Introduction
The continuous development of economy and technology
have led to improvements in both living standard and quality of
life, yet this has been coupled with an increase in several
diseases. Fang et al suggested that currently, malignant
tumor has become the fourth ranked disease, followed by
cardiovascular diseases, hypertension and diabetes (1). Additionally, Ren et al suggested
that malignant tumor is a major disease that affects healthy
living, and its incidence is 24.6% of the total incidence in China
(2). Wang et al showed that in
comparison to tumors on other sites, gastrointestinal tumors
(gastric, colon and esophageal cancer) are more accelerated in
growth, which leads to difficulty in treating advanced stages, and
high recurrence (3). The current
treatments of gastrointestinal malignant tumors include surgery,
radiation therapy and chemotherapy, the latter of which treatment
constitutes the primary treatment.
Oshikata et al identified that different
tumor cells have different resistance which influence
chemotherapeutic effects to some extent (4). Consequently, it is crucial for studies
to be conducted on tumor cell resistance. A study by Correia and
Bissell showed that the multidrug resistance (MDR) of tumors causes
many different metabolism transduction mechanisms, cytotoxicity
(toxic effects of different drugs gather in cells) and other
factors, as the resistance of tumor cells is very complex (5). Xuemin and Shusheng demonstrated that as
the MDR gene-coded product, P-glycoprotein is mainly located on the
surface of the membrane and can participate in intercellular
communication and communication among cells as an ATP-dependent
transmembrane transporter to some extent (6). Findings have shown that P-glycoprotein
can be regarded as cells that indicate a type of channel protein of
one-way drug output, which can transport intracellular medicine out
to decrease intracellular concentrations of relevant drug in the
cell (7,8). In this study, according to the
expression levels of MDR1 gene and P-glycoprotein of
gastrointestinal tumors in different tumor environments, we studied
the association between MDR1 gene, P-glycoprotein and
resistance of gastrointestinal tumors in order to provide
theoretical and experimental references for reasonable
chemotherapeutic drugs in the clinic.
Patients and methods
General information
In total, 126 cases of patients with
gastrointestinal tumors (colon cancer, esophageal cancer and
gastric cancer) admitted to the Liaocheng People's Hospital
(Shandong, China) from February 2013 to February 2015 were selected
for the present study. Prior to the experiment, the subjects did
not receive any chemotherapy, radiation therapy or immunotherapy,
to treat the gastrointestinal tumors. Following surgery, CT scan
and other detection methods, there were 38 samples with colon
cancer, 46 samples with esophageal cancer and 42 samples with
gastric cancer in 126 tissue samples. The average age of patients
was 48.6±27.3 years, the disease duration was 4.3±1.2 years and
there were 87 males and 39 females. There were 47
well-differentiated cases, 38 moderate differentiation cases and 41
poor differentiation cases. This study was approved by the Ethics
Committee of Shandong University. Signed written informed consents
were obtained from all participants before the study.
Methods
Clinical specimens
We randomly divided the selected samples into three
equal parts and preserved them in liquid nitrogen at −196°C. One
part was preserved for mRNA extraction, one for detection of the
expression of P-glycoprotein and one for ATP-TCA (tumor
chemosensitivity assay).
Extraction of RNA in tissue samples and
RT-PCR
We extracted RNA from the controls and patients with
gastrointestinal tumors in samples (before and after treatment) as
described below. Small tissue sample (0.1 g) was thawed on ice,
followed by the addition of 0.45 ml of RNA Plus. The tissue was
ground in a pre-cooling mortar in a 1.5-ml tube, followed by the
addition of 0.45 ml RNA Plus. The contents were then centrifuged at
1,500 × g for 5 min, followed by the addition of 200 µl of
chloroform and the tube was agitated for 15 sec. The solution was
centrifuged again at 10,000 × g for 15 min at 4°C. The supernatant
was transferred into a new EP tube containing RNase, equal volumes
of isopropanol were added and the samples were placed on the ice
for 10 min. The contents were centrifuged again at 10,000 × g for
10 min at 4°C. The supernatant was removed, 750 µl of 75% ethanol
was added and gently mixed. The contents were centrifuged again at
10,000 × g for 10 min at 4°C. The supernatant was removed, and
residual ethanol was also removed as much as possible. Finally, a
moderate volume of water was added to recover the RNA pellet.
