Introduction
Pancreatic cancer (pancreatic ductal carcinoma; PDC)
is one of the most devastating types of disease, as the early-stage
tumors are difficult to detect but have a high potential for
dissemination and distant metastasis (1,2). A
previous autopsy study revealed that 70% of patients succumb to the
disease due to widespread metastasis, most frequently in the liver,
peritoneum and lung, and that 30% succumb due to locally
destructive disease (3). The control
of hematogenous metastasis has been a long-standing concern for the
prevention of cancer-associated mortality, but has yet to be
achieved.
Chemotherapy regimens for PDC have improved over the
past few years, but the prognosis for patients with metastatic
pancreatic cancer remains poor, with an associated median survival
time of 8–12 months (4–6). Surgical resection is the only definitive
treatment available; however, the cancer recurs postoperatively in
80% of patients with PDC (7). For
~20% of these patients with surgical resection, recurrence and
mortality occurred within 6 months, and their survival time
differed by several months compared with patients who could not
undergo surgery due to pancreatic cancer metastasis (4–8). When
lesions are identified preoperatively through diagnostic imaging,
those indicative of local disease must be differentiated from
occult distant metastases, which cause recurrence subsequent to
surgery (9,10); pancreatectomy may successfully treat
local lesions, but offers little benefit to patients with
metastatic disease. Local disease and metastatic disease are
associated with different treatment regimens and survival times
(4). If identified, a
metastasis-specific biomarker may better indicate when resection is
appropriate.
In order to understand the mechanisms underlying
hematogenous metastasis in PDC, the molecular expression profile of
the BxPC-3 PDC cell line, which demonstrates weak metastasis to the
liver, was previously compared with that of the highly metastatic
LM-BxPC-3 subline (11). This led to
the isolation of ZNF185 as a metastasis-associated protein by
global quantitative proteome analysis (12). ZNF185 contains two zinc-finger motifs
in the C-terminus that fit the consensus pattern of a LIM domain
(13). The LIM-domain is a
protein-protein interaction motif. A diverse group of proteins
containing LIM domains have been identified with a range of
functions. The majority of identified cytoplasmic LIM-domain
proteins interact with the cytoskeleton or extracellular adhesion
components to control cell morphology, motility and
integrin-dependent adhesion and signaling (13). Since ZNF185 was observed to
co-localize primarily with F-actin and partially with vinculin and
paxillin, it was assumed that ZNF185 serves a role in cell adhesion
(14).
At present, the biological and clinical significance
of ZNF185 in cancer has not been investigated extensively. Only a
small number of studies have focused on ZNF185 in the context of
cancer: One group concluded that ZNF185 serves a tumor-suppressive
role in prostate cancer (14),
whereas another group considered ZNF185 to be an unfavorable
prognostic indicator in colon cancer (12). In the present study, the
clinicopathological significance of ZNF185 in PDC was
investigated.
Materials and methods
Cells
The BxPC-3 human PDC cell line was purchased from
the American Type Culture Collection (Manassas, VA, USA). A highly
liver-metastatic cancer cell subline, LM-BxPC-3, was established
via the intrasplenic transfer of cells derived from poorly
developed but visible hepatic tumor foci formed following
transplantation of the parental BxPC-3 cells and transfer to
NOD/Shi-scid/IL-2Rγnull (NOG) mice at the Central
Institute for Experimental Animals (CIEA; Kanagawa, Japan)
(11,15,16). The
cells were cultivated in RPMI-1640 medium (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) supplemented with 10% fetal bovine serum
(BioWest, Nuaillé, France) in a humidified (37°C, 5%
CO2) incubator, and passaged on reaching 80%
confluence.
Xenograft models
A total of 6 NOG mice (9–12 weeks of age, male)
obtained from the CIEA were used in the present study. All animals
were housed in plastic cages (136×208×115 mm) within a
pathogen-free vinyl isolator system (1,150×500×500 mm) maintained
at 22±1°C with 45±10% humidity, and there was a 12-h light/dark
cycle. To create subcutaneous models, 1×104 BxPC-3 tumor
cells suspended in 0.1 ml serum-free RPMI-1640 medium
(Sigma-Aldrich; Merck KGaA) were injected into the subcutis of the
mice. The experimental liver metastasis models were generated via
intrasplenic injection of 1×104 LM-BxPC-3 cells in 0.1
ml serum-free medium, followed by splenectomy after 1 min, as
previously described (11,15,16). The
mice were sacrificed under anesthesia and necropsied 6–8 weeks
after inoculation, according to the standard protocols of the CIEA.
Tumor tissues were surgically removed for standard histological
examinations. The expression of ZNF185 in the tumors was evaluated
using immunohistochemistry. Subsequent to immune-labeling as
described in the immunohistochemical analysis section, the number
of ZNF185-positive tumor cells per 1,000 tumor cells was counted in
five distinct areas in each BxPC-3 subcutaneous tumor sample using
an optical microscope at ×200 magnification. Similarly, at the same
magnification, the number of ZNF185-positive cells per 200 tumor
cells were counted in 9 defined areas in the LM-BxPC-3
liver-metastatic tumor samples. The cells were counted separately
in two areas of the cancer cell nests: Central and peripheral
(Fig. 1). The peripheral area was
defined as the outer two layers of cells in contact with the
surrounding extracellular matrix, and the central area as the cells
present in a central position within a cell nest, or the cells in
areas other than the peripheral area. All experiments involving
laboratory animals were performed in accordance with the care and
use guidelines of the CIEA. The present study was approved by the
Animal Committee of the CIEA (permit no. 08018A).
