Introduction
The worldwide incidence of thyroid malignancy has
increased >2-fold since the 1970s (1). Improvements in the diagnostic
capabilities for microcarcinomas, for which long-term outcomes are
generally excellent, has contributed to this increase (2,3). Surgical
excision is the gold-standard method for differentiating malignant
from benign thyroid disease. Ultrasound (US) -guided fine needle
aspiration (FNA) with cytology (FNAC) is not warranted in the
evaluation of all thyroid nodules, >95% of which are benign
(4–6).
US is non-invasive and does not expose patients to
ionizing radiation, and high-resolution US has become the
gold-standard modality for imaging thyroid nodules. In its
conventional two-dimensional application, US is useful for guiding
FNA, for distinguishing cystic from solid nodules and for
elucidating other aspects of nodular morphology. Malignancy is more
likely in nodules exhibiting hypoechogenicity, punctate
microcalcifications, elevated central color flow, rough edges, a
high anteroposterior to transverse diameter or lack of a halo
(7). However, as a number of these
features lack accuracy in distinguishing benign lesions from
malignancy, the usefulness of conventional US is limited (8–11).
Tissue elasticity is another important index for
assessing the risk of malignancy. When a thyroid nodule is
palpable, an elevated index of suspicion is justified when physical
examination reveals that it is firm compared with the surrounding
tissue. Only a minority of thyroid nodules are palpable, however,
and assessment of firmness is subjective and influenced markedly by
the location and size of the lesion (4). Although the Doppler mode adds a valuable
dimension to conventional US, it provides no useful information
with respect to nodule elasticity (4,12–14).
First utilized to evaluate thyroid nodules by
Lyshchik et al (15), US
elastography (USE) has been used in other tissues since the early
1990s (16,17). By quantifying the degree of distortion
resulting from a force applied to tissue, USE generates estimates
of the stiffness/elasticity of a tissue and comparisons of the
stiffness of different tissues (4,7).
Consequently, the technique has been termed ‘electronic palpation’
(4). In comparison with soft nodules,
firm nodules are less prone to deformation when compressive forces
are applied (i.e. they are less elastic) and the lower elasticity
is associated with the risk of malignancy (4,7,18–20). In
addition to selecting high-risk nodules for further evaluation by
FNAC, the elasticity information from USE may aid in the diagnosis
of nodules that are deemed cytologically indeterminate (21).
The aim of the present study was therefore to
compare effectiveness of USE with that of conventional US in the
differential diagnosis of benign vs. malignant thyroid nodules.
Materials and methods
Patients
The present retrospective study was approved by the
Institutional Review Board of The First Affiliated Hospital of
Medical College (Xi'an Jiaotong University, Xi'an, China) who
waived the requirement for informed consent due to the
retrospective nature of the study. A total of 123 consecutive
patients [101 females and 22 males; age range, between 23 and 56
years (median, 40 years)] who underwent examination at The First
Affiliated Hospital of Medical College for thyroid nodules from
January 2012 to February 2013 were included. None of these patients
had any environmental exposure or genetic predisposition to thyroid
carcinoma.
The nodule diameters ranged between 0.5 and 1.6 cm.
All patients underwent routine preoperative conventional US and
elastography examinations. All nodules were confirmed by pathology.
The length of time between US and surgery was ~2 weeks.
A number of the 150 nodules were surgically excised
and a number of diagnoses were made using FNAC. Accepted criteria
for FNAC biopsy results were used and the pathologists who made the
histological diagnosis were blinded to the results of conventional
US and USE. No prior imaging was reviewed before enrollment.
Inclusion/exclusion criteria
To be included in the present study, each nodule
from each patient was required to meet the following requirements:
A maximum diameter <2 cm, and no obvious abnormal echo within
the surrounding thyroid tissue.
If the nodule volume was too large (maximum length
>2 cm), the cystic component of a nodule was >50% of the
total volume, or the nodule was located within the thyroidal
isthmus, the patient was excluded, because the above conditions
would result in poor elastographic image quality.
On the basis of parameters used in the routine US
diagnosis (22), all masses were
suspicious for malignancy if they contained solid hypoechoes,
unclear boundary, no peripheral halo, irregular shape, posterior
echo attenuation, an aspect ratio ≥1, increased perfusion or
diffuse internal sand-like microcalcifications. Therefore, those
patients with such nodules were considered high-risk patients.
Nodules suspected of malignancy were included in the present study.
