Introduction
Malignant glioma is the most common form of brain
cancer (1). Patients with malignant
glioma are typically treated with a combination of surgery,
chemotherapy and radiation therapy (1–4); however,
the median survival time is only 12–15 months (2). Therefore, an improved understanding of
the molecular mechanisms underlying glioma pathogenesis is
essential in order to identify novel molecular targets for the
treatment of glioma.
Protocadherins (PCDHs) are classified into three
subgroups: δ1 (PCDH1, 7, 9, 11 and 20), δ2 (PCDH8, 10, 12, 17, 18
and 19) and ε (PCDH15, 16, 21 and cadherin related family member 5)
(5). PCDHs are predominantly
expressed in the central nervous system during embryonic
development and in adulthood (6–9). The loss
of PCDH family proteins has been demonstrated to contribute to the
development of various types of human cancer, including colon,
liver, renal, prostate, breast, nasopharyngeal and lung cancer, and
astrocytoma (10–17). The loss of PCDH is due to gene
deletion, mutation or promoter methylation (13–15).
Overexpression of PCDH family proteins can inhibit tumor cell
migration, in addition to anchorage-dependent and
anchorage-independent proliferation (13–15,17),
suggesting that PCDH family proteins function as tumor
suppressors.
The human PCDH8 gene is located at 13q21.1 (18), a putative tumor suppressor gene-rich
chromosome. The inactivation of the PCDH8 gene caused by promoter
methylation has previously been reported in various types of human
cancer (16,19–22).
However, the biological functions and clinical significance of the
PCDH8 protein in glioma remains unclear. In the present study, the
expression of PCDH8 mRNA and protein was detected in glioma and
normal brain tissues. PCDH8 expression was subsequently knocked
down or overexpressed in U87 and U251 glioma cell lines in order to
assess how PCDH8 alters tumor cell phenotype and gene expression
in vitro.
Materials and methods
Antibodies
The anti-PCDH8 antibody was purchased from Abcam
(ab55507; 1:2000; Cambridge, UK). Antibodies specific for Rac-α
serine/threonine-protein kinase (AKT; #4691; 1:1,000),
phosphorylated (p)-AKT [Threonine (T)308; #13038; 1:1,000), p-AKT
[Serine (S)473; #4060; 1:1,000], glycogen synthase kinase (GSK)-3β
(#9315; 1:1,000) p-GSK3β [(S9); #9322; 1:500], β-catenin (#8480;
1:1,000), p-β-catenin (#4176; 1:500) and β-actin (#3700; 1:4,000)
were purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA). The anti-FLAG antibody (F3165; 1:5,000) was from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Tissue samples
A total of 10 samples of human glioma tissue
(surgical resection) and 4 samples of non-tumorous brain tissue
(internal decompression in cerebral trauma) were collected from
February 2015 to January 2016 at The Affiliated Hospital of Xuzhou
Medical University (Xuzhou, China). Surgically removed tissues
histologically diagnosed, and the remaining fresh tissues were
immediately frozen in liquid nitrogen and stored at −80°C until
required. Written informed consent was obtained from all patients
and the present study was approved by the Research Ethics Committee
of the Affiliated Hospital of Xuzhou Medical University.
Cell culture
Human glioma cell lines U87 and U251 were purchased
from the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). The cells were cultured in Dulbecco's modified
Eagle medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% fetal bovine serum (Tiangen Biotech
Co., Ltd., Beijing, China) in a humidified incubator with 5%
CO2 at 37°C.
RNA extraction, complementary (c)DNA
synthesis and reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) analysis
RNA was extracted from the fresh frozen tissue
specimens using TRIzol® reagent (Shenggong, Shanghai,
China) and cDNA was synthesized using the M-MLV reverse
transcription reagents (Roche Diagnostics, Basel, Switzerland)
according to the manufacturer's protocol. PCR reactions were
performed with 1 µl template from each reaction using an ABI 7300
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The reaction components include: 100 mM KCl, 4 mM MgCl2, 400
µM dNTP, 2.5 U Taq DNA Polymerase (Roche Diagnostics), 0.4 µM
Primer, 1xSYBR® Green (Roche Diagnostics). The
thermocycling conditions were: 5 min at 95°C for pre-denaturation,
30 sec at 95°C, 30 sec at 55°C, 30 sec at 72°C, the three steps (30
sec at 95°C, 30 sec at 55°C, 30 sec at 72°C) were repeated for 25
cycles and the final step was 5 min at 72°C. The primers used for
the amplification of PCDH8 and β-actin were as follows: PCDH8
forward, 5′-GCCCAACATGTTCGACGTGC-3′; and reverse,
5′-GGAGTGTCCTTTCCACACCG-3′; β-actin forward,
5′-CATGTACGTTGCTATCCAGGC-3′; and reverse,
5′-CGCTCGGTGAGGATCTTCATG-3′. The products were 256 and 195 bp long,
respectively. For each sample, the threshold cycle (Ct) was
determined and normalized to the average of the housekeeping gene
(ΔCt=Ct Unknown-Ct Housekeeping gene). The determination of gene
transcript levels in each sample was determined using the
2−ΔΔCq method (23).