Study population
The present study included 182 patients who had
undergone surgical resection for PDC at the National Cancer Center
Hospital (NCC; Tokyo, Japan) between January 1990 and December
2005. All patients provided written informed consent. The patient
cohort was comprised of 116 males and 67 females with a mean age of
64.1 years (range, 27–87 years). The pathological stages were
classified according to the Union for International Cancer Control
tumor-node-metastasis staging system (17). A total of 2 patients (1.0%) were at
Stage IA, 2 (1.0%) at Stage IB, 30 (16.5%) at Stage IIA, 129
(70.9%) at Stage IIB and 19 (10.4%) at Stage IV. No patient with
Stage III disease involving the celiac artery or superior
mesenteric artery underwent surgical resection. All patients with
Stage IV disease were diagnosed on the basis of para-aortic lymph
node metastasis. The clinicopathological features of the patients
are summarized in Table I.
 | Table I.Correlation between the expression of
ZNF185 on the plasma membrane and clinicopathological
variables. |
Table I.
Correlation between the expression of
ZNF185 on the plasma membrane and clinicopathological
variables.
|
| ZNF185 expression on
the plasma membrane |
|---|
|
|
|
|---|
| Categories | Negative (n=111) | Positive (n=71) | P-value |
|---|
| Age (years) |
|
| 0.84 |
|
<65 | 50 | 32 |
|
| ≥65 | 61 | 39 |
|
| Gender |
|
| 0.96 |
| Male | 70 | 45 |
|
|
Female | 41 | 26 |
|
| Location |
|
| 0.26 |
| Pancreas
head | 78 | 45 |
|
| Pancreas
body/tail | 33 | 26 |
|
| Tumor size |
|
| 0.34 |
| <30
mm | 42 | 22 |
|
| ≥30
mm | 69 | 49 |
|
| Histologic grade |
|
| 0.46 |
| G1 | 32 | 17 |
|
|
G2/G3 | 79 | 54 |
|
| Lymphatic
invasion |
|
| 0.56 |
| ly0,
1 | 33 | 24 |
|
| ly2,
3 | 78 | 47 |
|
| Venous
invasion |
|
| 0.03a |
| v0,
1 | 52 | 22 |
|
| v2,
3 | 59 | 49 |
|
| Intra-pancreatic
nerve invasion |
|
| 0.12 |
| ne0,
1 | 55 | 27 |
|
| ne2,
3 | 56 | 44 |
|
| Cancer-stroma
association |
|
| 0.70 |
|
Medullary or Intermediate | 72 | 48 |
|
|
Scirrhous | 39 | 23 |
|
| Portal vein
invasion |
|
| 0.14 |
|
Negative | 67 | 35 |
|
|
Positive | 44 | 36 |
|
| Extra-pancreatic
nerve plexus invasion |
|
| 0.43 |
|
Negative | 72 | 42 |
|
|
Positive | 39 | 29 |
|
| Lymph node
metastasis (UICC) |
|
| 0.20 |
| N0 | 24 | 10 |
|
| N1 | 87 | 61 |
|
| Distant metastasis
(UICC) |
|
| 0.19 |
| M0 | 102 | 61 |
|
| M1 | 9 | 10 |
|
| Stage (UICC) |
|
| 0.09 |
| IA | 0 | 2 |
|
| IB | 1 | 1 |
|
|
IIA | 23 | 7 |
|
|
IIB | 78 | 51 |
|
| IV | 9 | 10 |
|
Pathological examination
Histological diagnosis was performed according to
the WHO classification (2) and the
Japan Pancreas Society Classification (18). Papillary adenocarcinoma, mucinous
carcinoma and well-differentiated tubular adenocarcinoma were
defined as well-differentiated (G1); moderately-differentiated
tubular adenocarcinoma was defined as moderately-differentiated
(G2); poorly-differentiated adenocarcinoma and adenosquamous
carcinoma were defined as poorly-differentiated (G3). The following
histopathological variables were evaluated according to the Japan
Pancreas Society: Extra-pancreatic nerve plexus invasion,
cancer-stroma association, lymphatic invasion, venous invasion and
intrapancreatic nerve invasion. The grading of lymphatic invasion,
venous invasion and intrapancreatic nerve invasion was as
determined as follows: 0, no invasion; 1, slight invasion; 2,
moderate invasion; 3, marked invasion.
Immunohistochemical analysis
Sections (4-µm-thick) of representative
formalin-fixed paraffin-embedded tissue blocks stained with a
rabbit antibody specific to ZNF185 (Sigma-Aldrich; Merck KGaA) were
used, as per the protocol of our previous study (12). The sections were incubated in 0.3%
H2O2 in methanol at room temperature for 30
min to block endogenous peroxidase reaction. Subsequent to washing
in PBS, sections were incubated with rabbit serum (Nichirei
Biosciences Inc., Tokyo, Japan) at room temperature for 20 min to
block non-specific binding. The sections were then incubated
overnight in a humid chamber at 4°C with primary antibodies against
ZNF-185 (dilution, 1:200; cat. no. HPA0004000; Sigma-Aldrich; Merck
KGaA). Following three washes in PBS, the sections were incubated
with a peroxidase-labeled polymer-conjugated rabbit anti-goat
antibody (Histofine Simple Stain Max PO; dilution, ready-to-use;
cat. no. 414162F; Nichirei Biosciences, Inc.). The amplified immune
products were visualized using the 3,3′-diaminobenzidine
tetrahydrochloride reaction as described previously (19). The number of ZNF185-positive tumor
cells was counted in 5 distinct areas using an optical microscope
at ×100 magnification. Quantification of ZNF185 expression was
performed using a scale of negative, weakly positive, moderately
positive and strongly positive; normal acinar cell staining was
defined as moderately positive. Tissues that stained
moderately-strongly positive in >5% of the cells were defined as
immunopositive.