Nodules that were highly suspicious for malignant tumors were
surgically resected. The subjects were also included in the study
group due to results of indeterminate FNAC and referral for
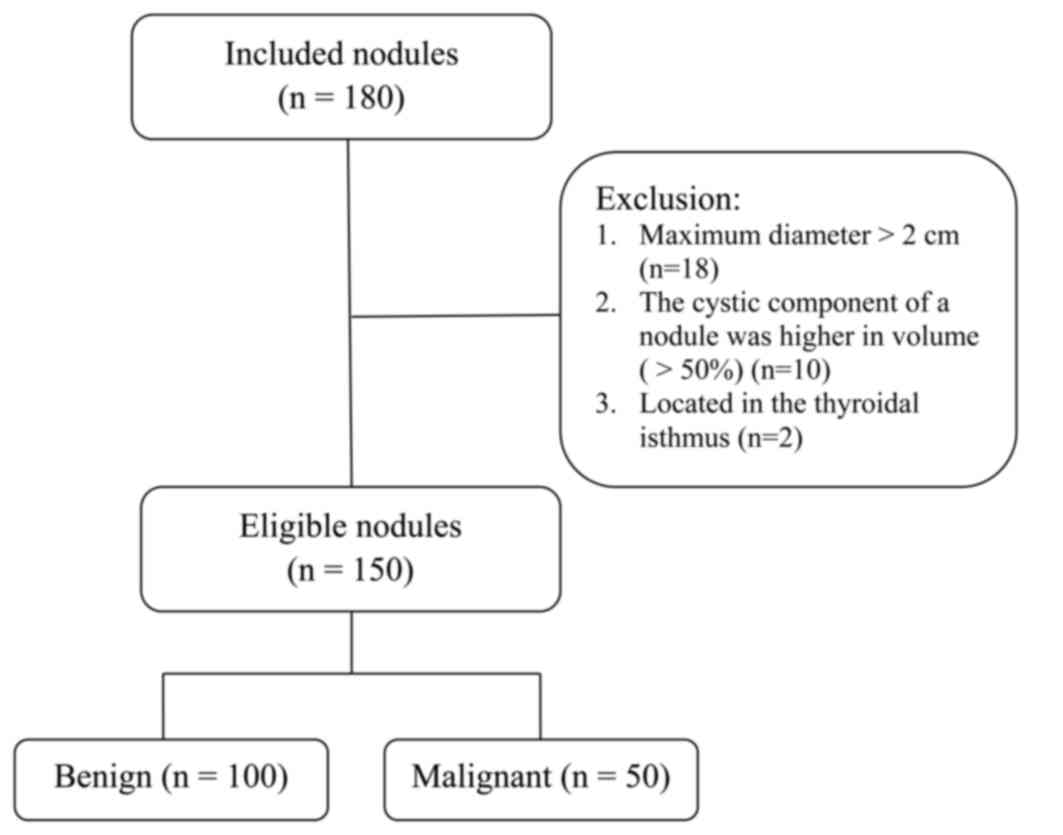
surgery. Of 180 nodules evaluated, 30 did not meet the inclusion
criteria and were therefore excluded. The remaining 150 nodules
from 123 patients were included in the present study for further
analysis (Fig. 1).
The presence of a single US criterion for malignancy
was sufficient for inclusion. Not all high-risk nodules exhibit all
the features of malignancy, i.e. a number exhibited only one or two
malignant features. Not all nodules suspicious for malignancy
exhibited internal microcalcifications. Masses with solid
hypoechoes, unclear boundary, irregular shape, posterior echo
attenuation, length/width ratio ≥1 and diffuse microcalcifications
were diagnosed as malignant lesions. Non-high-risk subjects did not
undergo USE. Only high-risk subjects were assessed using USE.
In order to avoid subjectivity of scoring, each
patient was diagnosed by two radiologists with >5 years of
experience in US diagnosis. If the two radiologists differed in
their diagnoses, they conferred to reach a final consensus. The
radiologists were blinded to the clinical data, but knew the
results of the traditional US.
Instrumentation
Elastographic data were obtained using an EUB-7500
platform and Hi Vision Preirus color Doppler ultrasonography
instrument (both Hitachi, Ltd., Tokyo, Japan) equipped with
real-time USE (RE) software. The probe frequency was between 6 and
13 MHz.
US evaluation
The lesions were first examined by grayscale US, and
routine horizontal and vertical scans were performed. Tumor
location, size, shape, boundary, internal echoes and cervical lymph
nodes were evaluated. Subsequently, color and pulsed Doppler
ultrasonography were performed to observe blood flow within the
lesions, followed by elastography. The sampling frame [i.e. the
region of interest (ROI)] was increased between 2- and 3-fold
compared with the lesion. A handheld probe was placed at the site
of the lesion and the pressure was maintained at between 3 and 4
according to the digital display. The dual-amplitude real-time
display function was used to display the two-dimensional and
elastography maps simultaneously to enable comparison between the
hardness of each lesion and its surrounding normal thyroid tissues.
When stable elasticity images were obtained, ROIs were selected in
both the lesional area and surrounding normal thyroid tissues to
calculate the strain ratio between the two.
Hardness grade of lesions on
elastography images
According to the color display of the lesions (i.e.
relative hardness), the lesion elasticity was divided into four
grades (23): Grade I, lesions and
surrounding tissues had uniform green color; grade II, lesions were
mainly green, and the periphery and a small amount of inside areas
were blue; grade III, lesions were mainly blue in color, exhibiting
disordered interlacing blue and green color; and grade IV, lesions
were completely blue.
Statistical analysis
Data are represented as the median with an
inter-quartile range (IQR, the range between the 25th and 75th
percentiles) for continuous variables due to non-normal data
distributions, and as frequencies with percentages for categorical
variables. The comparisons between benign and malignant groups were
performed by Wilcoxon's rank sum test for continuous data and by
χ2 test or Fisher's exact test for categorical data. The
receiver operating characteristic (ROC) curves were used to examine
the diagnostic performance characteristics of USE (i.e. grade and
strain ratio) vs. traditional US to distinguish benign and
malignant thyroid nodules. To compare the distinguishing ability
among three parameters, the point estimate with 95% confidence
interval (CI) of the area under the ROC curve (AUC) was provided as
an index to compare global test performance; pairwise comparison of
AUC among three parameters was also performed. Youden's index was
used to determine the optimal cut-off point to differentiate benign
from malignant thyroid nodules, which were determined by maximizing
the sum of sensitivity and specificity (24). The corresponding sensitivity,
specificity, positive predictive value (PPV), negative predictive
value (NPV) and accuracy were provided at the optimal threshold
values. The statistical analyses were performed using SAS software
(version 9.2; SAS Institute Inc., Cary, NC, USA), and two-tailed
P<0.05 was considered to indicate a statistically significant
difference.
