Introduction
Gastric cancer (GC) remains a major public health
problem, since it was the fourth most common type of cancer and the
third most common cause of cancer-associated mortality worldwide in
2012 (1). In China, GC is the third
frequently diagnosed cancer and the third leading cause of
cancer-associated mortality in 2011 (2). The understanding of molecular mechanisms
involved in the development and the progression of GC is important
for the diagnosis and treatment of this disease.
Calpains are a group of calcium-activated,
non-lysosomal neutral cysteine proteases (3). At present, the best-characterized
calpain isoforms are calpain-1 (µ-calpain or CAPN1) and calpain-2
(m-calpain or CAPN2), which require µM and mM concentrations of
Ca2+ for their intracellular activity, respectively
(3). CANP1 and CAPN2 are composed of
a distinct large catalytic subunit (80 kDa) and a small common
regulatory subunit (28 kDa) (3).
Calpains are responsible for the cleavage of a broad spectrum of
protein substrates, resulting in the generation of functional
fragments rather than total degradation products (4,5). It has
been confirmed that calpains are involved in cell differentiation,
migration and transformation, and perform an important role in
cancer pathogenesis and progression (4,6,7).
The proteolytic activity of calpain is inhibited by
an endogenous inhibitor, calpastatin, in a substrate-competitive
manner (8). The expression level of
calpastatin directly effects the calpain activity (9). In addition, calmodulin (CaM) is another
endogenous regulator of proteolytic activity of calpains (10). By binding to the PEST sequence [a
sequence that is rich in proline (P), glutamic acid (E), serine (S)
and threonine (T)], which is the target sequence of calpain, CaM
protects the protein to be cleaved by calpain (11–14).
The role that calpain system performs in cancer is
complicated. Calpain has been reported to be involved in apoptosis
of cancer cells (15). In addition,
the upregulation of calpain may be involved in cancer development
and migration (16), and calpain
expression is upregulated in a number of tumors, including
colorectal adenocarcinoma (17),
breast cancer (18) and ovarian
cancer (19). In GC, it has been
reported that the calpain activity was upregulated compared with
normal tissue (20), and it is
speculated that higher calpain and calpastatin expression may be a
favorable prognostic marker for GC (21). However, there is still no direct
experimental data about the comparison of the protein expression
levels of the members of calpain system in GC and normal gastric
mucosa.
In order to understand the roles that calpain,
calpastatin and CaM serve in GC, the current study compared the
expression levels of CAPN1, CAPN2, calpastatin and CaM in human GC
tissues and normal glandular tissues with immunohistochemistry. The
present results demonstrated that CAPN2 protein level increased in
GC compared with normal tissue, while calpastatin and CaM protein
level decreased. Calpain-1 appeared unchanged. None of the protein
expression levels were found to be associated with the clinical
variables of GC in the present study. The ratio of (CAPN1 ×
CAPN2)/(calpastatin × CaM) best predicted GC.
Materials and methods
Materials
Polyclonal antibodies specific for CANP1 (dilution,
1:1,000; cat no. PA5-27681), CAPN2 (dilution, 1:1,000; PA5-27720)
and CaM (cat no. MA3-917; 1:20) were obtained from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA) and anti-calpastatin (cat no.
sc-20779; 1:500) was purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Immunol Staining Primary Antibody Diluent Buffer
and Hematoxylin were from Nanjing KeyGen Biotech Co., Ltd.
(Nanjing, China). UltraSensitive™ S-P kit and 3,3′-diaminobenzidine
(DAB) kit were purchased from Fuzhou Maixin Biotech Co., Ltd.
(Fuzhou, China).
Clinical samples
Cancerous tissues and normal peritumoral gastric
mucosa (5 cm away from the tumor) were collected from patients
(n=51) treated at Northern Jiangsu People's Hospital (Yangzhou,
China) between January 2014 and December 2015. The age and gender
distribution, and the clinicopathological variables of the patients
are summarized in Table I. The study
design was approved by the local ethics committee of Northern
Jiangsu People's Hospital, in accordance with the guidelines of the
1975 Declaration of Helsinki. Written informed consent was obtained
from all patients. Surgical pathology specimens of 51 patients with
GC who had undergone resection were collected directly from surgery
and fixed in formalin (10%) immediately at 4°C for 24 h.