Statistical analysis
The associations between ZNF185 expression levels
and various clinicopathological parameters were evaluated
statistically using the χ2 test. Survival curves were
plotted according to the Kaplan-Meier method and statistical
comparisons among the groups were performed using the log-rank
test. Multivariate analysis of survival was conducted using the Cox
proportional hazards model. All statistical analyses were performed
using SPSS v. 21 (IBM SPSS, Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
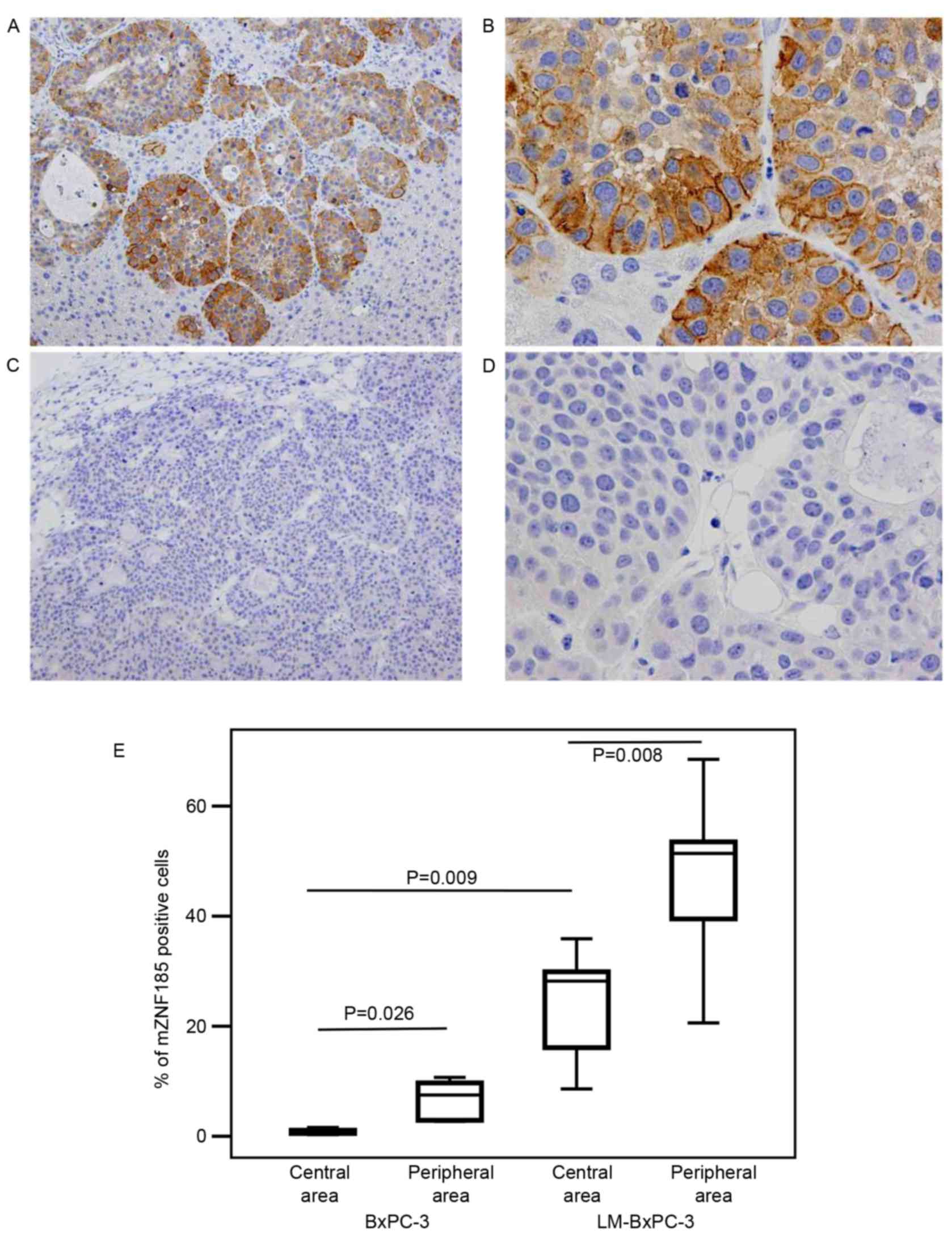
Unique distribution of ZNF185
expression in pancreatic cancer cells
LM-BxPC-3 cells were established as a highly
liver-metastatic subline derived from the poorly-metastatic BxPC-3
cells, and appeared to express ZNF185 more abundantly compared with
the parental cells. When the expression of ZNF185 was examined in
xenotransplanted tumors in immunocompromised NOG mice, it was
identified that the subcellular location of ZNF185 varied depending
on the position of the cancer cells expressing it in the cancer
cell nests (Fig. 1). ZNF185 was
expressed in the cytoplasm of 76.1±15.5% of the parental cells
located at the periphery of the cancer cell nests, whereas only
5.8±2.4% of the parent cancer cells at the center of the nests
expressed cytoplasmic ZNF185. A small population of the cells
expressed ZNF185 on the plasma membrane, representing 6.7±3.7 and
0.8±0.5% of the parent cancer cells located at the periphery and
center of the nests, respectively (Fig.
