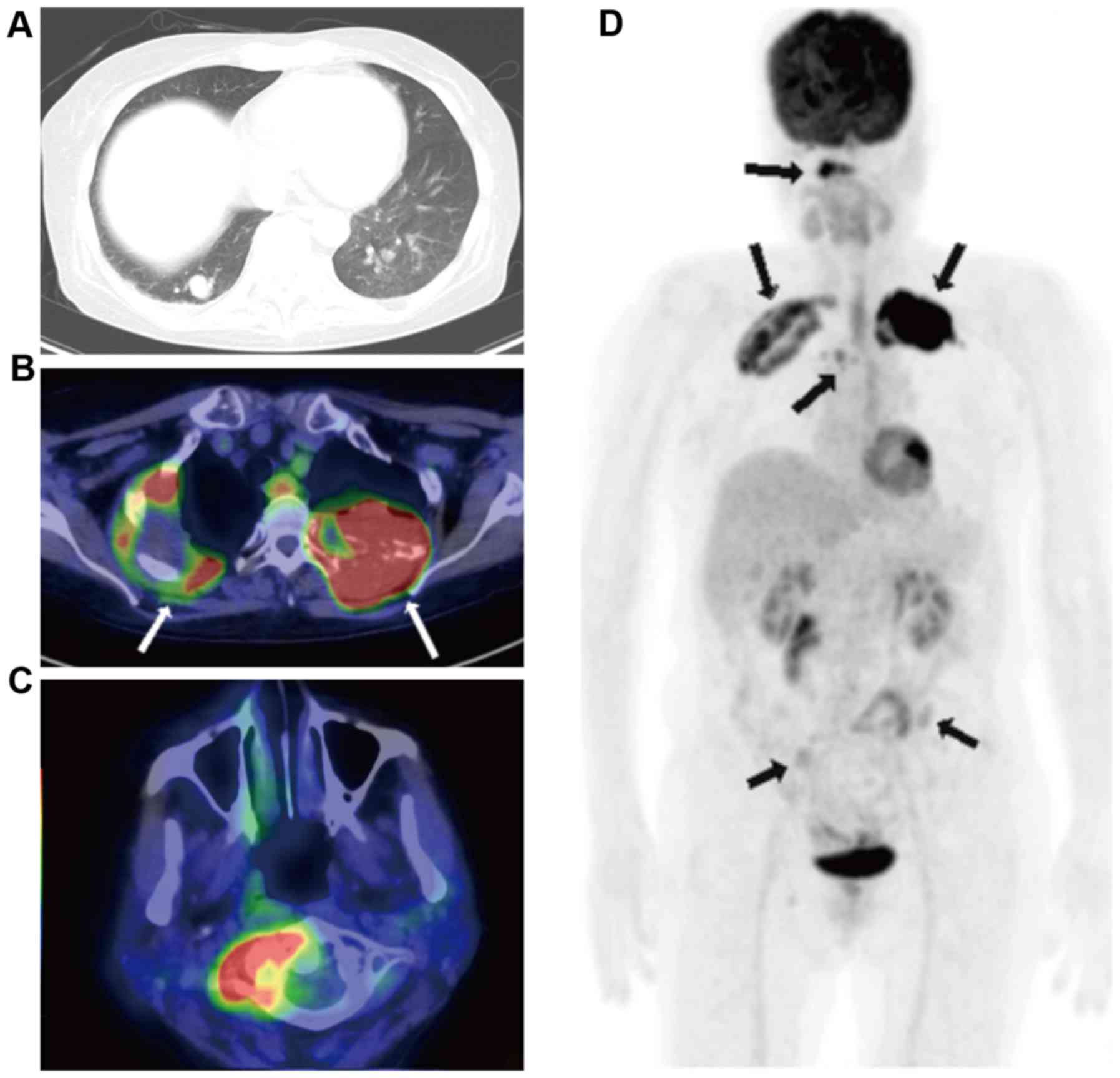
myoma. A chest CT revealed the presence of a tumor in the right
upper lung that was 60 mm in diameter and numerous nodular lesions
of varying sizes in each of the lungs, in addition to a soft tissue
mass that was 70 mm in diameter in the left second rib (Fig. 3A). The parameters assessed included
complete blood count and standard clinical laboratory examinations,
and the expression levels of specific tumor markers were within
normal limits, including carcinoembryonic antigen, serum
cytokeratin-19 fragments and Pro-gastrin-releasing pepetide.