Normal-appearing peritumoral gastric mucosa was similarly dissected
and fixed.
 | Table I.Clinicopathological variables of
patient sample. |
Table I.
Clinicopathological variables of
patient sample.
| Variables | Patients, n |
|---|
| Age, years (mean ±
SD) | 62.5±10.1 |
| Gender |
|
| Male | 41 |
|
Female | 10 |
| Tumor size,
cm3 (mean ± SD) | 6.4±1.6 |
| T classification |
|
| 1 | 1 |
| 2 | 6 |
| 3 | 39 |
| 4 | 5 |
| N classification |
|
| 0 | 10 |
| 1 | 16 |
| 2 | 20 |
| 3 | 5 |
| Clinical stage |
|
| I | 1 |
| II | 7 |
| IIIa | 16 |
| IIIb | 20 |
| IV | 7 |
Immunohistochemistry
Sections (4 µm) of the formalin-fixed,
paraffin-embedded tissues were cut, followed by deparaffinizing in
xylene and rehydrating in an alcohol gradient. Antigen retrieval
was performed in citrate buffer (pH 6.0) using an electric pressure
cooker for 2 min at 120°C, and cooled naturally in the buffer for
~20 min. Immunostaining was performed according to the
streptavidin-peroxidase method using a kit (Nanjing KeyGen Biotech
Co., Ltd.) according to the manufacturer's protocol. Subsequent to
blocking of the samples with blocking solution in the kit, the
primary antibodies anti-CAPN1 (dilution, 1:1,000; cat no.
PA5-27681; Thermo Fisher Scientific, Inc.), anti-CAPN2 (dilution,
1:1,000; cat no. PA5-27720; Thermo Fisher Scientific, Inc.),
anti-calpastatin (dilution, 1:500; cat no. sc-20779; Santa Cruz
Biotechnology, Inc.) and anti-CaM (1:20; cat no. MA3-917; Thermo
Fisher Scientific, Inc.) were diluted using primary antibody
diluents and applied to the tissues at 4°C overnight. Staining was
achieved using the DAB dye for 1 min at 25°C.
The staining was observed with an upright microscope
(magnification, ×40, 10 fields of view/slice) equipped with a CCD
camera (Olympus Corporation, Tokyo, Japan). The images were
document with cellSens Entry software (Ver. 1.5; Olympus
Corporation, Tokyo, Japan). The expression of the four proteins was
quantified by mean optical density (MOD) using Image-Pro Plus 6.0
software (Media Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
The results were presented as mean ± standard
deviation. Based on whether the distribution was normal or not,
paired t-test or Wilcoxon matched-pairs signed rank test were used
to compare the MOD variance in cancer and its peritumoral normal
mucosa tissues. The association between calpain system protein
expressions with each other was subjected to Pearson's rank
correlation. Receiver operator characteristic (ROC) curves were
used to investigate which protein could be the best indicator for
GC, and the cut-off points were generated according to ROC curve
and Youden's index. The association between protein expression and
clinicopathological variables was assessed using unpaired t-test
(gender) and Spearman's rank correlation (other parameters).
P<0.05 was considered to indicate a statistically significant
difference. Statistical analysis was performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA).