Fluorescence quantitative PCR was used to detect the
expression of MDR1 gene. Oligon 7.0 software was used to
design primers (upstream, 5-'CCCATCATTGCAATAGC AGG-3′ and
downstream, 5′-G3TCAAACTYCTGCTCC TGA-3′). Reaction set up for
fluorescence quantitative PCR consisted of 5 µl of SYBR-Green and
0.5 µl of forward primer, 0.5 µl of reverse primer, 1 µl of RT
product and 3 µl of ddH20. The reaction was conducted at
95°C for 5 min, at 95°C for 10 sec, at 60°C for 30 sec, 40 cycles,
at 65°C for 10 sec and at 95°C for 10 sec.
P-glycoprotein expression
The expression of P-glycoprotein was measured using
enzyme-linked immunosorbent assay (ELISA) (Roche Diagnostics,
Basel, Switzerland). Absorbance was measured at 450 nm after the
reaction, and the protein expression was calculated according to
the standard protein curve. In addition, we verified the results of
ELISA by western blotting, as described elsewhere (9). All kits were purchased from Thermo
Fisher Scientific (Waltham, MA, USA). Rabbit monoclonal
P-glycoprotein antibody (dilution, 1:500; cat. no. ab170904) and
secondary goat anti-rabbit (HRP) IgG antibody (dilution, 1:2,000;
cat. no. ab6721) were purchased from Abcam (Cambridge, MA, USA).
The procedure was conducted as per instructions of the kit.
Selection of chemotherapeutic drugs
We selected chemotherapeutic drugs with obvious
effects on colonic, esophageal and gastric cancer, by including 10
types of drugs, including methotrexate, cisplatin, fluorouracil,
adriamycin, paclitaxel, oxaliplatin, calcium folinate, etoposide,
xeloda and irinotecan. We used proper concentrations of these
chemotherapeutic drugs to conduct related experiments and set
proper experimental concentrations. According to pharmacokinetic
guidance, we calculated the concentration of plasma value as
(µg/ml) = 50 × daily dosage in clinic (mg/kg) 2 ×
103/5,000.
Detection of tumor resistance
We used currently accepted ATP-TCA to perform
experiments of chemosensitivity on related tissues. First, we
dissected tumor tissues into small sections of 0.5–1 mm3
on ice. Subsequently, pancreatin was used for digestion at 37°C,
with the concentration adjusted to 2×104/ml.
Subsequently, single cell suspension was cultivated in a 96-well
plate with 2×104 cells in each well. The corresponding
anticarcinogen was added into each well as described in the
experimental design (Table I).
 | Table I.Experimental design of tumor
resistance. |
Table I.
Experimental design of tumor
resistance.
| Control group | Drug groups |
|---|
|
|
|
|---|
|
| Concentration 1
(2X) | Concentration 2
(1X) | Concentration 3
(0.5X) | Concentration 4
(0.25X) | Concentration 5
(0.125X) |
|---|
| Parallel repeat in
three groups | Parallel repeat in
three groups | Parallel repeat in
three groups | Parallel repeat in
three groups | Parallel repeat in
three groups | Parallel repeat in
three groups |
The plates were placed in a 5% CO2
incubator for 3–4 days at 37°C, and 0.1 ml of ATP extract of tumor
cells was added to the incubator. The plates were placed at room
temperature for 20–30 min, and were mixed gently. Mixture (0.05 ml)
was taken into the detecting board, and detected after the addition
of 0.05 ml of LU-LU. The inhibition ratio was calculated as: (1 -
(x - Mi)/(Mo - Mi), where x, M0 and Mi were inhibitory rates of
fluorescence intensity, and IC50 and IC90 were calculated on the
basis of the above data. The effects of different drugs on the
inhibitory rates of tumors were evaluated according to the
Kurbacher standard.
Statistical analysis
SPSS 20.0 statistical software (Chicago, IL, USA) to
analyze obtained data. The data were presented as mean ± standard
deviation to indicate related measurement results, and
χ2 was used for the measurement data. P<0.05 was
considered statistically significant.