Results
Demographic and pathological
characteristics of patients and thyroid nodules
The characteristics of patients and thyroid nodules
are summarized in Table I. Of 123
patients (a total of 150 thyroid nodules), 50 patients were
diagnosed with malignant nodules (papillary carcinoma, n=48;
follicular carcinoma, n=1; medullary carcinoma, n=1) and 100
patients were diagnosed with benign nodules (nodular goiter, n=70;
adenoma, n=28; Hürthle cell thyroid tumors, n=2). A total of 50
patients were identified to exhibit ≥1 malignant thyroid nodule (6
patients exhibited malignant and benign thyroid nodules) and 73
patients were identified to exhibit all benign thyroid nodules. The
patients in the malignant group were significantly younger compared
with those in the benign group [median (IQR), 36 (31–40) years vs.
44 (39–48) years; P<0.001]. The malignant thyroid nodules were
significantly smaller compared with the benign nodules [median
(IQR), 8.0 (6.0–9.0) mm vs. 9.0 (8.0–11.0) mm; P<0.001].
 | Table I.Demographic and pathological
characteristics of patients and thyroid nodules. |
Table I.
Demographic and pathological
characteristics of patients and thyroid nodules.
| Characteristic | Benign | Malignant | P-value |
|---|
| Patient
characteristics |
|
|
|
| No. of
patients | 73 | 50a |
|
| Median
age, years (IQR) | 44 (39–48) | 36 (31–40) |
<0.001b |
| Sex, n (%) |
|
|
|
|
Female | 64 (87.7) | 37 (74.0) | 0.052c |
| Male | 9 (12.3) | 13 (26.0) |
|
| Thyroid nodule
characteristics |
|
|
|
| No. of
thyroid nodules | 100 | 50 |
|
| Median
size, mm (IQR) | 9.0 (8.0–11.0) | 8.0 (6.0–9.0) |
<0.001b |
| Pathology, n
(%) |
|
|
|
|