Results
Stain location and frequency
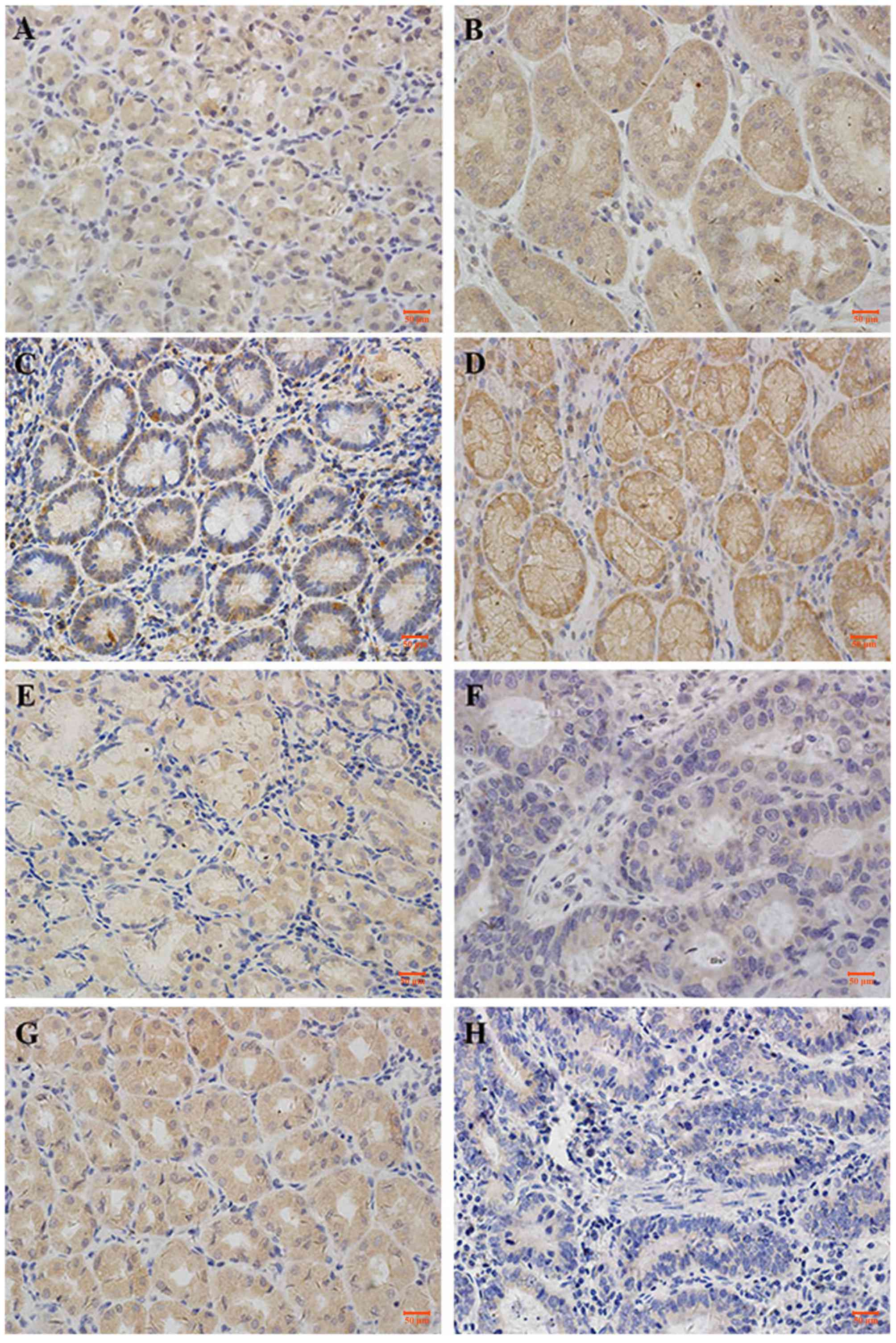
CAPN1, CAPN2, calpastatin and CaM demonstrated
cytoplasmic staining, with a certain degree of heterogeneity within
samples. Typical staining patterns are shown in Fig. 1. In normal tissues, CAPN1 had a median
MOD value of 2.7×10−2, and ranged between
2.9×10−3 to 6.1×10−2; CAPN2 had a median MOD
value of 5.0×10−2 and ranged between 1.8×10−2
to 1.1×10−1; calpastatin had a median MOD value of
3.3×10−2 and ranged between 1.9×10−3 to
9.1×10−2; and CaM had a median MOD of
1.2×10−2 and ranged between 2.0×10−8 to
8.1×10−2. In GC tissues, CAPN1 had a median MOD value of
3.2×10−2 and ranged between 3.2×10−3 to
1.4×10−1; CAPN2 had a median MOD value of
8.1×10−2 and ranged between 2.0×10−2 to
1.4×10−1; calpastatin had a median MOD value of