Results
mRNA expression of MDR1 in different
gastrointestinal tumors
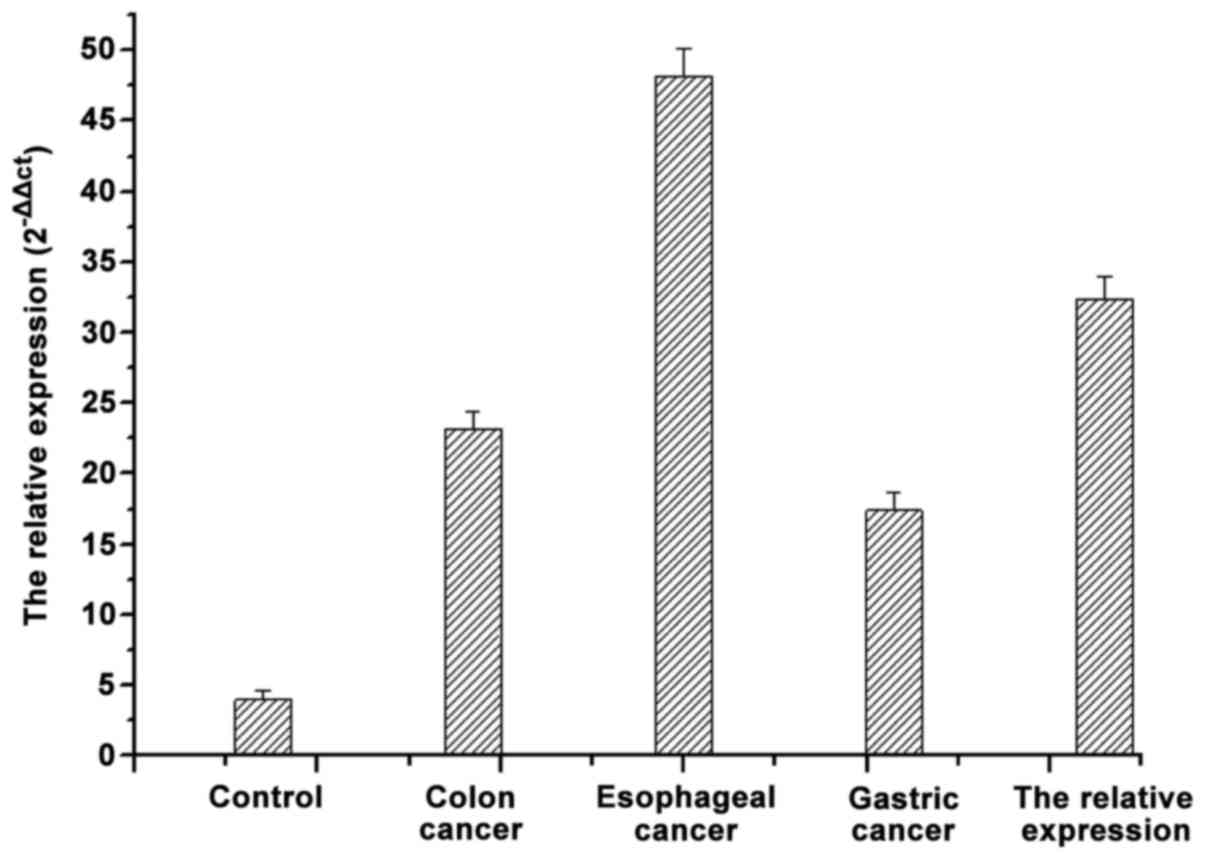
As shown in Fig. 1, in
comparison to the mRNA expression of MDR1 in tissues of the normal
digestive tract, the mRNA expression of MDR1 in samples of
patients with gastrointestinal tumor were significantly higher
(p<0.05). The mRNA of MRD1 was differentially expressed in the
different gastrointestinal tumors.
Expression of P-glycoprotein in different
gastrointestinal tumors
We detected the expression of P-glycoprotein in
different gastrointestinal tumors according to ELISA (Table II). In comparison to the control
group, the expression of P-glycoprotein in different
gastrointestinal tumors was significantly enhanced (p<0.05)
(Table II). We found a similar
observation from the western blotting results (Fig. 2). The expression of P-glycoprotein in
different gastrointestinal tumors was significantly higher than
that in tissues of controls (p<0.05).
 | Table II.Expression of P-glycoprotein in
different gastrointestinal tumors. |
Table II.
Expression of P-glycoprotein in
different gastrointestinal tumors.
| Group | Cases | Expression of
P-glycoprotein | χ2 | P-value |
|---|
| Controls | 36 | 4.3–5.2 |
|
|
| Esophageal
cancer | 46 | 26.4–36.7 | 98.6 | <0.05 |
| Colonic cancer | 38 | 16.4–25.8 | 95.3 | <0.05 |
| Gastric cancer | 42 | 14.3–20.2 | 94.7 | <0.05 |
Experiments of chemosensitivity by ATP-TCA
We found that different gastrointestinal tumors have
different sensitivities to different chemotherapeutic drugs, with
gastric cancer having the lowest sensitivity (Table III).
 | Table III.Detection of sensitivity for different
gastrointestinal tumors to different chemotherapeutic drugs. |
Table III.