Transient transfection of the PCDH8
plasmid and small interfering (si)RNAs
Transfection of the PCDH8 plasmid and siRNAs was
performed using Lipofectamine® 2000 Transfection reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. A FLAG-tagged PCDH8 construct
(3xFLAG-PCDH8) was purchased from Youbao Biotechnology Co., Ltd.
(Changsha, China) for overexpression of PCDH8 in glioma cell,
p3xFLAG-CMV-14 empty vector was used as a control. Three sets of
siRNA duplexes that target human PCDH8 were obtained from Shanghai
GenePharma Co., Ltd. (Shanghai, China). For silencing of PCDH8,
three siRNA sequences were designed as follows: siPCDH8 #1,
5′-GCCGUGUCCACUUAUGUCUTT-3′; siPCDH8 #2,
5′-GCAGCUUCGACUAUGAGACTT-3′; and siPCDH8 #3,
5′-GCUGAUCGUCAUCAUCGUGTT-3′, non-targeting sequence:
5′-UUCUCCGAACGUGUCACGUTT-3′ served as a negative control.
Western blotting
Total protein was extracted from tissues or U251/U87
cells using RIPA buffer (50 mM Tris-HCl pH 7.4, 150 Mm NaCl, 1%
Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 1 mM EDTA, and 1
µg/ml each aprotinin, leupeptin and pepstatin). Equal amounts of
protein lysates (80 µg) were run on 8% or 10% gels using SDS-PAGE
and then transferred to 0.45-µm-pore-size polyvinylidene difluoride
membranes (EMD Millipore, Billerica, MA, USA). Following blocking
with 5% non-fat milk, the membranes were probed with primary
antibodies (PCDH8, AKT, p-AKT(308), p-AKT(473), GSK3β, p-GSK3β and
β-actin) at 4°C overnight and secondary antibodies (Goat Anti Mouse
IgG-HRP; 1:5,000; Cat. AP124P; EMD Millipore; Goat Anti Rabbit
IgG-HRP; 1:5,000, Cat. AP132P; EMD Millipore) at room temperature
for 1 h. Bound antibodies were detected using the Pierce ECL Plus
Western Blotting substrate (Pierce; Thermo Fisher Scientific, Inc.)
and exposed to X-ray films. Where band densities were quantified,
ImageJ software (Version 2.1.4.7; National Institutes of Health,
Bethesda, MD, USA) was used. The relative amounts of protein were
determined by normalizing the densitometry value of the band of
interest to that of the loading control (β-actin).
EdU incorporation cell proliferation
assay
Cells were seeded into 96-well plates at a density
of 4×103 cells/well and incubated at 37°C for 24 h prior
to exposure to 50 µM EdU (Guangzhou RiboBio Co., Ltd., Guangzhou,
China) for an additional 2 h at 37°C. Cells were subsequently fixed
with 4% paraformaldehyde for 20 min and treated with 0.5%
Triton-X-100 for another 20 min at room temperature. The cells were
washed 5 times with PBS, then 100 µl 1X Apollo® Reaction
Cocktail (Guangzhou RiboBio Co., Ltd.) was added to each well and
the cells were incubated at room temperature for 30 min. Cellular
DNA was stained with 100 µl of Hoechst 33342 (5 µg/ml) at room
temperature for 20 min and visualized under a fluorescent
microscope (IX71; Olympus Corporation, Tokyo, Japan).
MTT cell viability assay
A total of 2×103 cells in 200 µl of
culture medium were seeded into 96-well plates and culture. MTT
(Sigma-Aldrich; Merck KGaA) was added into the medium to achieve a
final concentration of 0.5 mg/ml. Following incubation at 37°C for
4 h, 150 µl of dimethyl sulfoxide was added to dissolve the
crystals that had formed. Viable cells were counted every day by
reading the absorbance at 490 nm using a Synergy™ Mx Multi-Mode
Microplate Reader (BioTek Instruments, Inc., Winooski, VT,
USA).
Colony formation assay
A total of 400 cells were seeded into a 6 cm dish
and cultured for 10 days at 37°C prior to fixation with methanol
and staining with 0.05% crystal violet to assess colony formation.
The cells were washed with PBS and images of the plates were
captured using a camera. Colonies containing >50 cells were
counted.