1.7×10−2 and ranged between 2.8×10−3 to
6.6×10−2; and CaM had a median MOD of
1.6×10−3 and ranged between 1.0×10−8 to
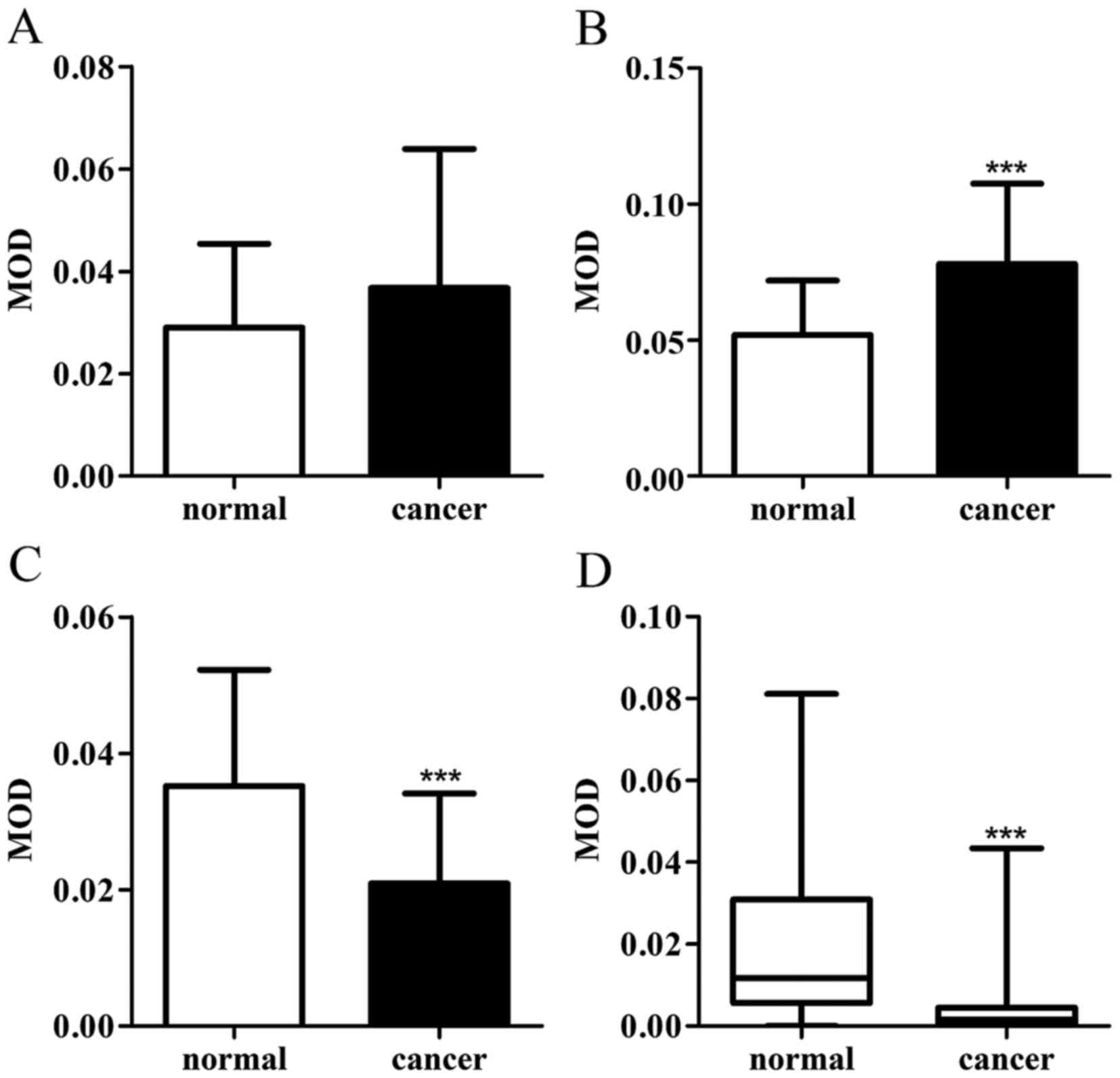
4.3×10−2. Paired t-test demonstrated that calpain-2
exhibited higher scores in GC compared with the normal tissue
(Fig. 2B), while the expression of
calpastatin and CaM decreased in cancer tissues (Fig. 2C and D). Wilcoxon matched-pairs signed
rank test indicated that the expression of calpain-1 exhibited no
difference in GC tissues against gastric mucosa (Fig. 2A). The association between the
expression of the proteins with each other was assessed using the
Pearson's rank correlation coefficient. As exhibited in Table II, in GC tissues, the expression of
CAPN1, CAPN2, calpastatin and CaM proteins significantly correlated
with each other (P<0.05), though these correlations were weak.