Contrast-enhanced magnetic resonance imaging of the head revealed a
tumor in the left parietal lobe that was 20 mm in diameter, with an
edema. As the patient was suspected to have a lung cancer with
pulmonary, bone and brain metastases, an 18-FDG-PET/CT scan was
performed. The results indicated abnormally high 18-FDG uptake in
the right upper lung tumor, the left third rib tumor, atlas
vertebra, fourth thoracic vertebra, bilateral ilia and multiple
bilateral pulmonary nodules in the lungs (Fig. 3B-D; SUVmax, 20.1).
Subsequently, a CT-guided needle biopsy of the right upper lung
tumor was performed. Histological examination of the lung biopsy
tissue specimen revealed a disordered arrangement of spindle-shaped
tumor cells with mild atypia (Fig.
2D). Immunohistochemical examination revealed that the cells
stained positive for α-SMA (Fig. 2E).
By contrast, CD34, S-100, estrogen receptor (ER) and progesterone
receptor (PgR) expression was not detected in these cells. The
Ki-67 ratio was <1% (Fig. 2F).
These findings are consistent with the phenotypic characteristics
of benign leiomyoma. In order to confirm the pathological
diagnosis, an additional CT-guided needle biopsy of the tumor of
the left rib was performed. The results of the second biopsy tissue
specimen from the tumor of the left rib corroborated the findings
from the first biopsy specimen obtained from the tumor in the right
lung. Consequently, a definitive diagnosis of PBML was established.
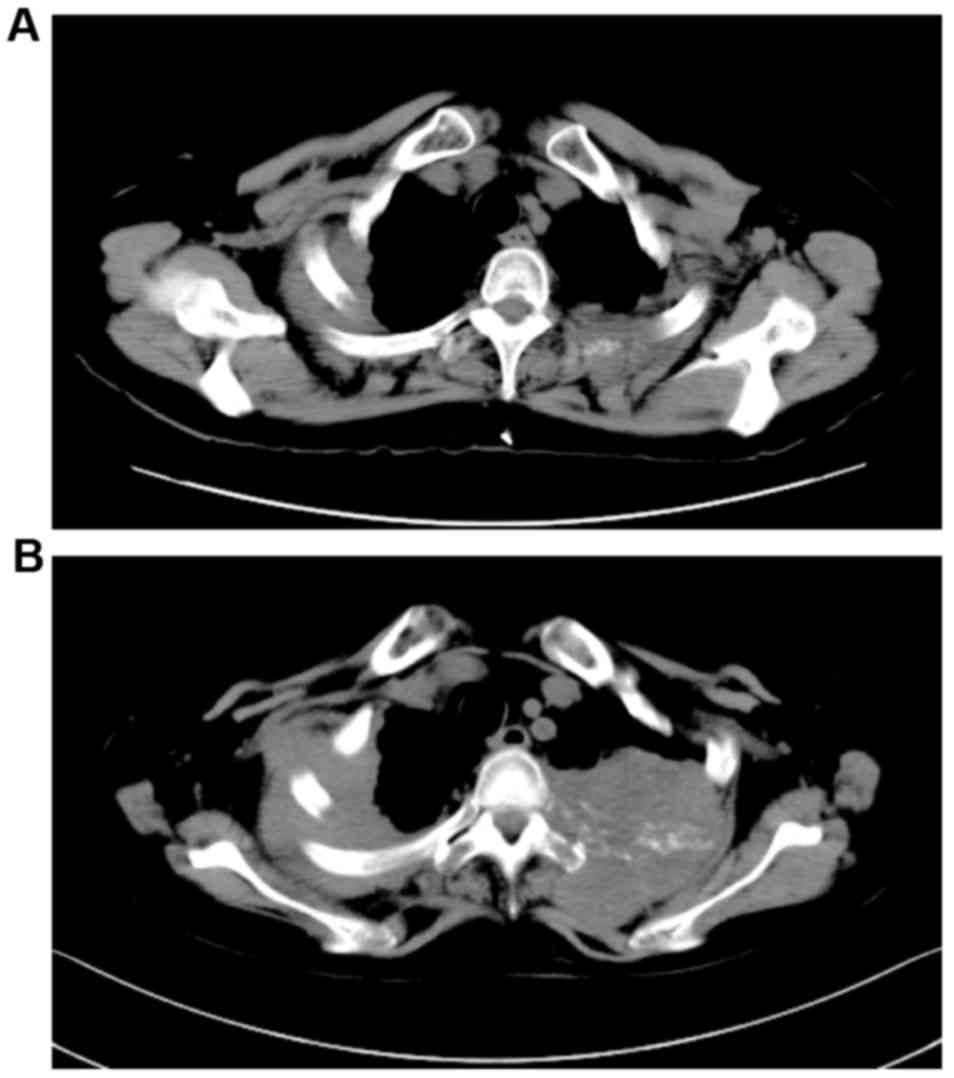
The patient received whole brain irradiation followed by palliative
irradiation for the pulmonary nodules in the right upper lung and
left second rib. However, no treatment response was observed and
the tumors were identified to be growing (Fig. 4). After three months, the patient was
re-admitted to hospital due to a consciousness disorder.