Statistical analysis
All statistical analyses were performed using SPSS
software (version 13.0; SPSS Inc., Chicago, IL, USA). Data were
presented as the mean ± standard error of the mean. Statistical
significance was determined using a Student's t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of PCDH8 in human glioma
tissue and normal brain tissues
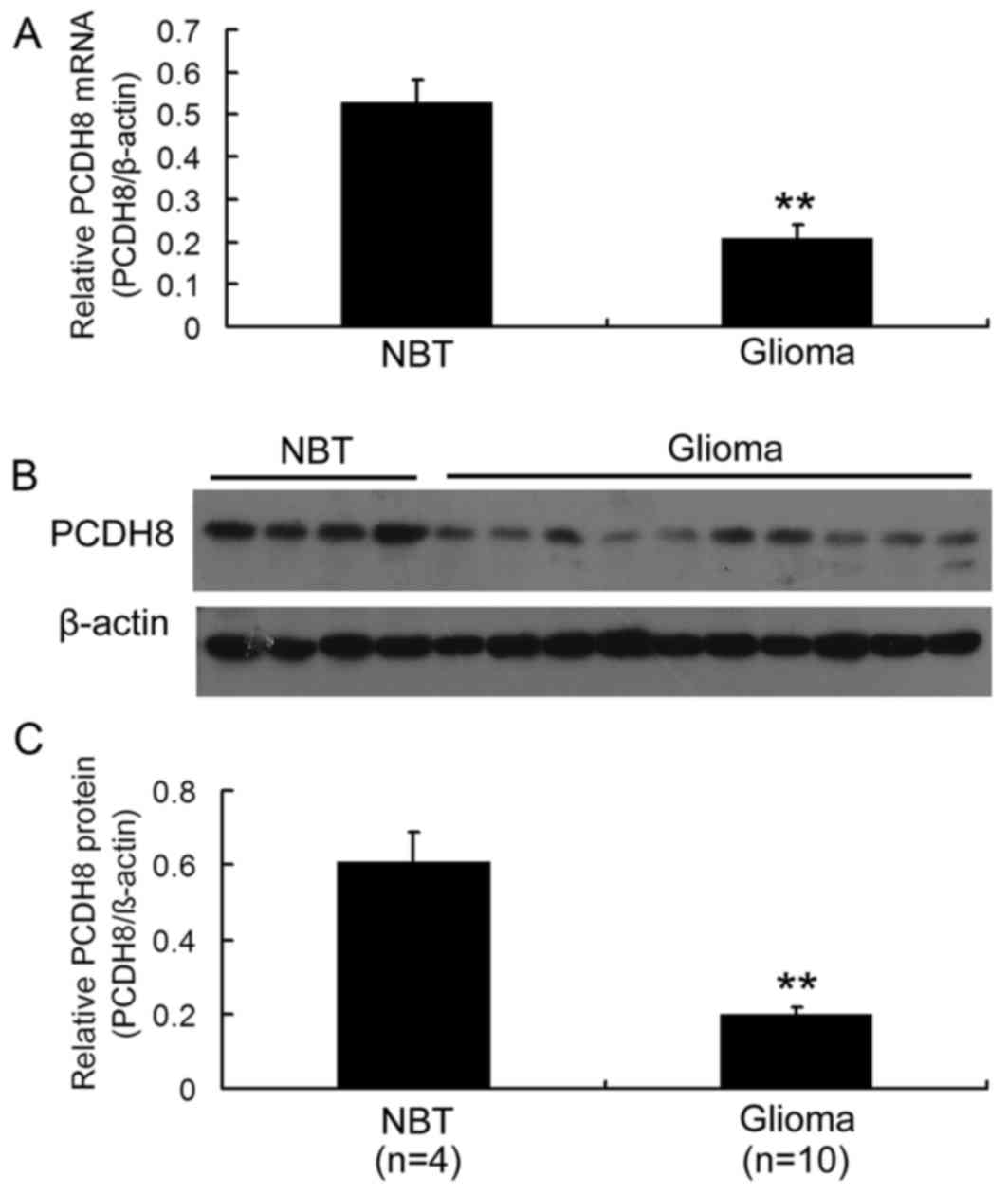
In order to investigate the expression of PCDH8 in
human glioma, the mRNA and protein levels of PCDH8 in 10 samples of
glioma tissue and 4 samples of normal brain tissue were examined.
RT-qPCR analysis demonstrated that PCDH8 mRNA levels were
significantly decreased in glioma tissue compared with normal brain
tissue (P<0.01; Fig. 1A). In
addition, western blot analysis revealed that PCDH8 protein levels
were decreased in glioma tissue compared with normal brain tissue
(Fig. 1B and C; P<0.01). These
data suggest that PCDH8 serves a role in gliomagenesis.