In normal-appearing peritumoral gastric mucosa, the expression of
these four proteins also correlated with each other. The expression
of calpastatin exhibited a stronger correlated with calpain-1
(r=0.741; P<0.001), while that of the others were weak.
 | Table II.The correlation between expression of
the proteins in normal-appearing peritumoral gastric mucosa and
cancer tissue with each other. |
Table II.
The correlation between expression of
the proteins in normal-appearing peritumoral gastric mucosa and
cancer tissue with each other.
|
| Normal tissue | Cancer tissue |
|---|
|
|
|
|
|---|
| Protein | Pearson value | P-value | Pearson value | P-value |
|---|
| Calpain-1 with
calpaspatin | 0.741 | <0.0001 | 0.342 | 0.014 |
| Calpain-2 with
calpaspatin | 0.535 | <0.0001 | 0.553 | <0.0001 |
| CaM with
calpaspatin | 0.551 | <0.0001 | 0.396 | 0.004 |
| Calpain-1 with
calpain-2 | 0.419 | 0.002 | 0.441 | 0.001 |
| Calpain-1 with
CaM | 0.443 | 0.001 | 0.428 | 0.002 |
| Calpain-2 with
CaM | 0.402 | 0.003 | 0.401 | 0.004 |
Decisions
ROC curves
ROC curves were employed to determine whether the
calpain system proteins are potential GC specific biomarkers. Area
under the curve (AUC) of ROC curves, cut-off points and positive
likelihood ratio were generated (Table
III). Among the four proteins, CaM appeared to be the best
discriminating marker for GC (AUC=83.2%). Since the calpain
activity is regulated by calpastatin and the activity modulator
CaM, the ratios of calpains to the regulators were also
investigated (Table III). The
results demonstrated that when the ratios of these protein levels
were used, the AUCs of ROC curves increased. The MOD value ratio of
(CAPN1 xCAPN2)/(calpastatin × CaM) demonstrated the best
discrimination power (AUC=89.7%) compared with the situation when
single protein expression was used (Table III). Since the ratio of (CAPN1 ×
CAPN2)/(calpastatin × CaM) may better reflect the calpain activity,
calpain activity has the potential to be developed as a new
diagnostic indicator for GC.
 | Table III.Cut-off points, sensitivity,
specificity and AUC of ROC. |
Table III.
Cut-off points, sensitivity,
specificity and AUC of ROC.
| Protein | Cut-off point | Sensitivity, % | Specificity, % | LR | AUC of ROC, % |
|---|
| CAPN1 | 0.056 | 25.49 | 94.12 | 4.33 | 56.56 |
| CAPN2 | 0.077 | 52.94 | 92.16 | 6.75 | 75.24 |
| Calpastatin | 0.022 | 86.27 | 66.67 | 2.59 | 76.78 |
| CaM | 0.007 | 72.55 | 86.27 | 5.29 | 83.22 |
| CAPN1 × CAPN2/(CaM
× calpastatin) | 25.000 | 84.31 | 86.27 | 6.14 | 89.73 |
Association with clinicopathological
criteria
The expression levels of CAPN1, CAPN2, calpastatin
and CaM were assessed for their correlation with a number of
clinicopathological variables, including age, gender, tumor size,
postoperative pathological stage and clinicopathological stage
(cTNM) (Table IV). None of the
proteins investigated in GC or normal tissues showed significant
correlation with the clinicopathological variables.
 | Table IV.Association between calapin-1,
calpain-2, calpastatin and CaM protein expression and
clinicopathological variables. |
Table IV.