Corticosteroids and glycerin were administered, and the
neurological symptoms were temporarily improved. However, the
patient succumbed to septicemia with Clostridium perfringens.
Discussion
To date, the metabolic behavior of PBML in
18-FDG-PET/CT has attracted a high degree of interest. A number of
previous reports have demonstrated that there is a lack of 18-FDG
uptake in PBML. However, to the best of our knowledge, there has
not yet been a systematic review with respect to the 18-FDG-PET/CT
findings for PBML. Therefore, a literature review of prior case
reports concerning patients with PBML and 18-FDG-PET/CT findings
was conducted. In total, 34 cases were identified in 29 reports
(3,6–33). In
addition to these 34 cases, the present two cases were added and a
total of 36 cases of PBML were reviewed. The results are summarized
in Table I. The median age of the
patients included was 47 years (range, 34–69 years). A total of 5
patients were pre-menopausal, 2 were post-menopausal and 19
patients had previously received a hysterectomy. The conditions of
the reproductive systems of the remaining 10 patients were
unconfirmed. All of the total 36 cases were of benign leiomyoma.
However, only one patient exhibited malignant transformation
(16). With regard to the
18-FDG-PET/CT findings, the accumulation of 18-FDG varied
significantly. According to the discretion of the researchers, the
patients were assigned into the following three groups: No
(minimal) uptake, low (moderate) uptake and high (positive) uptake.
A total of 25 patients (69.4%) were assigned to the no uptake
group, 8 patients (22.2%) were assigned to the low uptake group and
3 patients (8.3%) were assigned to the high uptake group. However,
only one patient out of those 3 had a tumor that demonstrated
aggressive behavior. The SUVmax values were identified
in 13/36 cases and the median value was determined to be 2.2
(range, 1.4–20.1). The Ki-67 proliferation index ranged from 0–5%
in those 13 cases and a total of 7/13 cases had a Ki-67 index
<1%. Consequently, there was no significant correlation observed
between the uptake of 18-FDG in the tumors and the Ki-67 index. The
expression of ER and PgR within the tumors was also assessed. In
total, 21/23 patient tissue samples exhibited positive staining for
ER (91.3%) and 20/21 had positive staining for PgR (95.2%).
However, the patient in case 2 was determined to be double negative
for ER and PgR.
 | Table I.Characteristics of patients with PBML
with 18-FDG-PET/CT findings. |
Table I.
Characteristics of patients with PBML
with 18-FDG-PET/CT findings.
| Author, year | Age | Status | FDG uptake
(SUVmax) | Ki-67 index | ER | PgR | (Refs.) |
|---|
| Chan et al,
2005 |
|
|
|
|
|
| (6) |
| Case
1 | 49 | Pre | No uptake (ca.
1.4) | NE | + | + |
|
| Case
2 | 45 | HRT | no uptake (ca.