Restoration of PCDH8 inhibits the
proliferation of human glioma cells
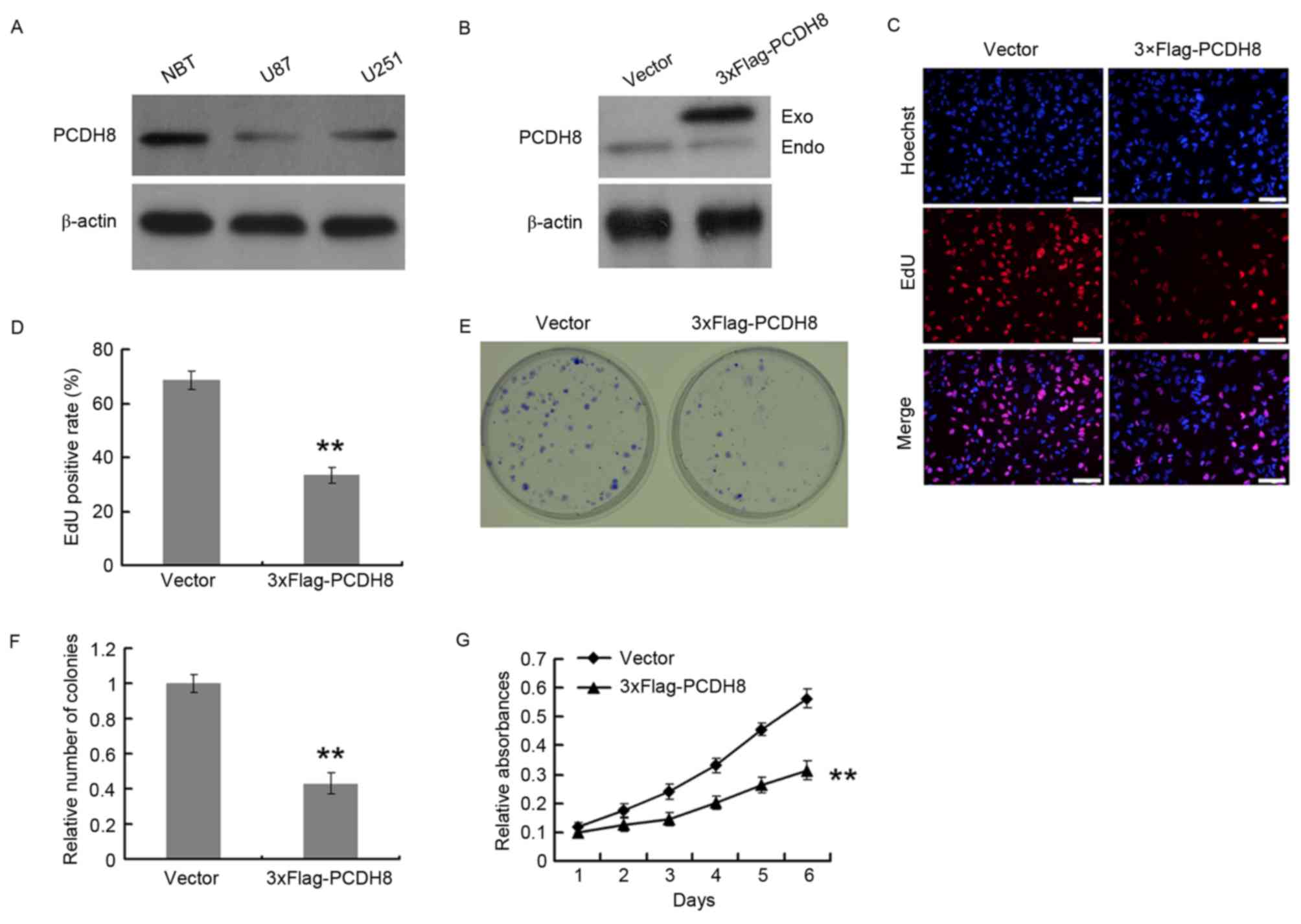
The expression of PCDH8 in U251 and U87 glioma cell
lines was quantified by western blot analysis, using normal brain
tissue as a control for PCDH8 expression. U251 and U87 cells
expressed decreased levels of PCDH8 compared with that of normal
brain tissue; however, PCDH8 expression in U251 cells was increased
compared with that in U87 cells (Fig.
2A). U87 cells were transfected with a FLAG-tagged PCDH8
plasmid to restore PCDH8 expression (Fig.
2B) and the effect on cell proliferation was examined using an
EdU incorporation assay (Fig. 2C and
D). The EdU positive rate of the FLAG-tagged PCDH8
plasmid-transfected group was significantly decreased by 51.3%
compared with the empty vector control group (P<0.01; Fig. 2D). The colony formation ability
(Fig. 2E) of U87 cells was also
significantly reduced upon expression of FLAG-PCDH8 compared with
the control group (P<0.01; Fig.
2F). In addition, the MTT assay demonstrated that FLAG-PCDH8
expression significantly decreased the viability of U87 cells,
compared with control group (P<0.01; Fig. 2G).