Association between calapin-1,
calpain-2, calpastatin and CaM protein expression and
clinicopathological variables.
|
| Calpain-1 | Calpain-2 | Calpastatin | CaM |
|---|
|
|
|
|
|
|
|---|
| Parameter | Normal | Cancer | Normal | Cancer | Normal | Cancer | Normal | Cancer |
|---|
| Gender | 0.333 | 0.439 | 0.780 | 0.348 | 0.159 | 0.678 | 0.051 | 0.719 |
| Age | 0.861 | 0.466 | 0.534 | 0.161 | 0.111 | 0.417 | 0.938 | 0.447 |
| Size | 0.376 | 0.946 | 0.479 | 0.519 | 0.330 | 0.619 | 0.122 | 0.134 |
| T | 0.253 | 0.767 | 0.115 | 0.413 | 0.209 | 0.869 | 0.135 | 0.962 |
| N | 0.873 | 0.391 | 0.333 | 0.332 | 0.752 | 0.185 | 0.711 | 0.124 |
| cTNM | 0.285 | 0.926 | 0.051 | 0.418 | 0.215 | 0.454 | 0.627 | 0.590 |
Discussion
The current study investigated the expression of
four calpain system proteins, CAPN1, CAPN2, calpastatin and CaM
between GC vs. uninvolved mucosa tissues. The expression level of
CAPN2 was higher in GC tissues, while the levels of calpastatin and
CaM, which inhibit calpain activity, were higher in uninvolved
mucosa tissues. However, CAPN1 protein levels were similar in the
two tissues. The alteration of these protein expression levels is
in accordance with previous enzyme activity investigation 20). In
addition, the alteration of calpain system protein levels was also
observed in other types of cancer. In colorectal adenocarcinomas,
similar to our results in GC, upregulation of CAPN2 and
downregulation of calpastatin was observed (17). In human prostate cancer, the mRNA
level of CAPN1 increased (22). These
results suggested that calpains may be an important factor in
tumorigenesis and tumor progression, and higher calpain enzyme
activity may be a negative prognostic marker. Studies demonstrated
that calpains were responsible for the generation of active forms
of proteins that facilitate the progress of cancer: In breast
cancer, calpain was associated with the cleavage of human epidermal
growth factor receptor 2 (23) and
E-cadherin (24), and the two
products were responsible for the progression of cancer. In
prostate cancer, calpain was hypothesized to be responsible for the
generation of active androgen receptor cleavage form (25). In accordance, high-level calpain
indicates poor clinical outcome in human breast cancer (18,26).
However, in GC Storr et al reported that high level of
CAPN1, CAPN2 and calpastatin each indicated a favorable clinical
outcome (21). According to the
present results, the expression of calpastatin was in positive
correlation with calpain in GC. The correlation between the protein
level of calpain and its endogenous inhibitor, calpastatin, may be
reason that the clapains and its inhibitor, which exert opposite
functions, both indicated a favorable prognostic result of GC.
Since calpain activity in the prognostic investigation is unknown,
whether the higher calpain activity performs a favorable role in GC
requires additional investigation.
To explore whether calpains and their associated
proteins may be used as biomarkers that possess diagnostic and
treatment potentials, ROC curves were employed in the present
study. According to the AUCs (Table
III), when using a single protein, CaM provides the best
prediction (AUC =83.2%). In addition, the four proteins in
combination, (CAPN1 × CAPN2)/(calpastatin × CaM), improved the
prediction ability (AUC =89.7%). Since the ratio of (CAPN1 ×
CAPN2)/(calpastatin × CaM) may better reflect the calpain activity,
the present results suggest that intra-tumor calpain activity may
provide a potential biomarker for GC. It has been reported that
calpain was responsible for platelet secretion (27). Galectin-3 secretion is also dependent
on the calpain activity (28). In
addition, calpain itself can be secreted by tubular epithelial
cells (29). Thus, detecting serum
secreted calpain or screening serum secreting proteins direct or
indirect regulated by calpain may provide a new diagnostic
target.