1). By contrast, all of the LM-BxPC-3 cells expressed
cytoplasmic ZNF185 irrespective of their position in the cancer
cell nests; 47.0±14.2 and 24.4±9.6% of the peripheral and central
cells, respectively, demonstrated membrane expression of ZNF185
(Fig. 1). These data suggest that the
amount and subcellular location of ZNF185 are correlated with the
position of the cells expressing it within the cancer cell nests,
and also that expression of ZNF185 on the plasma membrane is
associated with hematogenous metastasis. The clinicopathological
significance of the characteristic pattern of ZNF185 expression in
PDC was subsequently investigated.
Expression of ZNF185 in pancreatic
cancer tissues
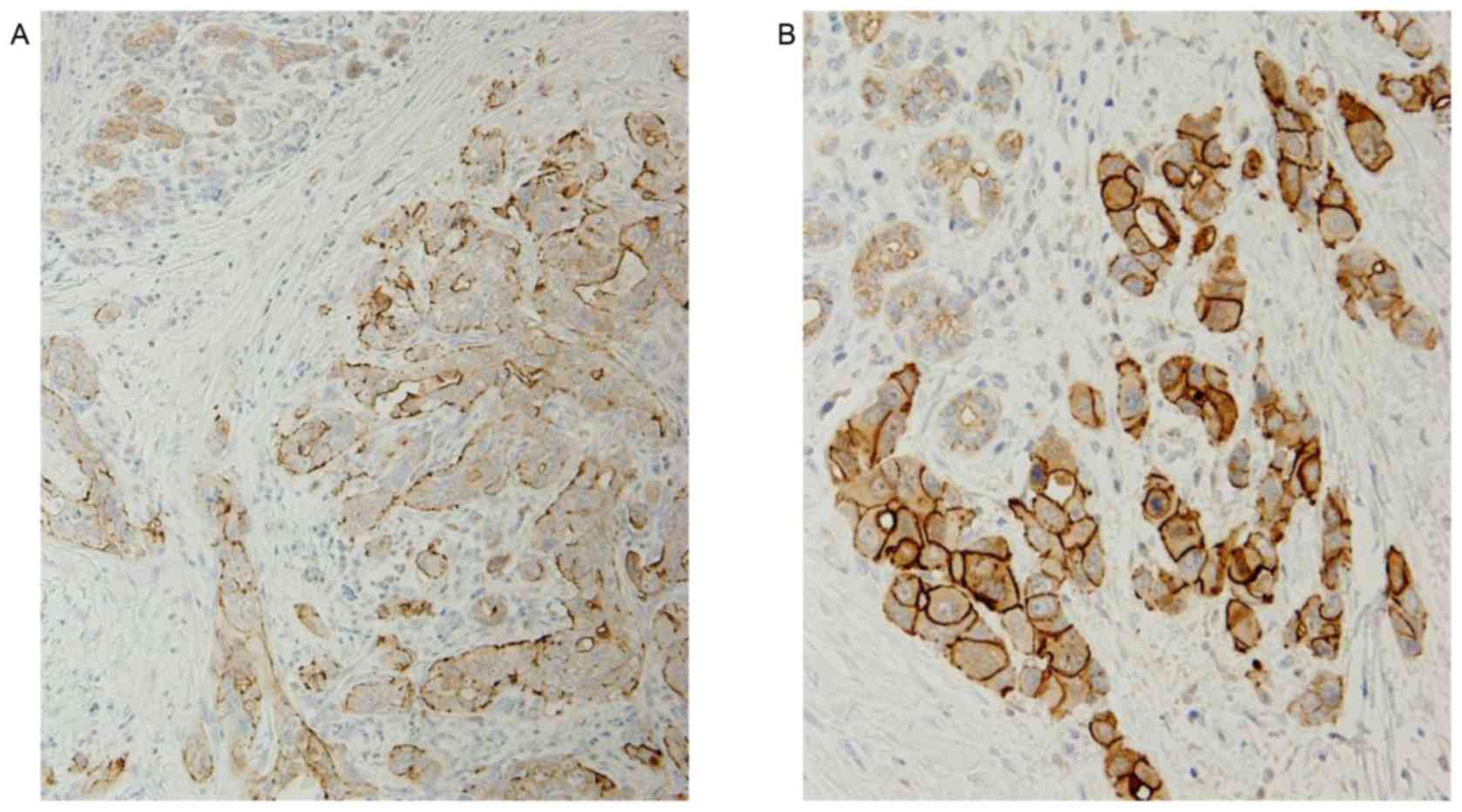
In clinical samples of PDC tissues, ZNF185 was
identified to be expressed in the cytoplasm and on the plasma
membrane of cancer cells. ZNF185 expression was observed in the
cytoplasm of cancer cells (cZNF185) in 169/182 patients with PDC
(92.8%), and in the cytoplasm and plasma membrane (mZNF185) in 71
patients (39%). None of the patients with PDC exhibited expression
of ZNF185 exclusively on the plasma membrane of the cancer cells
(Fig. 2). ZNF185 was also expressed
in non-neoplastic acinar cells of the pancreatic exocrine
glands.