PCDH8 silencing promotes the
proliferation of human glioma cells
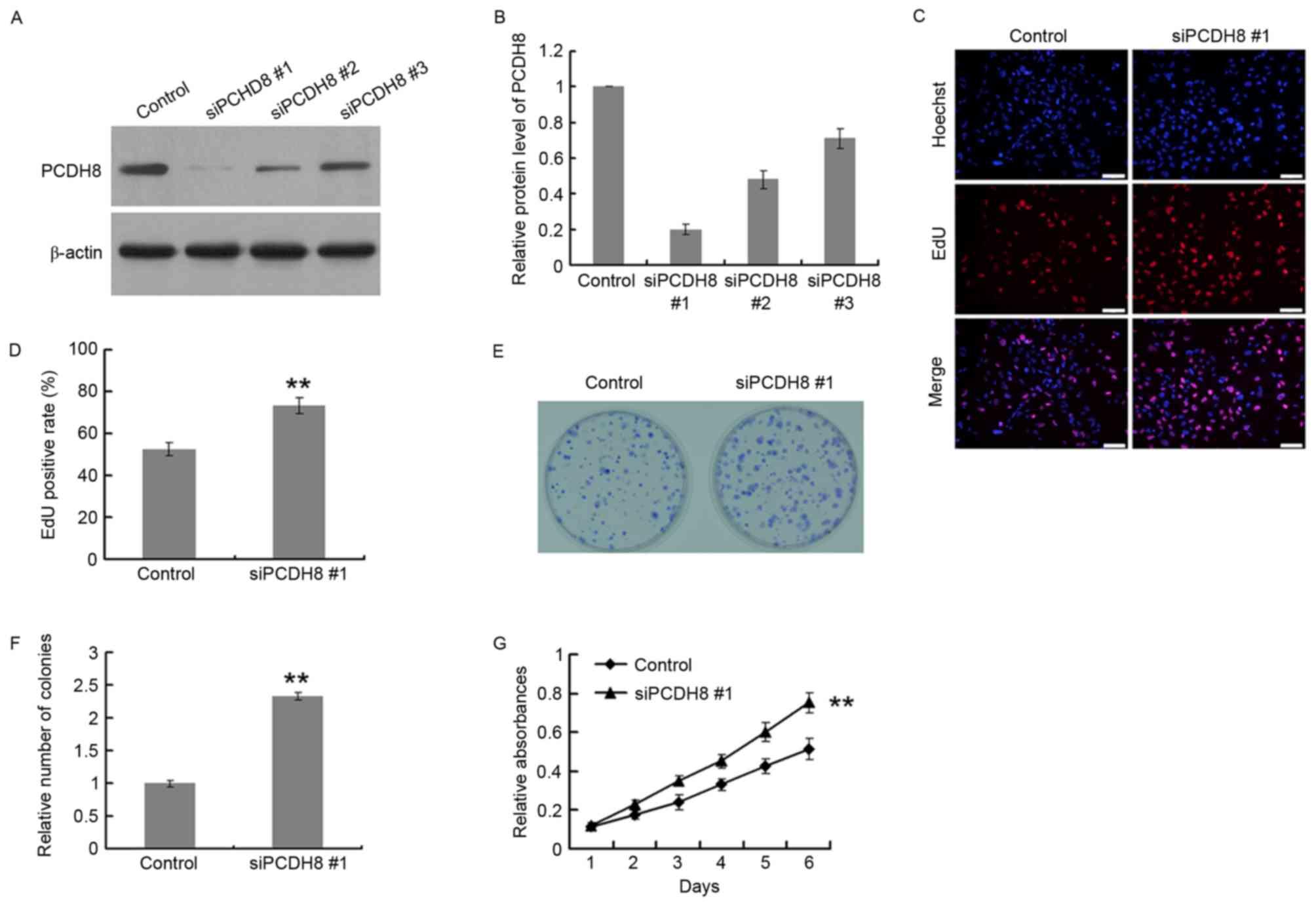
PCDH8 expression was downregulated using 3
PCDH8-specific siRNAs, using a control siRNA as the negative
control. The siRNAs were screened for their efficacy in suppressing
PCDH8 expression. The protein and mRNA silencing efficiency of
siPCDH8 #1 was ~80% in U251 cells (Fig.
3A and B); therefore, this siRNA was selected for use in
further experiments. An EdU incorporation assay was performed in
U251 cells to assess the effects of PCDH8 silencing on cell
proliferation (Fig. 3C and D). The
EdU positive rate of the siPCDH8 group was significantly increased
by 133.3% compared with the control group (P<0.01; Fig. 3D). The colony formation ability of
U251 cells (Fig. 3E) was also
significantly increased upon silencing of PCDH8 (P<0.01 vs. the
control group; Fig. 3F). In addition,
the MTT assay demonstrated that PCDH8 silencing significantly
increased the growth of U251 cells compared with the control group
(P<0.01; Fig. 3G).