Papillary carcinoma | 0 (0.0) | 48 (96.0) |
<0.001d |
|
Follicular carcinoma | 0 (0.0) | 1 (2.0) |
|
|
Medullary carcinoma | 0 (0.0) | 1 (2.0) |
|
| Nodular
goiter | 70 (70.0) | 0 (0.0) |
|
|
Adenoma | 28 (28.0) | 0 (0.0) |
|
| Hürthle
cell thyroid tumor | 2 (2.0) | 0 (0.0) |
|
Elastography grade and pathological
results of thyroid nodules
The distribution of elastography grades (25) between benign and malignant thyroid
nodules are presented in Table II.
In total, 86 (86%) benign thyroid nodules were classified as grade
I and II, and 45 (90%) malignant thyroid nodules were classified as
grade III and IV. A significant association between higher
elastography grade and malignant thyroid nodules was identified
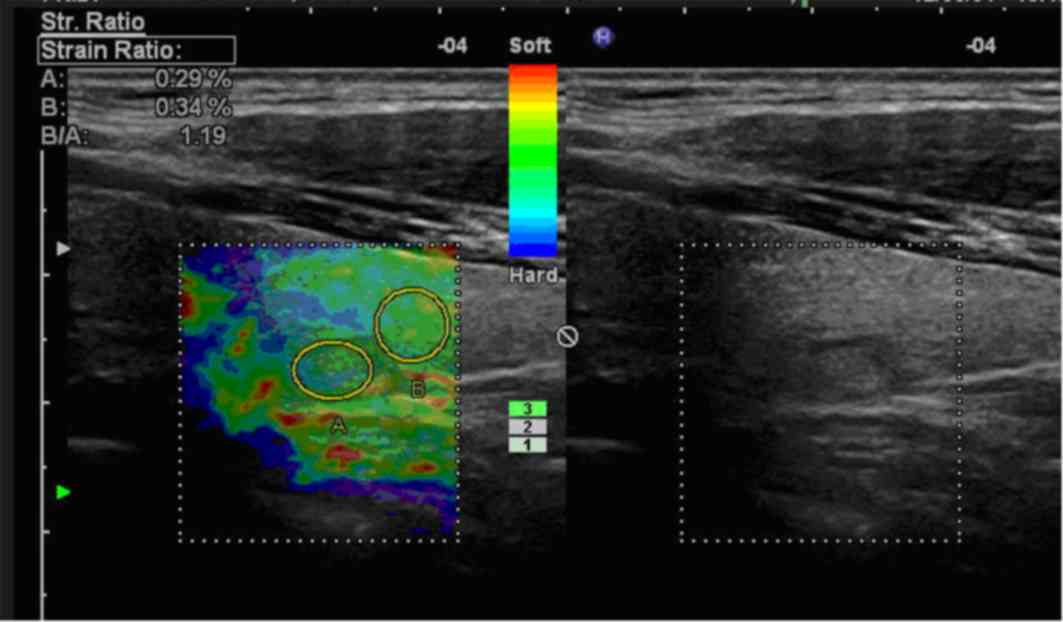
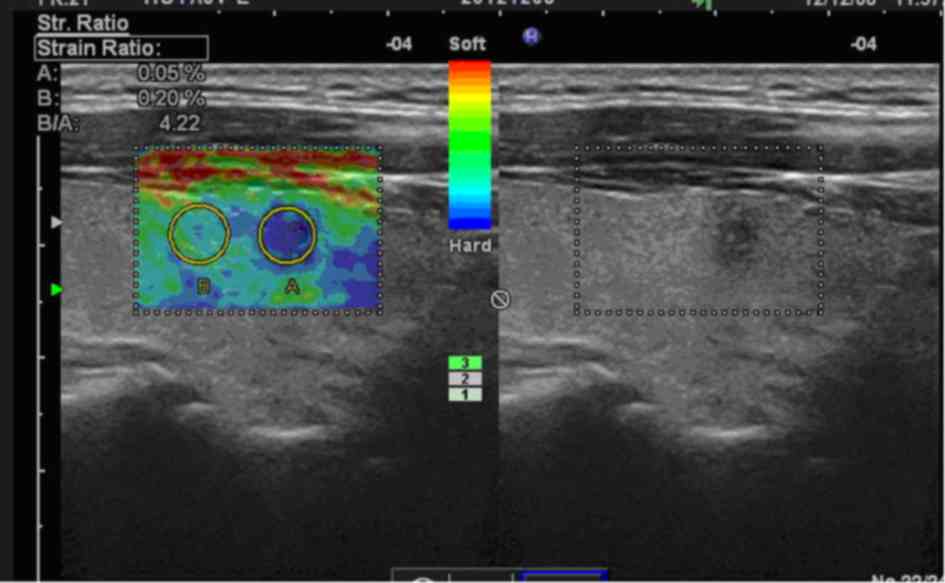
(P<0.001). Representative USE images are presented in Figs. 2 and 3.
 | Table II.Distribution of elastography grades
between benign and malignant thyroid nodules. |
Table II.
Distribution of elastography grades
between benign and malignant thyroid nodules.
| Elastography
grade | Benign, n (%) | Malignant, n
(%) |
|---|
| I | 46/100 (46.0) | 1/50 (2.0) |
| II | 40/100 (40.0) | 4/50 (8.0) |
| III | 10/100 (10.0) | 15/50 (30.0) |
| IV | 4/100 (4.0) | 30/50 (60.0) |
Strain ratio and pathological results
of thyroid nodules
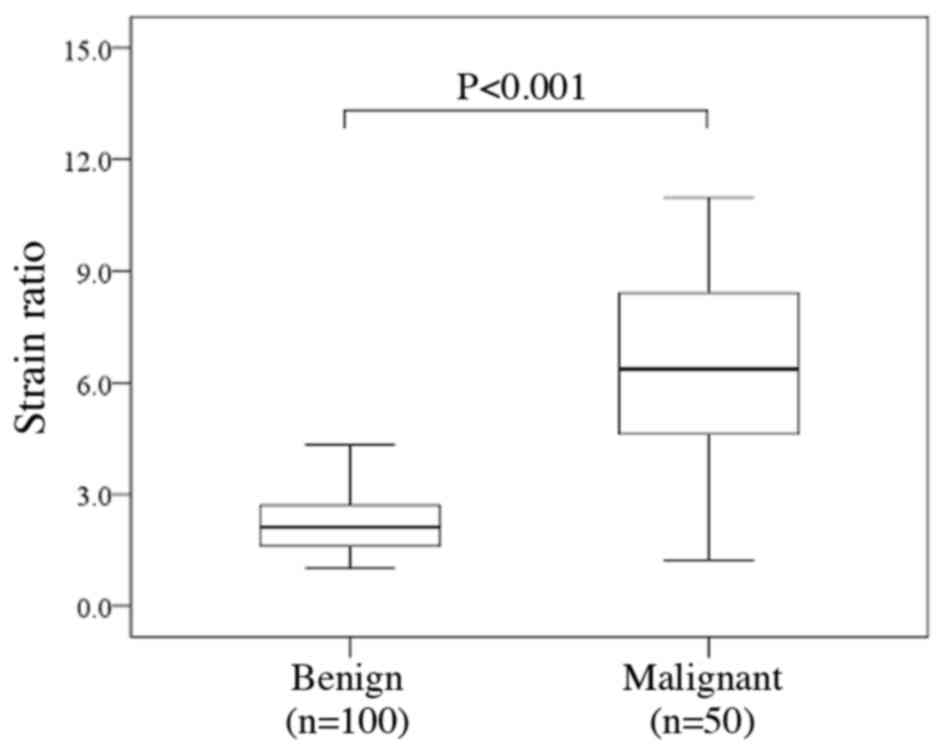
The distribution of strain ratio between benign and
malignant thyroid nodules is presented in Fig. 4. The malignant thyroid nodules
exhibited a significantly increased strain ratio compared with that
of benign nodules [median (IQR), 6.36 (4.62–8.42) vs. 2.12
(1.61–2.71); P<0.001].