Detection of sensitivity for different
gastrointestinal tumors to different chemotherapeutic drugs.
| Items | Cases | Insensitive to all
chemotherapeutic drugs | Sensitive to 1–4
types of chemotherapeutic drugs | Sensitive to 5–9
types of chemotherapeutic drugs | Sensitive to all
chemotherapeutic drugs |
|---|
| Colonic cancer | 38 | 6 | 11 | 21 | 0 |
| Esophageal
cancer | 46 | 0 | 21 | 20 | 5 |
| Gastric cancer | 42 | 16 | 26 | 0 | 0 |
| Total | 126 | 22 | 58 | 41 | 5 |
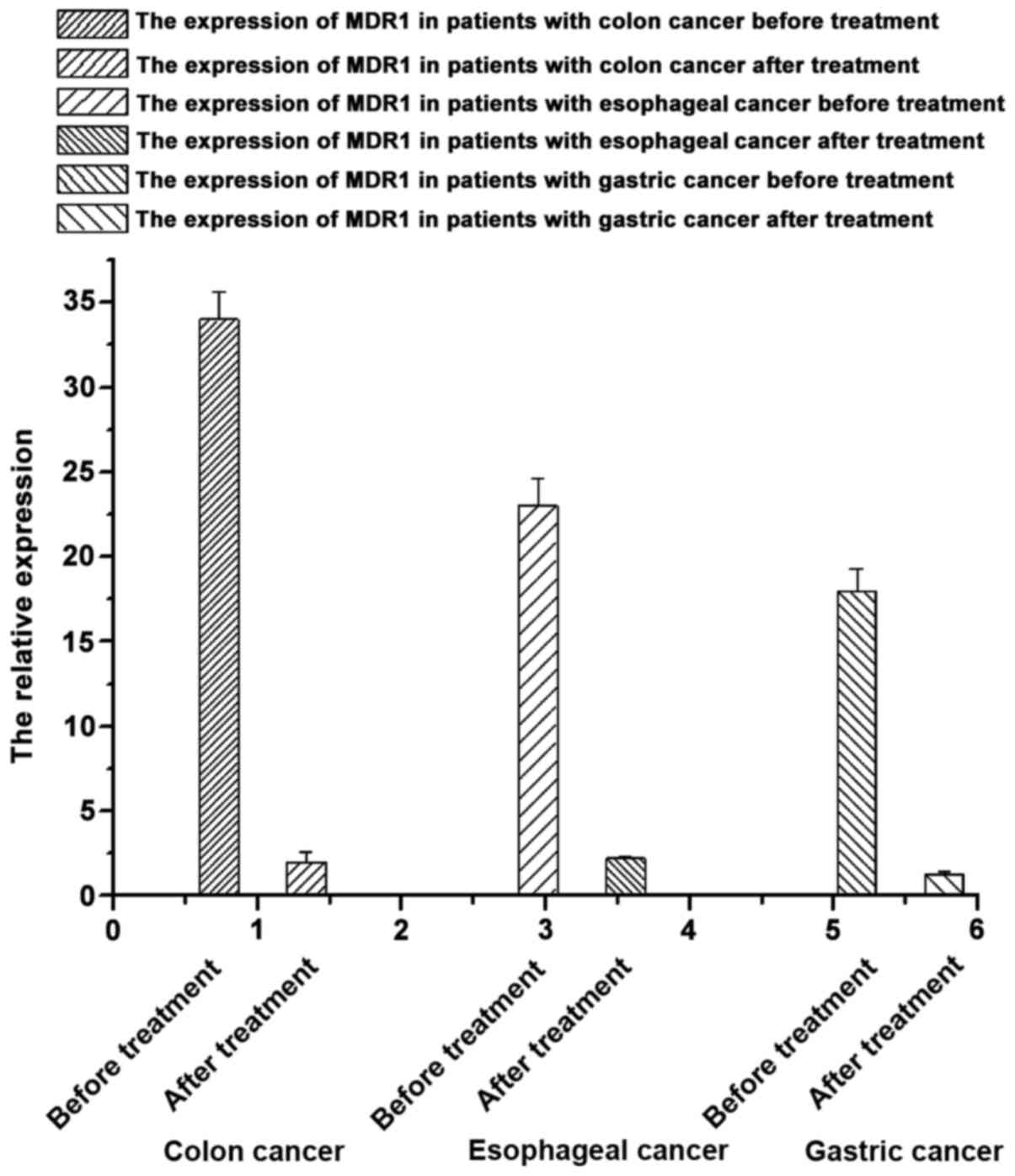
mRNA expression of MDR1 in patients with
gastrointestinal tumors using the same type of drugs before and
after chemotherapy
According to the results of the mRNA expression of
MDR1 in the tissue samples from patients with
gastrointestinal tumors before and after chemotherapy (Fig. 3), we found that the mRNA expression of
MDR1 in samples from patients with gastrointestinal tumors
after treatment were significantly lower than that before treatment
(p<0.05), suggesting that chemotherapeutic drugs can decrease
the mRNA expression of the MDR1 gene.