PCDH8 negatively regulates the
AKT/GSK3β/β-catenin signaling pathway
It has previously been reported that PCDH10, which
belongs to the same subfamily of proteins as PCDH8, can inhibit
tumor growth by regulating the AKT/GSK3β/β-catenin signaling
pathway (24); however, whether PCDH8
regulates this signaling pathway in human glioma cells remains
unclear. To investigate this, the expression levels of AKT, p-AKT,
GSK3β, p-GSK3β and β-catenin were detected in U87 cells expressing
FLAG-PCDH8 and U251 cells transfected with siPCDH8. Restoration of
PCDH8 led to a marked decrease in p-AKT (T308/S473) and p-GSK3β
(S9) expression, thereby reducing the level of β-catenin compared
with the control group (Fig. 4A).
Silencing of PCDH8 resulted in a notable increase in p-AKT
(T308/S473) and p-GSK3β (S9) expression, which led to an increased
level of β-catenin compared with the control group (Fig. 4B). These results suggest that PCDH8
inhibits glioma cell proliferation by negatively regulating the
AKT/GSK3β/β-catenin signaling pathway.
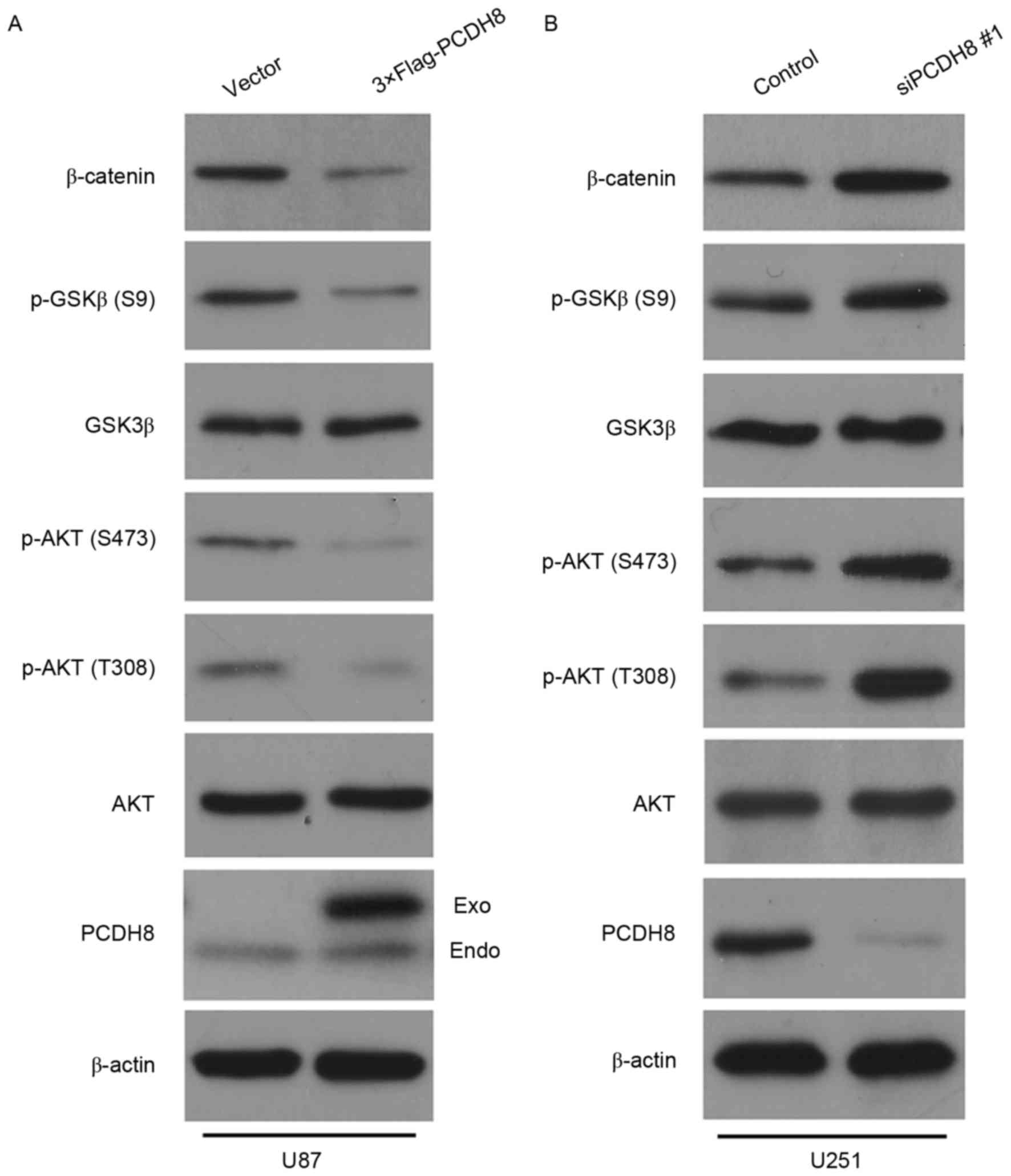 | Figure 4.PCDH8 negatively regulates the
AKT/GSK3β/β-catenin signaling pathway. (A) Western blot analysis of
p-AKT (T308), p-AKT (S473), p-GSK3β (S9) and β-catenin expression
in U87 cells following transfection with FLAG-PCDH8. (B) Western
blot analysis of p-AKT (T308), p-AKT (S473), p-GSK3β (S9) and
β-catenin expression in U251 cells transfected with siPCDH8. PCDH8,
protocadherin-8; AKT, Rac-α serine/threonine-protein kinase; p,
phosphorylated; GSK3β, glycogen synthase kinase-3β; T, threonine;
S, serine; siPCDH8, small interfering RNA targeting PCDH8. |
Discussion
Results from the present study demonstrated that
PCDH8 mRNA and protein expression is decreased in glioma tissues
compared with normal brain tissues. Restoration of PCDH8 expression
in glioma cells significantly inhibited cell proliferation, while
silencing of PCDH8 promoted cell proliferation. PCDH8 restoration
decreased p-AKT (T308/S473) and p-GSK3β (S9) expression, thereby
reducing the expression of β-catenin, while silencing of PCDH8
resulted in a significant increase in p-AKT (T308/S473) and p-GSK3β
(S9) expression, thereby elevating the level of β-catenin.