ROC curve analysis using elastography
grade and strain ratio to distinguish benign and malignant thyroid
nodules
As elastography grade and strain ratio demonstrated
significant differences between benign and malignant thyroid
nodules, further ROC curve analysis was performed to compare the
discriminating ability between these two diagnostic approaches and
traditional US (Fig. 5). Compared
with traditional US, elastography grade and strain ratio
demonstrated significantly increased AUCs (P<0.001; Fig. 5). Additionally, the strain ratio
exhibited an improved diagnostic utility compared with traditional
US (P<0.001; Fig. 5). However, no
significant difference was identified for AUC between elastography
grade and strain ratio (P=0.902). Furthermore, the corresponding
sensitivity, specificity, PPV, NPV and accuracy were 90.0, 86.0,
76.3, 94.5 and 87.3% for elastography grade, 88.0, 93.0, 86.3, 93.9
and 91.3% for strain ratio, and 82.0, 69.0, 56.9, 88.5 and 73.3%
for traditional US, respectively (Table
III). The optimal threshold values corresponded to the highest
sum of sensitivity and specificity.
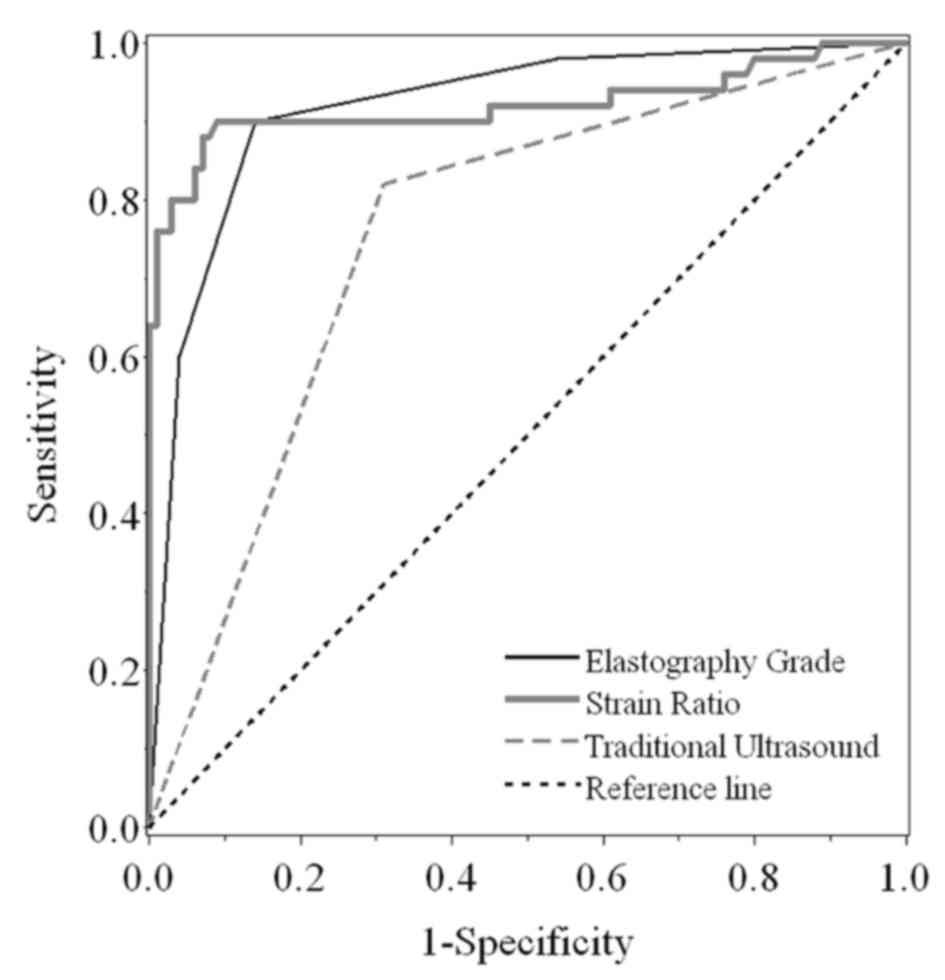 | Figure 5.Receiver operating characteristic
curve analyses. The specificity and sensitivity of using
elastography grade, strain ratio and traditional ultrasound to
distinguish benign from malignant thyroid nodules were compared.
The AUC was 0.918 (95% CI, 0.872–0.965), 0.921 (95% CI,
0.860–0.982) and 0.755 (95% CI, 0.685–0.826) for elastography
grade, strain ratio and traditional ultrasound, respectively. The
AUCs of elastography grade and strain ratio were significantly
increased compared with those of traditional ultrasound
(P<0.001). However, no significant difference in AUC was
identified between elastography grade and strain ratio (P=0.902).
AUC, area under the curve; CI, confidence interval. |
 | Table III.Comparison of diagnostic performance
in distinguishing benign from malignant thyroid nodules using
elastography grade, strain ratio and traditional ultrasound. |
Table III.