2.0) | NE | NE | NE |
|
| Moon et al,
2009 | 52 | Post | No uptake (NE) | NE | + | + | (7) |
| di Scioscio et
al, 2009 | 64 | HRT | no uptake (NE) | <1% | + | + | (8) |
| Lee, 2007 | 51 | NE | No uptake (NE) | NE | NE | NE | (9) |
| Londero et al,
2008 | 52 | HRT | low uptake (NE) | NE | + | + | (10) |
| Kasai et al,
2009 | 53 | HRT | No uptake (NE) | NE | − | + | (11) |
| Lin et al,
2010 |
|
|
|
|
|
| (12) |
| Case
1 | 38 | NE | low uptake
(0.2–2.2) | NE | NE | NE |
|
| Case
2 | 37 | HRT | Non avid
(0.7–2.9) | NE | NE | NE |
|
| Clément-Duchêne et
al, 2010 | 55 | HRT | no uptake (NE) | NE | NE | NE | (13) |
| Lin and Bradshaw,
2010 | 44 | Pre | No uptake (NE) | NE | + | + | (14) |
| Caminati et al,
2011 | 69 | NE | no uptake (NE) | NE | + | + | (15) |
| Ogawa et al,
2011 | 65 | NE | Low uptake
(ca. 3.8) | NE | NE | NE | (16) |
| Yoon et al,
2011 | 34 | Pre | faint uptake
(NE) | NE | NE | NE | (17) |
| Ni et al, 2012 | 46 | NE | Non avid
(ca. 3.1) | NE | NE | NE | (18) |
| Nakajo et al,
2012 | 50 | HRT | no uptake
(ca. 1.5) | <1% | + | + | (19) |
| Fu et al, 2012 | 46 | HRT | No uptake (NE) | <5% | NE | + | (20) |
| Alraiyes et al,
2013 | 40 | HRT | no uptake (NE) | NE | + | + | (21) |
| Okabe et al,
2013 | 47 | Pre | No uptake (NE) | NE | + | NE | (22) |
| Tsunooka et
al, 2013 |
|
|
|
|
|
| (23) |
| Case
1 | 51 | NE | no uptake (NE) | <1% | + | + |
|
| Case
2 | 58 | HRT | No uptake (NE) | <1% | + | + |
|
| Mogi et al,
2013 | 35 | NE | no uptake (NE) | NE | + | + | (24) |
| Chen et al,
2013 |
|
|
|
|
|
| (25) |
| Case
1 | 47 | NE | No uptake (NE) | NE | + | + |
|
| Case
2 | 47 | HRT | moderately
(NE) | <3% | + | + |
|
| Case
3 | 53 | NE | Moderately
(NE) | <5% | + | + |
|
| Ağaçkiran et al,
2013 | 44 | HRT | increased
(1.92–4.60) | <3% | + | + | (26) |
| Loukeri et al,
2014 | 47 | Pre | Low uptake
(ca. 2.2) | <3% | + | NE | (27) |
| Jeon et al,
2013 | 57 | HRT | low uptake
(NE) | NE | + | + | (28) |
| Okita et al,
2013 | 44 | HRT | No uptake (NE) | NE | NE | NE | (29) |
| Jin et al,
2013 | 43 | HRT | minimal
(0.9–1.8) | <1% | + | + | (30) |
| Taftaf et al,
2014 | 52 | HRT | Positive uptake
(NE) | NE | + | NE | (31) |
| Ma and Cao,
2015 | 45 | HRT | no uptake (NE) | <1% | + | + | (32) |
| Wei et al,
2015 | 47 | HRT | No uptake (NE) | <5% | NE | NE | (3) |
| Wang et al,
2016 | 48 | NE | low uptake
(0.5–2.1) | NE | NE | NE | (33) |
| Present case-1 | 38 | HRT | No uptake
(<1.6) | <1% | NE | NE |
|
| Present case-2 | 62 | Post | high uptake
(20.1) | <1% | − | − |
|
It has previously been reported that 18-FDG-PET/CT
is useful for distinguishing malignant leiomyosarcoma from benign
leiomyoma (34,35). In general, SUV values for
leiomyosarcoma were observed to be significantly greater than those
for leiomyoma. However, numerous reports for 18-FDG-avid leiomyoma
were also identified (36–38). Furthermore, a series of comprehensive
analyses for those patients who underwent 18-FDG-PET/CT revealed
that small proportion of benign uterine leiomyomas exhibited
positive 18-FDG uptake. In the present study, a case of 18-FDG-avid
PBML is detailed. Summarizing previous studies indicated that 33
PBML cases involved 18-FDG non-avid tumors, and only 3 were
18-FDG-avid tumors. No particular phenotype was identified to be
associated with 18-FDG-avid PBML. Further investigations are
required in order to improve present understanding of the
biological characteristics of PBML, thus leading to its optimal
management.
References
|
1
|
Steiner PE: Metastasizing fibroleiomyoma
of the uterus: Report of a case and review of the literature. Am J
Pathol. 15:89–110, 7. 1939.PubMed/NCBI
|
|
2
|
Ki EY, Hwang SJ, Lee KH, Park JS and Hur
SY: Benign metastasizing leiomyoma of the lung. World J Surg Oncol.