Therefore, the loss of PCDH8 expression may contribute to glioma
development. However, further studies are required to investigate
the role that PCDH8 serves in glioma in vivo. Data from the
present study indicates that the expression level of PCDH8 is
important for the development of glioma; therefore, PCDH8 may be a
potential biomarker for the early detection of or prognosis of
glioma.
It has previously been reported that the PCDH8 gene
is frequently methylated in the development of human cancer, which
is correlated with poor survival rates (7,14,18,22),
indicating that PCDH8 functions as a tumor suppressor. Methylation
is typically used to inactivate specific genes. Methylation of
PCDH8 may inhibit the transcription of PCDH8, thereby contributing
to the progression of cancer. The results from the present study
demonstrated that the mRNA and protein levels of PCDH8 were
decreased in human glioma tissue compared with normal brain tissue.
The loss of PCDH8 mRNA and protein expression is consistent with
previous reports on PCDH8 methylation in cancer (22).
The proto-oncogene Wnt/β-catenin signaling pathways
inhibit GSK3β activity through the promotion of AKT-mediated
phosphorylation of GSK3β at S9. This subsequently promotes the
accumulation of β-catenin, which translocates to the nucleus
(25,26). Data from the present study indicates
that PCDH8 inhibits the activation of AKT. The subsequent reduction
of GSK3β phosphorylation at S9 leads to the activation of GSK3β,
which in turn stimulates the degradation of β-catenin. These
results indicate that PCDH8 serves a role in the regulation of the
Wnt/β-catenin signaling pathway through AKT. However, the specific
molecular mechanism by which PCDH8 regulates AKT remains unclear.
Further studies should focus on investigation of the downstream
molecules directly regulated by PCDH8, which may further elucidate
the molecular mechanism of PCDH8 regulated AKT signaling.
Acknowledgements
The present study was supported by the Xuzhou
Municipal Science and Technology Bureau (Xuzhou, China; grant no.
XZZD1020 and KC16SH042), Xuzhou Municipal Health and Family
Planning Commission (Xuzhou, China; grant no. XWJ2011030) and
Jiangsu Province's Young Provincial Talents Program (Jiangsu,
China; grant no. QNRC2016383).
References
|
1
|
Meyer MA: Malignant gliomas in adults. N
Engl J Med. 359:18502008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Buonerba C, Di Lorenzo G, Marinelli A,
Federico P, Palmieri G, Imbimbo M, Conti P, Peluso G, De Placido S
and Sampson JH: A comprehensive outlook on intracerebral therapy of
malignant gliomas. Crit Rev Oncol Hematol. 80:54–68. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sherman JH, Hoes K, Marcus J, Komotar RJ,
Brennan CW and Gutin PH: Neurosurgery for brain tumors: Update on
recent technical advances. Curr Neurol Neurosci Rep. 11:313–319.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fiorentino A, Chiumento C, Caivano R,
Cozzolino M, Pedicini P and Fusco V: Adjuvant radiochemotherapy in
the elderly affected by glioblastoma: Single-institution experience
and literature review. Radiol Med. 118:870–881. 2013.(In Italian).
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim SY, Yasuda S, Tanaka H, Yamagata K and
Kim H: Non-clustered protocadherin. Cell Adh Migr. 5:97–105. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sano K, Tanihara H, Heimark RL, Obata S,
Davidson M, St John T, Taketani S and Suzuki S: Protocadherins: A
large family of cadherin-related molecules in central nervous
system. EMBO J. 12:2249–2256. 1993.PubMed/NCBI
|
|
7
|
Frank M and Kemler R: Protocadherins. Curr
Opin Cell Biol. 14:557–562. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Phillips GR, Tanaka H, Frank M, Elste A,
Fidler L, Benson DL and Colman DR: Gamma-protocadherins are
targeted to subsets of synapses and intracellular organelles in
neurons. J Neurosci. 23:5096–5104. 2003.PubMed/NCBI
|
|
9
|
Kim SY, Chung HS, Sun W and Kim H:
Spatiotemporal expression pattern of non-clustered protocadherin
family members in the developing rat brain. Neuroscience.