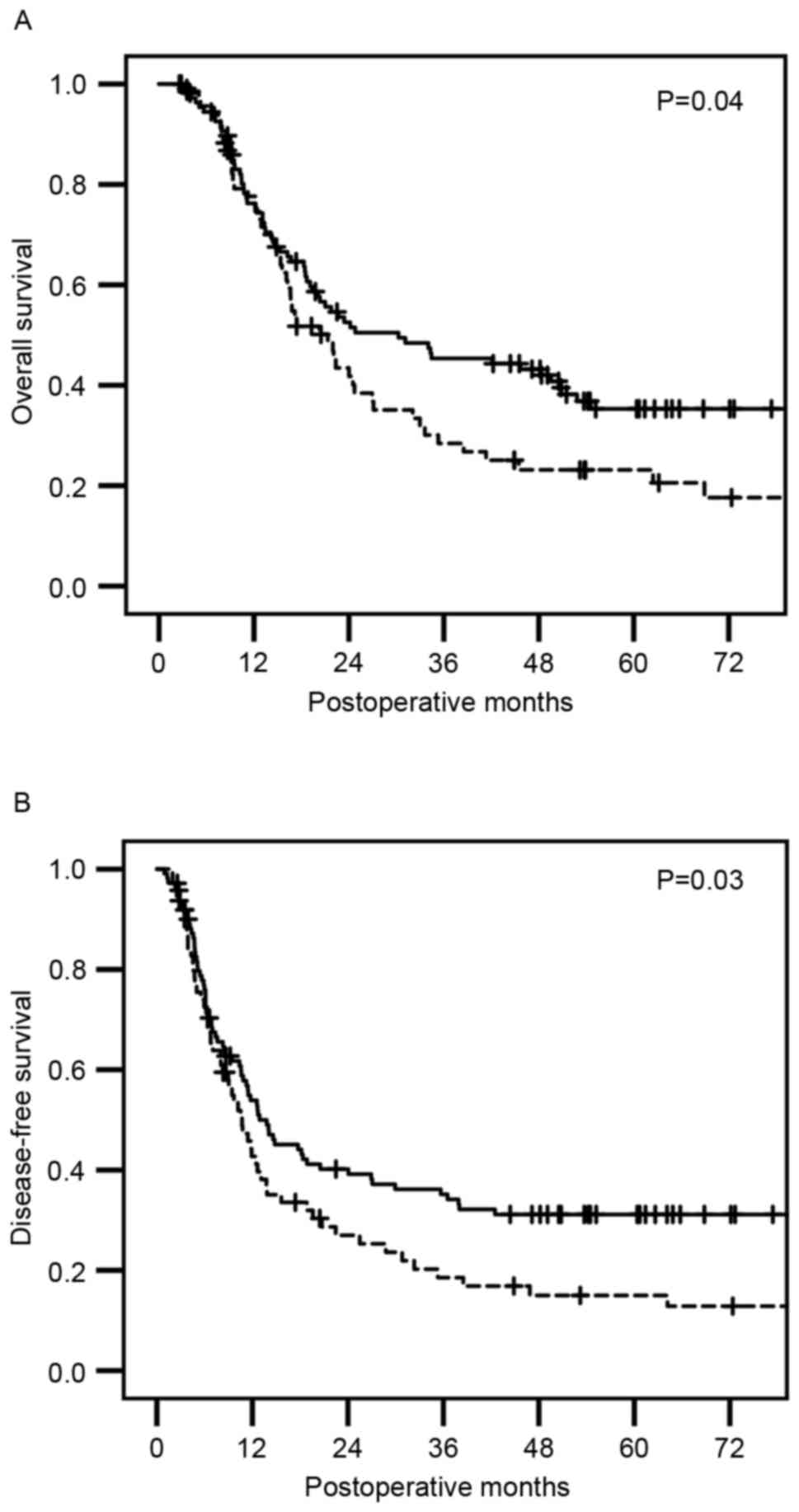
Significantly shorter survival time
for PDC patients with mZNF185-positive cancer cells
Patients with PDC with and without mZNF185-positive
cancer cells exhibited a median overall survival (OS) time of 21.3
months [95% confidence interval (CI)], 15.9–26.7 months) and 30.2
months (95% CI, 12.1–48.4 months), respectively, and a disease-free
survival (DFS) time of 10.7 months (95% CI, 7.9–13.4 months) and
12.9 months (95% CI, 7.9–13.4 months), respectively. Kaplan-Meier
survival analyses revealed that patients with mZNF185-positive
cancer demonstrated a significantly shorter OS (P=0.04; Fig. 3A) and a significantly shorter DFS
(P=0.03; Fig. 3B), as compared with
patients with mZNF185-negative cancer. There was no significant
difference in OS or DFS observed between patients with and without
cZNF185-positive cancer.