The protein expression levels of intra-tumor or
normal-appearing gastric mucosal calpain system showed no
correlation with various clinicopathological variables, including
gender, age, tumor size, T category, N category or cTNM. These
results are different from Storr's study (21). Whether this system is correlated with
the clinicopathological variables requires additional
investigation.
In conclusion, calpain may be a positive factor in
tumorigenesis and tumor progression. Although the protein
expression of all these four proteins was not in association with
the clinical variables of GC in the present study, higher calpain
enzyme activity may be a negative prognostic marker, since calpains
are responsible for the generation of active forms of certain
proteins that facilitate the progression of cancer. The ratio of
(CAPN1 × CAPN2)/(calpastatin × CaM) may serve as a potential
biomarker for GC.
Acknowledgements
The present study was supported by the National
Natural Foundation of China (grant no. 81102460), the College
Natural Foundation of Jiangsu Education Department (grant no.
14KJB310026), the Yangzhou Natural Foundation (grant no.
YZ2014020), the Fundamental Research Funds for the Central
Universities and the Yangzhou University Fundamental Research
Funds.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Zeng H, Zhang S and He J:
Annual report on status of cancer in China, 2011. Chin J Cancer
Res. 27:2–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moretti D, Del Bello B, Allavena G and
Maellaro E: Calpains and cancer: Friends or enemies? Arch Biochem
Biophys. 564:26–36. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carragher NO and Frame MC: Calpain: A role
in cell transformation and migration. Int J Biochem Cell Biol.
34:1539–1543. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sorimachi H, Hata S and Ono Y: Impact of
genetic insights into calpain biology. J Biochem. 150:23–37. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang Y and Wang KK: The calpain family
and human disease. Trends Mol Med. 7:355–362. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Storr SJ, Carragher NO, Frame MC, Parr T
and Martin SG: The calpain system and cancer. Nat Rev Cancer.
11:364–374. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang Z, Hoffmann FW, Norton RL, Hashimoto
AC and Hoffmann PR: Selenoprotein K is a novel target of m-calpain,
and cleavage is regulated by Toll-like receptor-induced calpastatin
in macrophages. J Biol Chem. 286:34830–34838. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li X, Li Y, Shan L, Shen E, Chen R and
Peng T: Over-expression of calpastatin inhibits calpain activation
and attenuates myocardial dysfunction during endotoxaemia.
Cardiovasc Res. 83:72–79. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marshall CB, Nishikawa T, Osawa M,
Stathopulos PB and Ikura M: Calmodulin and STIM proteins: Two major
calcium sensors in the cytoplasm and endoplasmic reticulum. Biochem
Biophys Res Commun. 460:5–21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barnes JA and Gomes AV: PEST sequences in
calmodulin-binding proteins. Mol Cell Biochem. 149–150:17–27. 1995.
View Article : Google Scholar
|
|
12
|
Sivanandam A, Murthy S, Chinnakannu K, Bai
VU, Kim SH, Barrack ER, Menon M and Reddy GP: Calmodulin protects
androgen receptor from calpain-mediated breakdown in prostate
cancer cells. J Cell Physiol. 226:1889–1896. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Molinari M, Anagli J and Carafoli E: PEST
sequences do not influence substrate susceptibility to calpain
proteolysis. J Biol Chem. 270:2032–2035. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang KK, Villalobo A and Roufogalis BD:
Calmodulin-binding proteins as calpain substrates. Biochem J.