147:996–1021. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okazaki N, Takahashi N, Kojima S, Masuho Y
and Koga H: Protocadherin LKC, a new candidate for a tumor
suppressor of colon and liver cancers, its association with contact
inhibition of cell proliferation. Carcinogenesis. 23:1139–1148.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stassar MJ, Devitt G, Brosius M, Rinnab L,
Prang J, Schradin T, Simon J, Petersen S, Kopp-Schneider A and
Zöller M: Identification of human renal cell carcinoma associated
genes by suppression subtractive hybridization. Br J Cancer.
85:1372–1382. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen MW, Vacherot F, De La Taille A,
Gil-Diez-De-Medina S, Shen R, Friedman RA, Burchardt M, Chopin DK
and Buttyan R: The emergence of protocadherin-PC expression during
the acquisition of apoptosis-resistance by prostate cancer cells.
Oncogene. 21:7861–7871. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Waha A, Güntner S, Huang TH, Yan PS,
Arslan B, Pietsch T, Wiestler OD and Waha A: Epigenetic silencing
of the protocadherin family member PCDH-gamma-A11 in astrocytomas.
Neoplasia. 7:193–199. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Imoto I, Izumi H, Yokoi S, Hosoda H,
Shibata T, Hosoda F, Ohki M, Hirohashi S and Inazawa J: Frequent
silencing of the candidate tumor suppressor PCDH20 by epigenetic
mechanism in non-small-cell lung cancers. Cancer Res. 66:4617–4626.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ying J, Li H, Seng TJ, Langford C,
Srivastava G, Tsao SW, Putti T, Murray P, Chan AT and Tao Q:
Functional epigenetics identifies a protocadherin PCDH10 as a
candidate tumor suppressor for nasopharyngeal, esophageal and
multiple other carcinomas with frequent methylation. Oncogene.
25:1070–1080. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu JS, Koujak S, Nagase S, Li CM, Su T,
Wang X, Keniry M, Memeo L, Rojtman A, Mansukhani M, et al: PCDH8,
the human homolog of PAPC, is a candidate tumor suppressor of
breast cancer. Oncogene. 27:4657–4665. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu J, Cheng YY, Tao Q, Cheung KF, Lam CN,
Geng H, Tian LW, Wong YP, Tong JH, Ying JM, et al: Methylation of
protocadherin 10, a novel tumor suppressor, is associated with poor
prognosis in patients with gastric cancer. Gastroenterology.
136:640–651, e1. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Strehl S, Glatt K, Liu QM, Glatt H and
Lalande M: Characterization of two novel protocadherins (PCDH8 and
PCDH9) localized on human chromosome 13 and mouse chromosome 14.
Genomics. 53:81–89. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He D, Zeng Q, Ren G, Xiang T, Qian Y, Hu
Q, Zhu J, Hong S and Hu G: Protocadherin8 is a functional tumor
suppressor frequently inactivated by promoter methylation in
nasopharyngeal carcinoma. Eur J Cancer Prev. 21:569–575. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang D, Zhao W, Liao X, Bi T, Li H and
Che X: Frequent silencing of protocadherin 8 by promoter
methylation, a candidate tumor suppressor for human gastric cancer.
Oncol Rep. 28:1785–1791. 2012.PubMed/NCBI
|
|
21
|
Lin YL, Ma JH, Luo XL, Guan TY and Li ZG:
Clinical significance of protocadherin-8 (PCDH8) promoter
methylation in bladder cancer. J Int Med Res. 41:48–54. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin YL, Wang YL, Ma JG and Li WP: Clinical
significance of protocadherin 8 (PCDH8) promoter methylation in
non-muscle invasive bladder cancer. J Exp Clin Cancer Res.
33:682014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu Y, Yang Z, Yuan H, Li Z, Li Y, Liu Q
and Chen J: PCDH10 inhibits cell proliferation of multiple myeloma
via the negative regulation of the Wnt/β-catenin/BCL-9 signaling
pathway. Oncol Rep. 34:747–754. 2015.PubMed/NCBI
|
|
25
|
Ge X and Wang X: Role of Wnt canonical
pathway in hematological malignancies. J Hematol Oncol. 3:332010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu YZ, Wu K, Huang J, Liu Y, Wang X, Meng
ZJ, Yuan SX, Wang DX, Luo JY, Zuo GW, et al: The PTEN/PI3K/Akt and
Wnt/β-catenin signaling pathways are involved in the inhibitory
effect of resveratrol on human colon cancer cell proliferation. Int
J Oncol. 45:104–112. 2014.PubMed/NCBI
|