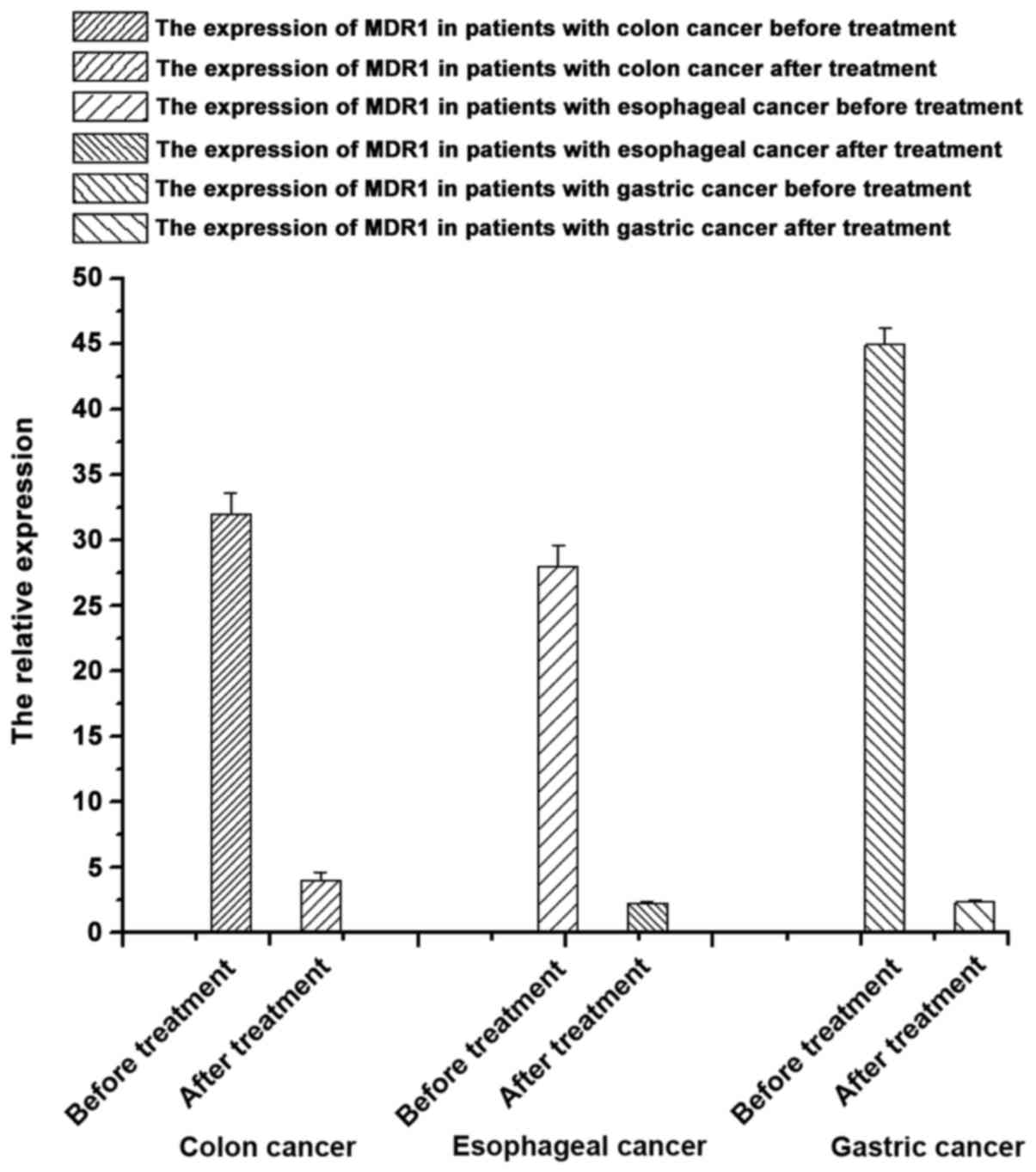
Expression of P-glycoprotein of patients with
gastrointestinal tumors using the same type of chemotherapeutic
drugs before and after chemotherapy
According to the results of the expression of
P-glycoprotein in samples from patients with different
gastrointestinal tumors before and after chemotherapy (Table III and Fig. 4), the expression of P-glycoprotein in
samples from patients with gastrointestinal tumors after
chemotherapy were significantly lower than that before treatment
(p<0.05), suggesting that chemotherapeutic drugs can decrease
the expression of P-glycoprotein of patients to some extent.