Comparison of diagnostic performance
in distinguishing benign from malignant thyroid nodules using
elastography grade, strain ratio and traditional ultrasound.
| Diagnostic
method | Sensitivity, %
(CI) | Specificity, %
(CI) | PPV, % (CI) | NPV, % (CI) | Accuracy, %
(CI) |
|---|
| Elastography
gradea | 90.0
(81.7–98.3) | 86.0
(79.2–92.8) | 76.3
(65.4–87.1) | 94.5
(89.8–99.2) | 87.3
(82.0–92.7) |
| Strain
ratiob | 88.0
(79.0–97.0) | 93.0
(88.0–98.0) | 86.3
(76.8–95.7) | 93.9
(89.2–98.6) | 91.3
(86.8–95.8) |
| Traditional
ultrasoundc | 82.0
(71.4–92.7) | 69.0
(59.9–78.1) | 56.9
(45.5–68.4) | 88.5
(81.4–95.6) | 73.3
(66.3–80.4) |
Discussion
US serves an important function in the detection,
diagnosis, prognosis and follow-up of a thyroid mass. However, its
value for the differentiation of benign from malignant masses is
limited. Microcalcifications, aspect ratio ≥1 and ragged edge have
been used as specific characteristics of malignant tumors (23,26). In
recent years, the incidence of micropapillary thyroid carcinoma has
increased (1,2). However, prognosis for this condition is
usually favorable provided that an early diagnosis is made, usually
via FNA, although it is an invasive technique (4–6).
Completely non-invasive real-time USE is able to differentiate
thyroid cancer from benignity by assessing tissue hardness and
elasticity, by grading the elasticity of thyroid nodules, and by
measuring the strain ratio (20,27–30).
In the present study, USE of 150 thyroid nodules
demonstrated primarily benign nodular goiter (n=100) with nodules
exhibiting a soft texture and significant deformation under
compression. Additionally, these nodules exhibited a large number
of follicles filled with colloid. Nodular texture was soft, and USE
demonstrated a lower grade of elasticity (grade I/II) in 86% of
benign nodules and a low strain ratio (2.30±1.01). Of the 100
nodules in the benign group, 86 nodules had an elasticity score
<3. However, 10 benign nodules had a score of 3, and 4 benign
nodules had a score of 4 and were misdiagnosed as false positives.
A number of nodules exhibited calcification, causing increased
stiffness, and nodule position may also have affected the
elasticity score.
In contrast, malignant nodules in the present study
were harder with less deformation under compression. The
pathological results demonstrated primarily papillary carcinoma,
with an interstitium accompanied by sand bodies yielding a hard
texture. USE of malignant nodules demonstrated a higher grade of
elasticity (grade III/IV) in 90% of nodules and an increased strain
ratio (6.39±2.50). Of the 50 nodules in the malignant group, 45
(90%) malignant thyroid nodules were classified as grade III and
IV. The elasticity scores of 15 malignant nodules was 3 and 30 had
elasticity scores of 4. The elasticity scores of the remaining five
malignant nodules were 1 in one nodule and 2 in four nodules; these
were misdiagnosed as false negatives. False negative results of USE
included a nodule whose histopathology exhibited medullary thyroid
cancer and another whose histopathology exhibited follicular
carcinoma. Cancer cells in a follicle-like arrangement with
deposition of amyloid-like substance within the interstitium may
create a soft texture causing a false negative USE reading. False
negative results on USE may also have resulted from large
quantities of fluid in cystic nodules, which may have decreased
their stiffness.
USE is particularly appropriate for the thyroid,
because of its accessible anatomic position (7,15,21,31,32). A
meta-analysis of eight studies evaluating real-time elastography
(RTE) and consisting a total of 639 thyroid nodules suggests that
RTE is able to differentiate malignancy accurately compared with
FNAC (33). Specifically, the
aforementioned meta-analysis associated malignancy with stiffness
with a sensitivity and specificity of 92% (CI 88–96%) and 90% (CI
85–95%), respectively. Additional trials have yielded similar
results for USE evaluation of thyroid nodules in groups of patients
who were later identified to have a high incidence of malignancy
(34).
A previous study by Vidal-Casariego et al
(35) failed to demonstrate accurate
differentiation of malignant and benign nodules when USE was used
on nodular goiter patients at low risk of thyroid malignancy.
However, this study involved a total of 128 patients and
examination of larger numbers of patients across multiple risk
groups is required prior to drawing definitive conclusions
regarding the utility of USE across risk groups. Additionally, the
accuracy of USE has been questioned in the setting of diffuse
thyroid disease, such as chronic Hashimoto thyroiditis (5,36). At the
same time, efforts to standardize USE protocols and enhance USE
imaging may lead to improved accuracy. These efforts include a
proposed classification system using linear discriminant analysis
to select possible malignant nodules detected by USE (37) and an innovation known as micropure
imaging (38).
The present study has identified that measuring the
strain ratio in USE may assist in the differentiation of benign
from malignant nodules. ROC curves were plotted for 123 patients
with benign and malignant nodules, and these provide a maximum
Youden index of 0.81, and a corresponding optimal cut-off point of
the elastic strain ratio of 3.68. Consequently, 3.68 was selected
as the threshold for differentiating benign from malignant thyroid
nodules. This value differs from strain ratio threshold values in
the literature possibly due to different measurement techniques and
operating practices. When two threshold points are identified from
two separate studies, it is necessary to determine whether study
participants come from populations with different sociodemographics
or clinical profiles, whether the diagnostic criteria of disease
(i.e. malignant vs. benign) were different or whether the
measurements of strain ratio (e.g. the instrument used and
parameter settings) were different. Therefore, owing to different
instruments and operating practices, the threshold values may vary.