262:693–706. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang H, Murthy S, Sarkar FH, Sheng S,
Reddy GP and Dou QP: Calpain-mediated androgen receptor breakdown
in apoptotic prostate cancer cells. J Cell Physiol. 217:569–576.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu T, Mendes DE and Berkman CE: Prolonged
androgen deprivation leads to overexpression of calpain 2:
Implications for prostate cancer progression. Int J Oncol.
44:467–472. 2014.PubMed/NCBI
|
|
17
|
Lakshmikuttyamma A, Selvakumar P, Kanthan
R, Kanthan SC and Sharma RK: Overexpression of m-calpain in human
colorectal adenocarcinomas. Cancer Epidemiol Biomarkers Prev.
13:1604–1609. 2004.PubMed/NCBI
|
|
18
|
Storr SJ, Lee KW, Woolston CM, Safuan S,
Green AR, Macmillan RD, Benhasouna A, Parr T, Ellis IO and Martin
SG: Calpain system protein expression in basal-like and
triple-negative invasive breast cancer. Ann Oncol. 23:2289–2296.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Storr SJ, Safuan S, Woolston CM,
Abdel-Fatah T, Deen S, Chan SY and Martin SG: Calpain-2 expression
is associated with response to platinum based chemotherapy,
progression-free and overall survival in ovarian cancer. J Cell Mol
Med. 16:2422–2428. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ivanova EV, Kondakova IV, Spirina LV,
Afanas'ev SG, Avgustinovich AV and Cheremisina OV:
Chymotrypsin-like activity of proteasomes and total calpain
activity in gastric and colorectal cancer. Bull Exp Biol Med.
157:781–784. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Storr SJ, Pu X, Davis J, Lobo D,
Reece-Smith AM, Parsons SL, Madhusudan S and Martin SG: Expression
of the calpain system is associated with poor clinical outcome in
gastro-oesophageal adenocarcinomas. J Gastroenterol. 48:1213–1221.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rios-Doria J, Day KC, Kuefer R, Rashid MG,
Chinnaiyan AM, Rubin MA and Day ML: The role of calpain in the
proteolytic cleavage of E-cadherin in prostate and mammary
epithelial cells. J Biol Chem. 278:1372–1379. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Panis C, Pizzatti L, Corrêa S, Binato R,
Lemos GF, Herrera AC, Seixas TF, Cecchini R and Abdelhay E: The
positive is inside the negative: HER2-negative tumors can express
the HER2 intracellular domain and present a HER2-positive
phenotype. Cancer Lett. 357:186–195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ye Y, Tian H, Lange AR, Yearsley K,
Robertson FM and Barsky SH: The genesis and unique properties of
the lymphovascular tumor embolus are because of calpain-regulated
proteolysis of E-cadherin. Oncogene. 32:1702–1713. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Libertini SJ, Tepper CG, Rodriguez V,
Asmuth DM, Kung HJ and Mudryj M: Evidence for calpain-mediated
androgen receptor cleavage as a mechanism for androgen
independence. Cancer Res. 67:9001–9005. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Storr SJ, Woolston CM, Barros FF, Green
AR, Shehata M, Chan SY, Ellis IO and Martin SG: Calpain-1
expression is associated with relapse-free survival in breast
cancer patients treated with trastuzumab following adjuvant
chemotherapy. Int J Cancer. 129:1773–1780. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Croce K, Flaumenhaft R, Rivers M, Furie B,
Furie BC, Herman IM and Potter DA: Inhibition of calpain blocks
platelet secretion, aggregation, and spreading. J Biol Chem.
274:36321–36327. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Menon S, Kang CM and Beningo KA:
Galectin-3 secretion and tyrosine phosphorylation is dependent on
the calpain small subunit, Calpain 4. Biochem Biophys Res Commun.
410:91–96. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peltier J, Bellocq A, Perez J, Doublier S,
Dubois YC, Haymann JP, Camussi G and Baud L: Calpain activation and
secretion promote glomerular injury in experimental
glomerulonephritis: Evidence from calpastatin-transgenic mice. J Am
Soc Nephrol. 17:3415–3423. 2006. View Article : Google Scholar : PubMed/NCBI
|