Discussion
Previous findings suggested that currently,
malignant tumors have become a major burden to society (10,11). In
recent years, its morbidity in China is on the increase. According
to statistical information from the Chinese Health Ministry in
2014, the lethality rate ranks second among urban and rural
residents in China, which is almost 25.45% of the total number of
deaths. The current treatments for gastrointestinal tumors are
mainly exairesis, radiation therapy and chemotherapy (12–14). Of
these treatments, exairesis cannot remove tumors that have formed
or transferred (15). Therefore, it
is mainly used in the early stage, and it is ineffective in
treating malignant tumors, or tumors in the late stages and cancer
(16). Compared with exairesis and
radiation therapy, chemotherapy has been widely applied in the
treatment of malignant tumors, especially gastrointestinal tumors.
Statistical information suggests that its total effective rate can
reach up to 30% (17).
During the progress of chemotherapy, the drugs with
chemical toxicity enter tumor cells to kill them. Therefore, tumor
cell resistance is very important for chemotherapy. Solbach et
al suggested that the occurrence of tumor cell resistance to
chemotherapeutic drugs can be divided into two categories (18). One is primary drug resistance, meaning
it is formed naturally and is not associated with medication in the
late stage, and the other is acquired drug resistance, meaning that
it is formed by related selectivity of chemotherapy drugs or
induction. Previous findings showed that P-glycoprotein containing
MDR1 gene encode can be regarded as one-way transport
protein in cells (19,20). Additionally, P-glycoprotein can remove
chemotherapeutic drugs in tumor cells in order that tumor cells are
not killed by chemotherapeutic drugs (21). In this study, we found that the mRNA
levels of MDRI in different gastrointestinal tumor cells had
substantially increased, and their contents were significantly
different from those in controls (p<0.05). The levels of
P-glycoprotein in the controls were also significantly different
from those in patients with gastrointestinal tumors (p<0.05).
These results suggest that MDR1 and P-glycoprotein are
related to gastrointestinal tumors. According to the analysis of
the experimental results of resistance of patients with different
gastrointestinal tumors by ATP-TCA, we identified that most
gastrointestinal tumors were sensitive to chemotherapeutic drugs,
albeit their sensitivity was different. According to detection of
the expression of MDR1 and P-glycoprotein in the samples of the
same patient before and after chemotherapy, the expression of MDR1
and P-glycoprotein after chemotherapy was decreased compared to
that before chemotherapy, suggesting chemotherapeutic drugs can
influence the expression of MDR1 and P-glycoprotein to some extent
to decrease related tumor cell resistance.
References
|
1
|
Fang H, Liang X, Cao Y, Luo H, Si Y and
Liu Z and Liu Z: Clinical effects of regional treating chemotherapy
on gastrointestinal tumors. Shaanxi Oncol Med. 3:23–32. 2001.(In
Chinese).
|
|
2
|
Ren R, Xin XY and Liu SJ: The expressions
of PKC-α and P-gp in in Taxol-resistant ovarian cancer cell line
A2780/Taxol. J Fourth Military Med Univ. 6:527–529. 2007.(In
Chinese).
|
|
3
|
Wang J, Jiang JW, Cai SJ, Peng H, Gao YQ,
Cao XD and Wu G: Clinical study on the effect of electroacupuncture
on cellular immune function in patients with gastrointestinal
tumor. J Acupunct Tuina Sci. 6:67–69. 2011.(In Chinese).
|
|
4
|
Oshikata A, Matsushita T and Ueoka R:
Enhancement of drug efflux activity via MDR1 protein by spheroid
culture of human hepatic cancer cells. J Biosci Bioeng.