Prognostic significance of mZNF185
expression in association with clinicopathological factors
Univariate analysis indicated that tumor size,
histologic grade, lymphatic invasion, venous invasion,
intra-pancreatic nerve invasion, portal vein invasion,
extra-pancreatic nerve plexus invasion, nodal metastasis and the
expression of mZNF185 were all factors significantly correlated
with a shorter OS time. Subsequent multivariate analysis of these
factors indicated that lymphatic invasion, portal vein invasion and
the expression of mZNF185 were significant (Table IIA). With regard to DFS, univariate
analysis indicated that tumor size, tumor-stroma ratio, lymphatic
invasion, venous invasion, intrapancreatic nerve invasion, portal
vein invasion, intra-pancreatic nerve invasion, nodal metastasis,
distant metastasis and the expression of mZNF185 were factors
significantly correlated with a shorter DFS time. Multivariate
analysis indicated that tumor-stroma ratio, lymphatic invasion,
venous invasion, lymph node metastasis and the expression of
mZNF185 were all significant (Table
IIB). Among the various clinicopathological variables, only
marked venous invasion was more prevalent in patients who were
mZNF185-positive, as compared with in patients who were
mZNF185-negative (Table I).
 | Table II.Univariate and multivariate analyses
of various clinicopathological factor and ZNF185 expression for
overall survival and disease-free survival. |
Table II.
Univariate and multivariate analyses
of various clinicopathological factor and ZNF185 expression for
overall survival and disease-free survival.
| A, Overall
survival |
|---|
|
|---|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
| Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Age (≥65) | 0.82
(0.57–1.19) | 0.31 |
|
|
| Gender (male) | 1.23
(0.83–1.81) | 0.29 |
|
|
| Location (pancreas
head) | 1.18
(0.80–1.74) | 0.39 |
|
|
| Size (≥30 mm) | 2.11
(1.40–3.17) |
<0.01a |
|
|
| Histologic type
(G2, G3) | 1.57
(1.01–2.42) | 0.04a |
|
|
| Stroma
(scirrhous) | 0.88
(0.60–1.30) | 0.54 |
|
|
| Lymphatic invasion
(ly2, ly3) | 2.65
(1.66–4.13) |
<0.01a | 2.46
(1.51–3.82) |
<0.01a |
| Venous invasion
(v2, v3) | 2.00
(1.35–2.96) |
<0.01a |
|
|
| Intrapancreatic
neural invasion (ne2, ne3) | 1.66
(1.14–2.43) | 0.008a |
|
|
| Portal vein
invasion (present) | 1.98
(1.34–2.86) |
<0.01a | 1.73
(1.20–2.51) |
<0.01a |
| Nerve plexus
invasion (present) | 1.47
(1.01–2.13) | 0.04a |
|
|
| Lymph node
metastasis (N1) | 1.91
(1.12–3.25) | 0.01a |
|
|
| Distant metastasis
(M1) | 1.30
(0.71–2.37) | 0.39 |
|
|
| ZNF185 expression
(positive on the plasma membrane) | 1.45
(1.008–2.11) | 0.04a | 2.19
(1.04–2.19) | 0.02a |
|
| B, Disease-free
survival |
|
| Age (≥65) | 0.90
(0.64–1.28) | 0.580 |
|
|
| Gender (male) | 1.14
(0.79–1.64) | 0.460 |
|
|
| Location (pancreas
head) | 1.07
(0.74–1.55) | 0.694 |
|
|
| Size (≥30 mm) | 2.16
(1.47–3.17) |
<0.001a |
|
|
| Histologic type
(G2, G3) | 1.48
(0.99–2.20) | 0.054 |
|
|
| Stroma
(scirrhous) | 0.68
(0.47–0.99) | 0.049a | 0.68
(0.46–1.004) | 0.053 |
| Lymphatic invasion
(ly2, ly3) | 1.98
(1.32–2.96) | 0.001a | 1.70
(1.12–2.57) | 0.012a |
| Venous invasion
(v2, v3) | 2.15
(1.48–3.12) |
<0.001a | 1.66
(1.13–2.45) | 0.009a |
| Intrapancreatic
neural invasion (ne2, ne3) | 1.62
(1.13–2.31) | 0.008a |
|
|
| Portal vein
invasion (present) | 1.45
(1.02–2.05) | 0.035a |
|
|
| Nerve plexus
invasion (present) | 1.68
(1.18–2.39) | 0.004a |
|
|
| Lymph node
metastasis (N1) | 2.29
(1.35–3.88) | 0.002a | 1.90
(1.12–3.24) | 0.017a |
| Distant metastasis
(M1) | 1.81
(1.08–3.03) | 0.023a |
|
|
| ZNF185 expression
(positive on the plasma membrane) | 1.44
(1.01–2.04) | 0.039a | 1.43
(1.003–2.05) | 0.048a |
Discussion
PDC has a typically poor prognosis due to its
aggressive behavior and extensive invasion and metastasis,
particularly hematogenous metastasis (1,2). ZNF185
has been isolated as a metastasis-associated protein by global
quantitative proteome analysis of the BxPC-3 and LM-BxPC-3 PDC cell
lines (11,15). In the present study, the expression of
ZNF185 in PDC was characterized clinicopathologically.
Firstly, using xenograft models, the unique
distribution of the ZNF185 molecule in cancer cells was observed.