11:2792013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wei WT and Chen PC: Benign metastasizing
leiomyoma of the lung: A case report and literature review. Oncol
Lett. 10:307–312. 2015.PubMed/NCBI
|
|
4
|
Mahmoud MS, Desai K and Nezhat FR:
Leiomyomas beyond the uterus; benign metastasizing leiomyomatosis
with paraaortic metastasizing endometriosis and intravenous
leiomyomatosis: A case series and review of the literature. Arch
Gynecol Obstet. 291:223–230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wada N, Nakayama H, Suganuma N, Masudo Y,
Rino Y, Masuda M and Imada T: Prognostic value of the sixth edition
AJCC/UICC TNM classification for differentiated thyroid carcinoma
with extrathyroid extension. J Clin Endocrinol Metab. 92:215–218.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chan JW, Law WL, Cheung SO, Lee MP, Ng CK,
Lee S, Ko KM, Ma CC, Liu JY, Chan TM and Mok TY: Benign
metastasising leiomyoma: A rare but possible cause of bilateral
pulmonary nodules in Chinese patients. Hong Kong Med J. 11:303–306.
2005.PubMed/NCBI
|
|
7
|
Moon H, Park SJ, Lee HB, Kim SR, Choe YH,
Chung MJ, Jin GY and Lee YC: Pulmonary benign metastasizing
leiomyoma in a postmenopausal woman. Am J Med Sci. 338:72–74. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
di Scioscio V, Feraco P, Miglio L, Toni F,
Malvi D, Pacilli AM, Fasano L, Fabbri M and Zompatori M: Benign
metastasizing leiomyoma of the lung: PET findings. J Thorac
Imaging. 24:41–44. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee SM: Incidental multiple pulmonary
nodules: Benign metastasizing leiomyoma and 18F-FDG
PET/CT. Nucl Med Mol Imaging. 41:258–259. 2007.
|
|
10
|
Londero AP, Perego P, Mangioni C, Lellé
RJ, Londero F and Marchesoni D: Locally relapsed and metastatic
uterine leiomyoma: A case report. J Med Case Rep. 2:3082008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kasai Y, Masuya D, Matsuoka H, Yoshimatsu
H and Suzuki Y: Value of FDG-PET in a case of benign metastasizing
leiomyoma. The J Jpn Assoc Chest Surg. 23:871–874. 2009. View Article : Google Scholar
|
|
12
|
Lin X, Fan W, Lang P, Hu Y, Zhang X and
Sun X: Benign metastasizing leiomyoma identified using 18F-FDG
PET/CT. Int J Gynaecol Obstet. 110:154–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clément-Duchêne C, Vignaud JM, Régent D
and Martinet Y: Benign metastasizing leiomyoma with lung cystic
lesions and pneumothoraces: A case report. Resp Med CME. 3:183–185.
2010. View Article : Google Scholar
|
|
14
|
Lin TK and Bradshaw DA: Benign
metastasizing leiomyoma in a patient without gynecologic symptoms.
Chest. 138:89A2010. View Article : Google Scholar
|
|
15
|
Caminati A, Cavazza A, Mirenda MR and
Harari S: A 69-year-old female with multiple, bilateral pulmonary
nodules. Eur Respir Rev. 20:56–59. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ogawa M, Hara M, Ozawa Y, Moriyama S, Yano
M, Shimizu S and Shibamoto Y: Benign metastasizing leiomyoma of the
lung with malignant transformation mimicking mediastinal tumor.
Clin Imaging. 35:401–404. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoon G, Kim TJ, Sung CO, Choi CH, Lee JW,
Lee JH, Bae DS and Kim BG: Benign metastasizing leiomyoma with
multiple lymph node metastasis: A case report. Cancer Res Treat.
43:131–133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ni Y, Shi G, Wan H, Shen J, Jiang X and
Yuan F: Pulmonary benign metastasizing leiomyoma: Case report and
review of the literature. Clin Exp Obstet Gynecol. 39:249–251.
2012.PubMed/NCBI
|
|
19
|
Nakajo M, Nakayama H, Sato M, Fukukura Y,
Nakajo M, Kajiya Y, Yanagi M, Tabata K and Higashi M: FDG-PET/CT
finding of benign metastasizing leiomyoma of the lung. Acta Radiol
Short Rep. 1:pii: arsr.2012.120012. 2012.PubMed/NCBI
|
|
20
|
Fu Y, Li H, Tian B and Hu B: Pulmonary
benign metastasizing leiomyoma: A case report and review of the
literature. World J Surg Oncol. 10:2682012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alraiyes AH, Kheir F, Hirsh S, Salerno D,
Bernal-Green L and Daroca P: A 40-year-old woman with multiple lung
nodules. Chest. 143:1826–1829. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okabe R, Shoji T and Huang CL: Benign
metastasizing leiomyoma of the lung with spontaneous pneumothorax.