AUC was also used to evaluate the diagnostic accuracy; the AUC of
the strain ratio value was 0.921, and the AUC of elasticity
classification was 0.761, demonstrating certain accuracy.
The present study exhibited several limitations
including its retrospective nature. In addition, the number of
patients with follicular carcinoma and medullary carcinoma was low.
Therefore, imaging features of follicular and medullary carcinoma
require further study. Furthermore, the number of patients with
papillary carcinoma was high. Therefore, future studies should
utilize more diverse patient groups. On the basis of our inclusion
and exclusion criteria, the present study was performed on
pre-selected high-risk patients as only nodules suspected of being
malignant were included. This pre-selection led to an increased
rate of malignancy compared with the normal rate (33%) in the
present study population as the denominator for our 33% malignancy
rate was a pre-selected population of thyroid nodules from
high-risk patients. In contrast, the denominator for the 95% benign
nodule rate is the general population (4–6). It is
anticipated that the present study involving 123 patients will be
expanded to include more patients, allowing for comparison of the
technique across various risk populations. Further large sample
studies are also required to investigate how to evaluate the USE
images of nodules complicated by diffuse thyroid disease. Finally,
owing to the study design, a comparative study on the intra- and
inter-examiner variation and reliability was not performed, which
may be included in the next phase of the investigation.
The results of the present study indicate that USE
demonstrates a high sensitivity for thyroid nodule classification
based on elasticity as it assessed the relative stiffness of
thyroid nodules accurately. USE may aid the differentiation of
benign from malignant thyroid nodules. However, the stiffness of
benign and malignant nodules overlaps to a certain extent,
particularly in the case of diffuse lesions, fibrosis or
calcification surrounding the nodules. In practice, USE may be
performed in combination with conventional US to facilitate
diagnosis.
Acknowledgements
The present study was supported by the Shanxi
Science and Technology Project Foundation (grant no.
2012k13-02-11).
References
|
1
|
Sipos JA and Mazzaferri EL: Thyroid cancer
epidemiology and prognostic variables. Clin Oncol (R Coll Radiol).
22:395–404. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Davies L and Welch HG: Increasing
incidence of thyroid cancer in the United States, 1973–2002. JAMA.
295:2164–2167. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li N, Du XL, Reitzel LR, Xu L and Sturgis
EM: Impact of enhanced detection on the increase in thyroid cancer
incidence in the United States: Review of incidence trends by
socioeconomic status within the surveillance, epidemiology, and end
results registry, 1980–2008. Thyroid. 23:103–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Andrioli M and Persani L: Elastographic
techniques of thyroid gland: Current status. Endocrine. 46:455–461.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhatia KS, Lee YY, Yuen EH and Ahuja AT:
Ultrasound elastography in the head and neck. Part II. Accuracy for
malignancy. Cancer Imaging. 13:260–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhan WW: Advances in ultrasound diagnosis
of thyroid nodules (J/CD). Chin J Med Ultrasound Electronic
Version. 8:1170–1179. 2011.
|
|
7
|
Mansor M, Okasha H, Esmat S, Hashem AM,
Attia KA and El-din Hussein H: Role of ultrasound elastography in
prediction of malignancy in thyroid nodules. Endocr Res. 37:67–77.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wong CK and Wheeler MH: Thyroid nodules:
Rational management. World J Surg. 24:934–941. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hegedüs L: Clinical practice. The thyroid
nodule. N Engl J Med. 351:1764–1771. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rago T, Vitti P, Chiovato L, Mazzeo S, De
Liperi A, Miccoli P, Viacava P, Bogazzi F, Martino E and Pinchera
A: Role of conventional ultrasonography and color flow-doppler
sonography in predicting malignancy in ‘cold’ thyroid nodules. Eur
J Endocrinol. 138:41–46. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tamsel S, Demirpolat G, Erdogan M, Nart D,
Karadeniz M, Uluer H and Ozgen AG: Power Doppler US patterns of
vascularity and spectral Doppler US parameters in predicting
malignancy in thyroid nodules. Clin Radiol. 62:245–251. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chan BK, Desser TS, McDougall IR, Weigel
RJ and Jeffrey RB Jr: Common and uncommon sonographic features of
papillary thyroid carcinoma. J Ultrasound Med. 22:1083–1090. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alexander EK, Marqusee E, Orcutt J, Benson
CB, Frates MC, Doubilet PM, Cibas ES and Atri A: Thyroid nodule
shape and prediction of malignancy. Thyroid. 14:953–958. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gharib H, Papini E, Valcavi R, Baskin HJ,
Crescenzi A, Dottorini ME, Duick DS, Guglielmi R, Hamilton CR Jr,
Zeiger MA, et al: American association of clinical endocrinologists
and associazione medici endocrinologi medical guidelines for
clinical practice for the diagnosis and management of thyroid
nodules. Endocr Pract. 12:63–102. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lyshchik A, Higashi T, Asato R, Tanaka S,
Ito J, Mai JJ, Pellot-Barakat C, Insana MF, Brill AB, Saga T, et
al: Thyroid gland tumor diagnosis at US elastography. Radiology.
237:202–211. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ophir J, Céspedes I, Ponnekanti H, Yazdi Y
and Li X: Elastography: A quantitative method for imaging the
elasticity of biological tissues. Ultrasonic Imaging. 13:111–134.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Havre R and Gilja OH: Elastography and
strain ratio imaging of the gastrointestinal tract. Eur J Radiol.