In contrast to the tumor foci formed by the poorly metastatic
parent cells, of which only a small number expressed ZNF185 on
their cell membrane, the liver metastatic foci that formed
subsequent to the transplantation of highly metastatic cells
expressed cytoplasmic ZNF185 in all patients, and half of the cells
present at the periphery of cancer cell nests also expressed ZNF185
on their cell membranes. It is suggested that the amount and
subcellular location of ZNF185 are correlated with the position of
the cancer cells expressing it within cell nests. As the expression
of mZNF185 was limited to cells at the periphery of the cancer cell
nests that were in direct contact with the surrounding environment,
it is assumed that mZNF185 may serve a role in tumor behavior,
including motility.
Next, the distribution of ZNF185 in patients with
PDC was clinicopathologically investigated and it was identified
that mZNF185, but not cZNF185, was an unfavorable prognostic factor
in OS and DFS. Only marked venous invasion was significantly
correlated with mZNF185 expression among the various
clinicopathological variables examined (Table I). When the rates of hematogenous
metastasis (to the liver, lung, bone, pleura and adrenal glands)
following surgical resection were analyzed in the same PDC cohort,
it was identified to occur more frequently in patients with
mZNF185-positive cancer [51.7% (31/60)] as compared with in
patients without mZNF185-positive cancer [37.9% (39/103) odds
ratio; 1.82; 95% CI, 0.92–3.60; P=0.081]. It has been suggested
that higher expression levels of ZNF185 in colon cancer are
significantly associated with an increased frequency of liver
metastasis and are an indicator of poor prognosis (14). These data suggest that higher
expression levels of mZNF185 are associated with accelerated
hematogenous spreading of PDC. The occurrence of distant metastasis
was not determined to be correlated with mZNF185 expression, as all
patients with distant metastasis in the present study only
exhibited distant lymph node metastasis, and not other types of
distant metastasis (Table I).
It has been suggested that, in prostate cancer
cells, ZNF185 is co-localized primarily with F-actin and partially
with paxillin and vinculin (14).
Paxillin and vinculin are components of the cell matrix adhesion
apparatus (20,21). Focal adhesion, which provides a
structural link between cells and the extracellular matrix, also
facilitates signal transduction for the mediation of various
biological processes (22). Focal
adhesion involves numerous kinases, adaptors and cytoskeletal
proteins. The dynamics of focal adhesion turnover is a key
regulatory determinant of cancer cell migration, invasion and
metastasis (23). Paxillin binds to
numerous proteins involved in the organization of the actin
cytoskeleton, which are necessary for motility events associated
with tumor metastasis (20). The
overexpression of paxillin has been suggested to be associated with
distant metastasis and poor prognosis in colorectal cancer
(24), salivary adenoid cystic
carcinoma (25) and hepatocellular
carcinoma (26). Vinculin has also
been implicated in cell invasion and metastasis (21). It is speculated that ZNF185 serves a
role in the acceleration of cancer invasion and metastasis, in
association with focal adhesion. In our previous study, it was
suggested that patients who expressed ZNF185 also exhibited a poor
prognosis in cases of colon cancer (12). To the best of our knowledge, only one
previous study has indicated that ZNF185 functions as a
tumor-suppressive protein through an association with actin
cytoskeletal dynamics in prostate cancer (14). The present study identified that
mZNF185 was localized not only between cells and the extracellular
matrix, but also between cells that were present only in the
peripheral areas of cell nests (Fig.
1). Further prospective studies must additionally investigate
the involvement of mZNF185 in hematogenous metastasis in PDC.
In conclusion, the expression of mZNF185 is
hypothesized to be an independent indicator of unfavorable
prognosis in patients with PDC, being particularly evident in
highly liver-metastatic cancer cells located at the periphery of
cancer cell nests in xenograft models. It is also suggested that
mZNF185 may be involved in cancer cell motility, particularly
hematogenous metastasis, and that the amount and the subcellular
location of ZNF185 are each correlated with the location of the
cells expressing it in the cell nests.
Acknowledgements
This study was supported by a grant-in-aid for
Scientific Research to Daisuke Furukawa (type C; grant no.
16K07096) and Masato Nakamura (type B; grant no. 15H04287) from the
Ministry of Education, Culture, Sports, Science and Technology,
Japan.
References
|
1
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hruban RH, Boffetta P, Hiraoka N,
Iacobuzio-Donahue C, Kato Y, Kern SE, Klimstra DS, Kloppel G,
Maitra A, Offerhaus GJA, et al: Ductal Adenocarcinoma of the
Pancreas. In: World Health Organization Classification of Tumours.
Pathology & GeneticsTumours of the Digestive System. Bosman FT,
Carneiro F, Hruban RH and Theise ND: IARC; Lyon: pp. 281–291.