Thorac Cardiovasc Surg Rep. 2:26–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsunooka N, Hirayama K and Inazawa K:
Benign lung metastasizing leiomyoma: A report of 2 cases. Nihon
Rinsho Geka Gakkai Zasshi (Journal of Japan Surgical Association).
74:2428–2433. 2013. View Article : Google Scholar
|
|
24
|
Mogi A, Hirato J, Kosaka T, Yamaki E and
Kuwano H: Benign metastasizing leiomyoma of the lung: Report of a
case. Gen Thorac Cardiovasc Surg. 61:719–722. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen S, Zhang Y, Zhang J, Hu H, Cheng Y,
Zhou J, Shen L and Chen H: Pulmonary benign metastasizing leiomyoma
from uterine leiomyoma. World J Surg Oncol. 11:1632013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ağaçkiran Y, Findik G, Ustün LN, Aydoğdu K
and Kaya S: Pulmonary benign metastasizing leiomyoma: An extremely
rare case. Turk Patoloji Derg. Apr 9–2014.(Epub ahead of
print).
|
|
27
|
Loukeri AA, Pantazopoulos IN, Tringidou R,
Giampoudakis P, Valaskatzi A, Loukeri PA and Kampolis CF: Benign
metastasizing leiomyoma presenting as cavitating lung nodules.
Respir Care. 59:e94–e97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jeon HW, Choi SH, Sung SW and Park JK:
Pulmonary benign metastasizing leiomyoma: Report of three cases.
World J Surg Oncol. 11:2812013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Okita R, Yasuda K, Nojima Y, Maeda A,
Yukawa T, Saisho S, Shimizu K, Akiyama T, Miyagi Y, Oda T and
Nakata M: CT, MRI and 18F-FDG PET-CT findings of
pulmonary benign metastasizing leiomyoma: A case report. Open J
Thoracic Surg. 3:127–129. 2013. View Article : Google Scholar
|
|
30
|
Jin X, Meng Y, Zhu Z, Jing H and Li F:
Elevated 99mTc 3PRGD2 activity in benign metastasizing leiomyoma.
Clin Nucl Med. 38:117–119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Taftaf R, Starnes S, Wang J, Shipley R,
Namad T, Khaled R and Abdel Karim N: Benign metastasizing
leiomyoma: A rare type of lung metastases-two case reports and
review of the literature. Case Rep Oncol Med.
2014:8428012014.PubMed/NCBI
|
|
32
|
Ma H and Cao J: Benign pulmonary
metastasizing leiomyoma of the uterus: A case report. Oncol Lett.
9:1347–1350. 2015.PubMed/NCBI
|
|
33
|
Wang HC, Wang YB, Chen XH and Cui LL:
Uterine intravenous leiomyomatosis with intracardiac extension and
pulmonary benign metastases on FDG PET/CT: A case report. Korean J
Radiol. 17:289–294. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schwarzbach MH, Dimitrakopoulou-Strauss A,
Willeke F, Hinz U, Strauss LG, Zhang YM, Mechtersheimer G, Attigah
N, Lehnert T and Herfarth C: Clinical value of [18-F]
fluorodeoxyglucose positron emission tomography imaging in soft
tissue sarcomas. Ann Surg. 231:380–386. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nagamatsu A, Umesaki N, Li L and Tanaka T:
Use of 18F-fluorodeoxyglucose positron emission tomography for
diagnosis of uterine sarcomas. Oncol Rep. 23:1069–1076.
2010.PubMed/NCBI
|
|
36
|
Ak I, Ozalp S, Yalcin OT, Zor E and
Vardareli E: Uptake of 2-[18F]fluoro-2-deoxy-D-glucose in uterine
leiomyoma: Imaging of four patients by coincidence positron
emission tomography. Nucl Med Commun. 25:941–945. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shida M, Murakami M, Tsukada H, Ishiguro
Y, Kikuchi K, Yamashita E, Kajiwara H, Yasuda M and Ide M: F-18
fluorodeoxyglucose uptake in leiomyomatous uterus. Int J Gynecol
Cancer. 17:285–290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vriens D, de Geus-Oei LF, Flucke UE, van
der Kogel AJ, Oyen WJ, Vierhout ME and van der Meer JW: Benign
uterine uptake of FDG: A case report and review of literature. Neth
J Med. 68:379–380. 2010.PubMed/NCBI
|