111:590–593. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Correia AL and Bissell MJ: The tumor
microenvironment is a dominant force in multidrug resistance. Drug
Resist Updat. 15:39–49. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xuemin L and Shusheng W: The relationship
between diversity of MDR1 gene in mammary cancer and its expression
and toxicity of hematology caused by chemotherapy. Chin J Gen Surg.
11:78–80. 2012.(In Chinese).
|
|
7
|
Vincent M: Tesmilifene may enhance breast
cancer chemotherapy by killing a clone of aggressive, multi-drug
resistant cells through its action on the p-glycoprotein pump. Med
Hypotheses. 66:715–731. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rayes N, Seehofer D, Theruvath T, Schiller
RA, Langrehr JM, Jonas S, Bengmark S and Neuhaus P: Supply of pre-
and probiotics reduces bacterial infection rates after liver
transplantation - a randomized, double-blind trial. Am J
Transplant. 5:125–130. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moser JJ, Chan EK and Fritzler MJ:
Optimization of immunoprecipitation-western blot analysis in
detecting GW182-associated components of GW/P bodies. Nat Protoc.
4:674–685. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hans N, Maya S, Erich S, Christoph M,
Manfred Z, Diethelm W and Tanja F: Predicting resistance to
platinum-containing chemotherapy with the ATP tumor
chemosensitivity assay in primary ovarian cancer. Anticancer Res.
4:68–72. 2008.
|
|
11
|
Hongzhi J and Suobao L: Progress in
progress in the study of human intestinal microecosystem. Nature.
26:88–91. 2003.
|
|
12
|
Ying F: Human-being's knowledge of
intestinal microecology steps into a new stage. China Food Daily.
1:32–37. 2010.
|
|
13
|
Loo TW and Clarke DM: Recent progress in
understanding the mechanism of P-glycoprotein, mediated drug
efflux. J Membr Biol. 206:173–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ambudkar SV, Kim IW and Sauna ZE: The
power of the pump: mechanisms of action of P-glycoprotein (ABCB1).
Eur J Pharm Sci. 27:392–400. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shanshan M, Fei J, Hongwen L, Qingjie Z
and Jie Z: Effects of P-gp on blood-brain barrier of rat model of
chronic Parkinson's disease induced by paraquat and dopaminergic
neuron. Apoplexy Neuropsychiatr Illnesses. 6:57–61. 2013.
|
|
16
|
Ma X, Cai Y, He D, Zou C, Zhang P, Lo CY,
Xu Z, Chan FL, Yu S, Chen Y, et al: Transient receptor potential
channel TRPC5 is essential for P-glycoprotein induction in
drug-resistant cancer cells. Proc Natl Acad Sci USA.
109:16282–16287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamada O, Ozaki K, Furukawa T, Machida M,
Wang YH, Motoji T, Mitsuishi T, Akiyama M, Yamada H, Kawauchi K, et
al: Activation of STAT5 confers imatinib resistance on leukemic
cells through the transcription of TERT and MDR1. Cell Signal.
23:1119–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Solbach TF, König J, Fromm MF and Zolk O:
ATP-binding cassette transporters in the heart. Trends Cardiovasc
Med. 16:7–15. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Binkhathlan Z and Lavasanifar A:
P-glycoprotein inhibition as a therapeutic approach for overcoming
multidrug resistance in cancer: current status and future
perspectives. Curr Cancer Drug Targets. 13:326–346. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tsang TY, Tang WY, Chan JY, Co NN, Au
Yeung CL, Yau PL, Kong SK, Fung KP and Kwok TT: P-glycoprotein
enhances radiation-induced apoptotic cell death through the
regulation of miR-16 and Bcl-2 expressions in hepatocellular
carcinoma cells. Apoptosis. 16:524–535. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jamroziak K, Młynarski W, Balcerczak E,
Mistygacz M, Trelinska J, Mirowski M, Bodalski J and Robak T:
Functional C3435T polymorphism of MDR1 gene: an impact on genetic
susceptibility and clinical outcome of childhood acute
lymphoblastic leukemia. Eur J Haematol. 72:314–321. 2004.
View Article : Google Scholar : PubMed/NCBI
|