2010
|
|
3
|
Iacobuzio-Donahue CA, Fu B, Yachida S, Luo
M, Abe H, Henderson CM, Vilardell F, Wang Z, Keller JW, Banerjee P,
et al: DPC4 gene status of the primary carcinoma correlates with
patterns of failure in patients with pancreatic cancer. J Clin
Oncol. 27:1806–1813. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ueno H, Ioka T, Ikeda M, Ohkawa S,
Yanagimoto H, Boku N, Fukutomi A, Sugimori K, Baba H, Yamao K, et
al: Randomized phase III study of gemcitabine plus S-1, S-1 alone,
or gemcitabine alone in patients with locally advanced and
metastatic pancreatic cancer in Japan and Taiwan: GEST study. J
Clin Oncol. 31:1640–1648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moor M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgon-Bourgade S, de
la Fouchardiére C, et al: FOLFILINOX versus gemcitabine for
metastatic pancreatic cancer. N Engl J Med. 364:1817–1825. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oettle H, Neuhaus P, Hochhaus A, Hartmann
JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J,
Arning MB, et al: Adjuvant chemotherapy with gemcitabine and
long-term outcomes among patients with resected pancreatic cancer:
The CONKO-001 randomized trial. JAMA. 310:1473–1481. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Groot VP, Rezaee N, Wu W, Cameron JL,
Fisgman EK, Hruban RH, Weiss MJ, Zheng L, Wolfgang CL and He J:
Patterns, timing, and predictors of recurrence following
pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg. Mar
23–2017.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yokoyama N, Otani T, Hashidate H, Maeda C,
Katada T, Sudo N, Manabe S, Ikeno Y, Tuyoda A and Katayanagi N:
Real-time detection of hepatic micrometastases from pancreatic
cancer by intraoperative fluorescence imaging: Preliminary results
of a prospective study. Cancer. 118:2813–2819. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Satoi S, Yanagimoto H, Yamamoto T,
Toyokawa H, Hirooka S, Yamaki S, Opendro SS, Inoue K, Michiura T,
Ryota H, et al: A clinical role of staging laparoscopy in patients
with radiographically defined locally advanced pancreatic ductal
adenocarcinoma. World J Surg Oncol. 14:142016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suemizu H, Monnai M, Ohnishi Y, Ito M,
Tamaoki N and Nakamura M: Identification of a key molecular
regulator of liver metastasis in human pancreatic carcinoma using a
novel quantitative model of metastasis in
NOD/SCID/γcnull (NOG) mice. Int J Oncol.
31:741–751. 2007.PubMed/NCBI
|
|
12
|
Furukawa D, Chijiwa T, Matsuyama M, Mukai
M, Matsuo EI, Nishimura O, Kawai K, Suemizu H, Hiraoka N, Nakagohri
T, et al: Zinc finger protein 185 is a liver metastasis-associated
factor in colon cancer patients. Mol Clin Oncol. 2:709–713.
2014.PubMed/NCBI
|
|
13
|
Zheng Q and Zhao Y: The diverse
biofunctions of LIM domain proteins: Determined by subcellular
localization and protein-protein interaction. Biol Cell.
99:489–502. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang JS, Gong A and Young CY: ZNF185, an
actin-cytoskeleton-associated growth inhibitory LIM protein in
prostate cancer. Oncogene. 26:111–122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hamada K, Monnai M, Kawai K, Nishime C,
Kito C, Miyazaki N, Ohnishi Y, Nakamura M and Suemizu H: Liver
metastasis models of colon cancer for evaluation of drug efficacy
using NOD/Shi-scid IL2Rγnull (NOG) mice. Int J Oncol.
32:153–159. 2008.PubMed/NCBI
|
|
16
|
Matsuyama M, Wakui M, Monnai M, Mizushima
T, Nishime C, Kawai K, Ohmura M, Suemizu H, Hishiki T, Suematsu M,
et al: Reduced CD73 expression and its association with altered
purine nucleotide metabolism in colorectal cancer cells robustly
causing liver metastases. Oncol Lett. 1:431–436. 2010.PubMed/NCBI
|
|
17
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours. Wiley-Blackwell;
Hoboken, NJ: 2011
|
|
18
|
Japan-Pancreas-Society: Classification of
Pancreatic Cancer. Kanehara, Tokyo, Japan: 2011
|
|
19
|
Oguro S, Ino Y, Shimada K, Hatanaka Y,
Matsuno Y, Esaki M, Nara S, Kishi Y, Kosuge T and Hiraoka N:
Clinical significance of tumor-infiltrating immune cells focusing
on BTLA and Cbl-b in patients with gallbladder cancer. Cancer Sci.
106:1750–1760. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schaller MD: Paxillin: A focal
adhesion-associated adaptor protein. Oncogene. 20:6459–6472. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mierke CT: The role of vinculin in the
regulation of the mechanical properties of cells. Cell Biochem
Biophys. 53:115–126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yam JW, Tse EY and Ng IO: Role and
significance of focal adhesion proteins in hepatocellular
carcinoma. J Gastroenterol Hepatol. 24:520–530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nagano M, Hoshino D, Koshikawa N, Akizawa
T and Seiki M: Turnover of focal adhesions and cancer cell
migration. Int J Cell Biol. 2012:3106162012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao CJ, Du SK, Dang XB and Gong M:
Expression of paxillin is correlated with clinical prognosis in
colorectal cancer patients. Med Sci Monit. 21:1989–1995. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi J, Wang S, Zhao E, Shi L, Xu X and
Fang M: Paxillin expression levels are correlated with clinical
stage and metastasis in salivary adenoid cystic carcinoma. J Oral
Pathol Med. 39:548–551. 2010.PubMed/NCBI
|
|
26
|
Li HG, Xie DR, Shen XM, Li HH, Zeng H and
Zeng YJ: Clinicopathological significance of expression of
paxillin, syndecan-1 and EMMPRIN in hepatocellular carcinoma. World
J gastroenterol. 11:1445–1451. 2005. View Article : Google Scholar : PubMed/NCBI
|