83:438–441. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li P, Song Y and Hu XT: Comparative study
on ultrasound elastography and conventional ultrasound for the
diagnosis of benign thyroid nodules. Tongji Univ Trans (Med Sci).
31:88–91. 2010.
|
|
19
|
Cochlin DL, Ganatra RH and Griffiths DF:
Elastography in the detection of prostatic cancer. Clin Radiol.
57:1014–1020. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Asteria C, Giovanardi A, Pizzocaro A,
Cozzaglio L, Morabito A, Somalvico F and Zoppo A: US-elastography
in the differential diagnosis of benign and malignant thyroid
nodule. Thyroid. 18:523–531. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rago T, Santini F, Scutari M, Pinchera A
and Vitti P: Elastography: New developments in ultrasound for
predicting malignancy in thyroid nodules. J Clin Endocrinol Metab.
92:2917–2922. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lv K, Jiang YX and Zhang JX: Ultrasound
diagnosis of thyroid nodules. Chin J Ultrasonography. 12:285–288.
2003.
|
|
23
|
Moon WJ, Jung SL, Lee JH, Na DG, Baek JH,
Lee YH, Kim J, Kim HS, Byun JS and Lee DH: Thyroid Study Group,
Korean Society of Neuro- and Head and Neck Radiology: Benign and
malignant thyroid nodules: US differentiation-multicenter
retrospective study. Radiology. 247:762–770. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Youden WJ: Index for rating diagnostic
test. Cancer. 3:32–35. 1950. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sande JA, Verjee S, Vinayak S, Amersi F
and Ghesani M: Ultrasound shear wave elastography and liver
fibrosis: A prospective multicenter study. World J Hepatol.
9:38–47. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wiest PW, Hartshorne MF, Inskip PD, Crooks
LA, Vela BS, Telepak RJ, Williamson MR, Blumhardt R, Bauman JM and
Tekkel M: Thyroid palpation versus high-resolution thyroid
ultrasonography in the detection of nodules. J Ultrasound Med.
17:487–496. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dan HJ, Wang Y and Dan HY: Use of
real-time ultrasound elastography in the diagnosis of small
solitary solid thyroid nodules. China Med Imaging Technol.
26:63–65. 2010.
|
|
28
|
Liu F and Xiao Y: Application of
Ultrasonic elastic strain ratio in the diagnosis of benign and
malignant thyroid nodules. Chin J Med Ultrasound Electronic
Version. 7:671–678. 2010.
|
|
29
|
Jeh SK, Jung SL, Kim BS and Lee YS:
Evaluating the degree of conformity of papillary carcinoma and
follicular carcinoma to the reported ultrasonographic findings of
malignant thyroid tumor. Korean J Radiol. 8:192–197. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luo BM, Zeng J, Ou B and Zhi H: Effects of
the size of the region of interest in breast ultrasound
elastography on the diagnostic result. Chin Med Imaging Technol.
23:1330–1332. 2007.
|
|
31
|
Ophir J, Alam SK, Garra B, Kallel F,
Konofagou E, Krouskop T and Varghese T: Elastography: Ultrasonic
estimation and imaging of the elastic properties of tissues. Proc
Inst Mech Eng H. 213:203–233. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hong YR, Liu XM, Li ZY, Zhang X, Chen M
and Luo Z: Real-time ultrasound elastography in the differential
diagnosis of benign and malignant thyroid nodules. J Ultrasound
Med. 28:861–867. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bojunga J, Herrmann E, Meyer G, Weber S,
Zeuzem S and Friedrich-Rust M: Real-time elastography for the
differentiation of benign and malignant thyroid nodules: A
meta-analysis. Thyroid. 20:1145–1150. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vorländer C, Wolff J, Saalabian S,
Lienenlüke RH and Wahl RA: Real-time ultrasound elastography-a
noninvasive diagnostic procedure for evaluating dominant thyroid
nodules. Langenbecks Arch Surg. 395:865–871. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vidal-Casariego A, López-González L,
Jiménez-Pérez A, Ballesteros-Pomar MD, Kyriakos G, Urioste-Fondo A,
Álvarez-San Martín R, Cano-Rodríguez I and de la Jiménez-García
Marina JM: Accuracy of ultrasound elastography in the diagnosis of
thyroid cancer in a low-risk population. Exp Clin Endocrinol
Diabetes. 120:635–638. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Magri F, Chytiris S, Capelli V, Alessi S,
Nalon E, Rotondi M, Cassibba S, Calliada F and Chiovato L: Shear
wave elastography in the diagnosis of thyroid nodules: Feasibility
in the case of coexistent chronic autoimmune Hashimoto thyroiditis.
Clin Endocrinol (Oxf). 76:137–141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Luo S, Kim EH, Dighe M and Kim Y: Thyroid
nodule classification using ultrasound elastography via linear
discriminant analysis. Ultrasonics. 51:425–431. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ciledag N, Arda K, Aribas BK, Aktas E and
Köse SK: The utility of ultrasound elastography and MicroPure
imaging in the differentiation of benign and malignant thyroid
nodules. AJR Am J Roentgenol. 198:W244–W249. 2012. View Article : Google Scholar : PubMed/NCBI
|